平滑肌细胞(smooth muscle cell, SMC)来自胚胎发育时期的中胚层, 分化为不同的细胞群并获得具有成年特征的分化表型, 即收缩型, 其主要功能是维持血管的弹性。分化成熟后的VSMC在内环境因子, 如血小板衍生生长因子-BB (platelet derived growth factor-BB, PDGF-BB)和血管紧张素Ⅱ(angiotensinⅡ, AngⅡ)等刺激下仍可以去分化, 成为分化程度较低的分泌表型, 这一过程称为SMC的表型转化, 表现为SMC异常增殖、迁移、凋亡及大量合成细胞外基质等。血管SMC表型转化是导致血管重构(包括血管弹力下降和血管管腔渐进性狭窄), 进而引起终末器官血流灌注不足、器官功能异常甚至衰竭的核心环节[1]。研究发现, 细胞信号传导途径与SMC表型转化有着密切的联系。microRNA (miRNA), 一类非编码单链RNA分子, 可通过碱基配对的方法, 作用于靶标基因的转录产物, 使其沉默或降解, 调控信号传导通路中重要基因, 如丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)[2]、转化生长因子β(transforming growth factor β, TGF-β)[3]和磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase, PI3K)[4]等的表达, 进而调控SMC表型的转化。microRNA调控信号传导通路在介导血管SMC表型转化中有重要作用[5]。
本文针对目前研究已经发现的调控SMC表型转化的信号传导通路(包括MAPK、TGF-β/Smad、PI3K/ Akt及其他信号传导通路), 及miRNA调控机制相关研究进展进行综述。
1 MAPK信号传导通路调控SMC表型转化中miRNA的调控作用真核细胞主要并存3条MAPK信号通路, 即细胞外调节蛋白激酶(ERK1/2)通路、C-JUN N末端激酶(JNK/SPAK)通路和p38分裂原通路。配体与酪氨酸激酶受体在细胞膜表面结合使酪氨酸残基磷酸化, 成为细胞内信号蛋白的结合位点, 通过大鼠肉瘤蛋白(rat sarcoma, Ras)依次激活迅速加速纤维肉瘤激酶(rapidly accelerated fibrosarcoma, Raf)、丝裂原活化蛋白激酶激酶(mitogen-activated protein kinase kinase, MEK)和MAPK, MAPK磷酸化下游底物分子、转录因子如血清反应因子(serum response factor, SRF)、krüppel样因子4 (krüppel-like factor 4, KLF4)等发挥生物学效应。miRNA作用于MAPK信号通路中的重要基因调节细胞周期蛋白(cyclin)、细胞周期素依赖性激酶(cyclin-dependent protein kinases, CDKs)、CDKs抑制剂(cyclin dependent kinase inhibitors, CKIs) p21、p27等调控细胞周期, 发挥调节VSMC表型转化的作用。
miRNA调控MAPK信号通路上关键分子表达, 同时也受MAPK信号通路调节, 共同决定VSMC表型转化(图 1)。正向调控中, qRT-PCR检测发现小鼠股动脉导丝损伤模型中损伤血管的miR-155表达是正常大鼠的3.5倍, 过表达的miR-155靶向蛋白哺乳动物不育系20样激酶2 (mammalian sterile 20-like kinase 2, MST2), 促进Raf-1和MEK通路之间的信号交联, 激活炎症和氧化应激反应, 诱导小鼠VSMC的增殖, 加重新生内膜生成[6], 但也有研究表示miR-155具有抑制AngⅡ诱导VSMC增殖的作用[7]。检测发现, 大鼠颈动脉球囊损伤模型中损伤血管的miR-181b与miR-31表达显著提高。miR-181b提高ERK和Akt磷酸化程度, 提高cyclin D1和CDK4表达, 降低p21、p27的表达, 从而促进SMC表型转化的作用, 该作用可被PI3K和MAPK信号通路的抑制剂LY294002和PD98059显著减弱[8]; 其中miR-31可降低靶标大肿瘤抑制基因同源物2 (large tumor suppressor homolog 2, LATS2)的转录与翻译, 抑制DNA聚合酶δ的辅助蛋白增殖细胞核抗原(proliferating cell nuclear antigen, PCNA)表达, 发挥促进血管重构的作用[9]。

|
Figure 1 microRNA play a two-way role in regulation of VSMC phenotype change mediated by MAPK signaling pathway. In the positive regulatory role, miR-155, miR-181b and miR-31 activate ERK/MAPK signaling pathway to promote the phenotype change of VSMC by direct target MST2, ERK and LATS2. In the negative regulatory role, miR-132/212, miR-182-3p, miR-133, miR-1298 and miR-185 inhibit the phenotype change of VSMC by targeting LRRIFIP 1, MYADM, Sp-1, Cx43 and P2Y6. MST2: Mammalian sterile 20-like kinase 2; LATS2: Large tumor suppressor homolog 2; LRRIFIP1: Leucine-rich repeat interacting protein-1; MYADM: Myeloid associated differentiation marker; Cx43: Connexin43; P2Y6: P2Y purinoceptor 6; VSMC: Vascular smooth muscle cell |
负向调控中, miRNA抑制ERK磷酸化, 抑制VSMC表型转化。在大鼠颈动脉球囊损伤模型中, 血管损伤后miR-132/212、miR-182-3p、miR-133和miR-1298都显著降低, 利用重组腺病毒混合胶孵育或慢病毒溶液浸润等方法使血管局部过表达相应的miRNA, 发现VMSC的增殖率与迁移能力显著减弱, 血管内膜损伤后的新生内膜增生得到有效抑制。其中, miR-132/212靶向作用于富含亮氨酸重复作用蛋白-1 (leucine-rich repeat interacting protein-1, LRRIFIP1), 转染LRRIFIP1可在ERK1/2总量不变的情况下显著提高磷酸化ERK1/2的水平, 诱导p27表达, 进而促进VSMC分化与凋亡[10]; miR-182-3p沉默髓系分化标记蛋白(myeloid associated differentiation marker, MYADM)表达, 抑制ERK1/2磷酸化水平, 逆转不对称二甲基精氨酸(asymmetric dimethylarginine, ADMA)诱导的人主动脉平滑肌细胞表型的转化[11]; 激活的ERK信号通路可上调血管中miR-133表达, miR-133沉默转录因子Sp-1, 促进SRF与心肌素(myocardin)形成转录复合物, 该复合物抑制平滑肌基因Tagln1、Acta2和Mhy11的表达[12]; 在硬化闭塞的动脉血管中, miR-1298因上游DNA CpG岛的甲基化表达显著低于正常血管, 实验发现miR-1298下调调控离子运动和信号分子的细胞间通道主要连接蛋白43 (connexin43, Cx43)的表达, 抑制Cx43对ERK信号通路的激活, 发挥抑制新生内膜的增生作用, 另外miR-1298可显著减弱PDGF-BB诱导的VSMC增殖与迁移[13]。在AngⅡ诱导下, 人主动脉平滑肌细胞中miR-185显著降低, 增加miR-185的表达可抑制嘌呤受体P2Y6 (P2Y purinoceptor 6)表达进而抑制ERK1/2磷酸化水平, 发挥抑制细胞的增殖作用[14]。
2 TGF-β/Smad信号传导通路调控VSMC表型转化中miRNA的调控作用TGF-β家族由一类结构、功能相关的多肽生长因子亚家族组成, 包括TGF-β、活化素(activin)和骨形态发生蛋白(bone morphogenetic protein, BMP)。TGF-β与TGF-β Ⅱ型受体(TGFBR2)结合后, 激活并募集TGF-βⅠ型受体(TGFR1)组合形成二聚体受体复合物。在该复合物中, TGFBR2自主磷酸化同时可激活TGFBR1, 进而磷酸化受体相关Smad蛋白, 启动胞内信号级联反应。Smads发挥着核-质穿梭作用, 与转录子共同调节靶基因转录。
miRNA包括miR-20a、miR-143/145、miR-24、let-7a、miR-33和miR-599抑制TGF-β/Smad信号通路(图 2), 发挥抑制VSMC表型转换的作用。在缺氧诱导的肺动脉高压中, 肺动脉中miR-143/145和miR-24表达显著增加。抑制血管平滑肌层miR-20a、miR-143/145和miR-24的表达可显著改善肺高压和右心室的肥厚。尽管缺氧条件下细胞中miR-20a没有统计学意义上的变化, 但胆固醇修饰寡核苷酸抑制剂antagomiR-20a抑制miR-20a的表达, 诱导BMPR2、转录因子Id-2、p21表达, 提高靶标Smad5蛋白含量和磷酸化水平, 显著改善肺高压的情况[15]; miR-145基因敲除小鼠动脉中TGFBR2表达降低, 细胞外基质(extracellular matrix, ECM)表达提高, miR-143/145靶向TGFBR2转录本, 阻断ECM沉积和Smad7表达[16]; 在缺氧诱导下, VSMC中miR-24的表达水平提高近两倍, 可作用于靶标脚手架蛋白Trb3 (tribbles-like protein-3)转录本, 减少Smad1和Smad2蛋白表达, 减弱BMP4对Smad1/5/8蛋白的磷酸化作用和TGF-β对Smad2的磷酸化作用[17]; 抑制miR-20a、miR-143/145和miR-24的表达, 可有效抑制VSMC表型的转化, 减轻肺动脉血管的重构。
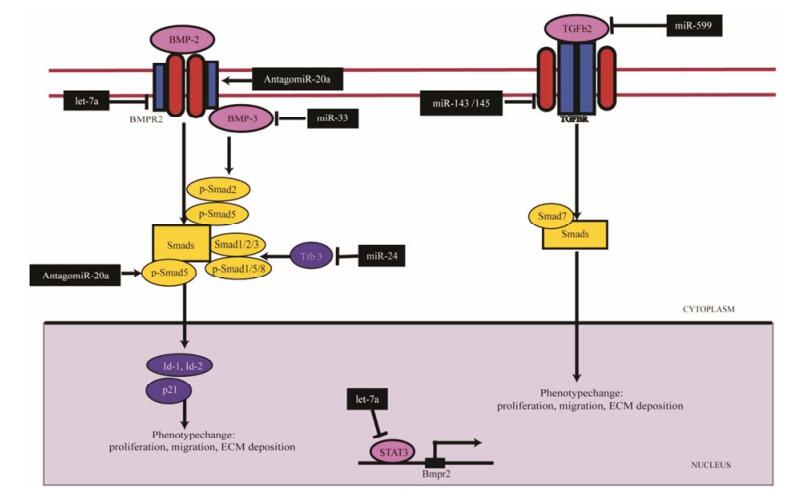
|
Figure 2 microRNA acts on TGF-β/Smad signaling pathway to regulate SMC phenotype change. miR-20a, miR-143/145 and miR-24 play a positive regulatory role in phenotypic change of VSMC by targeting Smad5, TGFBR2 and Trb3, while let-7a, miR-33 and miR-599 play a reverse regulatory role by targeting STAT3, BMP3 and TGFB2. Trb3: Tribbles-like protein-3; STAT3: Signal transducers and activators of transcription 3; BMP3: Bone morphogenetic protein 3; TGFBR2: TGF-β type Ⅱ receptor; TGFB2: Transforming growth factor β2 |
在抑制VSMC表型转化方面, 受损血管中let-7a和miR-33显著降低, 过表达的let-7a和miR-33可抑制VSMC增殖迁移, 发挥减缓血管重构的作用。在野百合碱(MCT)诱导的PAH大鼠肺动脉血管中, let-7a表达显著下降。let-7a靶向降低信号转导及转录激活因子3 (signal transducers and activators of transcription, STAT3)及下游分子BMPR2的表达, 用含let-7a的重组腺病毒的间充质干细胞(mesenchymal stem cells, MSC)作用于PAH大鼠可显著缓解血管的重构[18]; “套管法”静脉内膜移植引起的血管重构中, 移植血管中miR-33显著降低, 感染重组腺病毒过表达miR-33可抑制BMP3表达和下游分子Smad2和Smad5磷酸化水平, 降低细胞增殖率, 改善移植血管新生内膜增生[19]。此外, 在PDGF-BB刺激下, VSMC中miR-599表达降低, 过表达miR-599可抑制转化生长因子β2 (transforming growth factor β2, TGFB2)表达, 减弱ECM合成, 抑制VSMC的增殖和迁移[20]。
3 PI3K/Akt信号传导通路调控VSMC表型转化中miRNA的调控作用PI3K信号参与增殖、分化、凋亡和葡萄糖转运等多种细胞功能的调节。PI3K与生长因子受体或连接蛋白相互作用, 发生二聚体构象改变, 或通过和Ras直接结合激活, 在质膜上产生的第二信使PIP3促使细胞内信号蛋白Akt磷酸化, 进一步磷酸化下游靶蛋白哺乳动物雷帕霉素靶蛋白受体(mTOR)、p21等, 通过调节CDK、VSMC肌丝重组等方式, 诱导VSMC表型转化。
研究发现, 以PI3K-Akt信号通路关键分子为靶点的miRNA (图 3), 包括miR-138、miR-146、miR-761、miR-34c和miR-708等。在促进VSMC表型转化作用中, 缺氧条件下肺动脉平滑肌细胞(pulmonary artery smooth muscle cells, PASMC)中miR-138表达显著增加, miR-138可抑制细胞凋亡相关的哺乳动物不育系20样激酶1 (mammalian sterile 20-like kinase 1, MST1)的表达, 结合并活化Akt1, 促进细胞增殖迁移, 抑制凋亡, 加重PAH血管重构[21]。在抑制VSMC表型转化作用方面, miR-34c、miR-761、miR-10a和miR-708可抑制PI3K/Akt细胞信号通路发挥作用。在大鼠颈动脉球囊损伤血管重构模型中, 受损2周后血管中miR-34c-5p表达提高近4.5倍, miR-34c抑制干细胞因子(stem cell factor, SCF)表达, 逆转SCF与配体结合后Akt的磷酸化, 进一步抑制p21与p27表达而抑制血管新生内膜增生[22]。在AngⅡ诱导下, 增殖的VSMC中miR-761表达显著下降。miR-761降低mTOR的生理活性, 抑制下游p70S6激酶(p70S6K)作用, 干扰PI3K/Akt/mTOR/p70S6K信号通路对细胞G1期的调控, 使细胞停滞[23]。在胎牛血清(fetal bovine serum, FBS)和肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)表型转化刺激因子作用下, miR-10a和miR-708在人气道平滑肌(human airway smooth muscle, hASM)中表达都显著降低。miR-10a作用于PI3Ks, 降低Akt磷酸化水平, 抑制G2期/M期转化相关的cyclin B1和cyclin B2、G1/S期转化相关的CDK2和CDK6, 以及启动S期DNA合成的细胞分裂周期蛋白6 (cell division control protein 6, CDC6)的表达, 在人气道VSMC异常增殖中发挥重要作用[24]; qPCR检测发现哮喘患者气道平滑肌细胞在TNF-α作用下miR-708显著增高, miR-708作用于调控细胞钙和气道平滑肌收缩性的细胞表面白细胞分化抗原38 (cluster of differentiation 38, CD38), 增加Akt磷酸化水平和Akt2的表达, 增加阻断PI3K/Akt信号通路的人第10号染色体缺失的磷酸酶(phosphatase and tensin homolog deleted on chromosome ten, PTEN)的活性和表达, 从而发挥抑制VSMC增殖和血管的重构作用[25]。
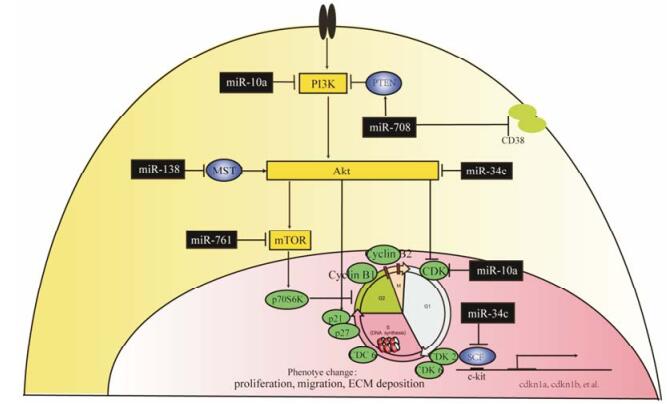
|
Figure 3 microRNA acts on the PI3K-Akt signaling pathway to regulate the phenotype change of VSMC. miR-138 target MST1 to activate PI3K/Akt signaling pathway to promote the phenotype change of VSMC, while miR-34c, miR-761, miR-10a and miR-708 inhibit PI3K/Akt signaling pathway by targeting SCF, mTOR, PI3K and CD38. MST1: Mammalian sterile 20-like kinase 1; SCF: Stem cell factor; PI3K: Phosphatidylino-sitol 3-kinases; CD38: Cluster of differentiation 38; mTOR: Mammalian target of rapamycin |
除上述信号通路外, miRNA通过AMP依赖的蛋白激酶(adenosine 5'-monophosphate-activated protein kinase, AMPK)、PDGF-BB、低氧诱导因子(hypoxia inducible factor-1, HIF-1)等关键分子对VSMC表型的转化也有着重要调节作用。
AMPK是一种保守的蛋白激酶, 在调控细胞生长、生物能量代谢等方面起着重要作用。AMPK通路活化, 促进p53磷酸化, 提高p21转录和翻译水平, 促进细胞周期停滞, 抑制VSMC增殖与迁移[26]。牵拉作用下小鼠的门静脉平滑肌中miR-144/451表达降低, miR-144/451可显著降低AMPK表达和5-氨基咪唑-4-甲酰胺核糖核苷酸(AICAR)诱导Akt的磷酸化, 沉默AMPK信号通路调控相关钙结合蛋白39 (calcium binding protein 39, CAB39)进而抑制VSMC表型转化[27]。在氧化修饰低密度脂蛋白(oxidized low density lipoprotein, oxLDL)作用下的人平滑肌细胞中, miR-195表达增高近13倍, miR-195抑制细胞分裂周期蛋白42 (cell division cycle 42, CDC42)表达, 抑制白介素-6、白介素-8等炎症因子诱导的VSMC增殖和迁移。在大鼠颈动脉球囊损伤模型中, 用含miR-195溶液孵育能显著改善受损血管的病理性重构[28], miR-195还能作用于AMPK通路抑制小鼠心肌的肥大[29]。
PDGF-BB与受体结合表现为细胞的增殖、迁移等生物学效应[30], PDGF活化后抑制平滑肌细胞特异基因的表达, 诱导VSMC的去分化作用, 增加细胞增殖和迁移, 促进损伤血管病理性重构。研究发现, 在PDGF-BB诱导下, VMSC中miR-221/222、miR-15b、miR-24/29a表达显著升高, 而miR-328表达降低。其中, miR-221/222下调p27的表达, 通过作用于心肌素抑制VMSC收缩型基因的转录, 促进细胞的增殖[31]; miR-15b沉默cyclin D、cyclin E从而抑制VSMC增殖[32]; 在小鼠颈动脉导丝损伤术和颈动脉结扎术所致的血管损伤中, 在心肌素诱导下, miR-24/29a下调血小板衍生生长因子受体(platelet-derived growth factor receptor, PDGFR)表达, 降低VSMC迁移, 抑制新生内膜的增厚[33]; 检测PCNA的表达和划痕实验发现, 有或者没有PDGF-BB的作用下, miR-328都可通过降低靶标Ser/Thr蛋白激酶-1 (Ser/Thr-protein kinase-1, PIM-1)转录和翻译, 从而抑制PASMC增殖与迁移[34]。
HIF-1由HIF-1α和HIF-1β两个亚基组成, 对低氧环境中基因的转录调控起主导作用, 调控一些对细胞适应性有着重要影响的miRNA[35], 同时, 一些miRNA也可以作用于HIF-1, 从而在血管重构中发挥重要作用。在缺氧诱导的大鼠PASMC中, miR-9和miR-206表达升高显著, 而miR-103/107表达降低。其中, miR-9作用于转录增强子促进HIF-1α转录, 抑制VSMC分化相关基因表达, 促进细胞增殖[36]; miR-206提高HIF-1α及其调控子Fhl-1 (four and a half LIM domains 1)表达, 下调cyclin D, 促进VSMC表型转化[37]; 而过表达的miR103/107可显著降低HIF-1β的转录和翻译水平, 抑制VSMC增殖和缺氧诱导下肺高压血管的重构[38]。
文中miRNA对信号通路和VSMC表型转化的作用列于表 1。
| Table 1 microRNA regulates the phenotypic change of smooth muscle cell mediated by signal pathways |
由于物理性质稳定, miRNA是个很有前景的疾病诊断与治疗的靶标。研究发现, miRNA在特发性肺纤维化[39]和肿瘤的多药耐药[40]中有着重要的调控作用, miRNA作为生物标记物也是个不错的选择, 据报道, miR-941和miR-19a可作为急性冠状动脉综合征和PAH生物标记物[41, 42]; miR-1、miR-133、miR-21、miR-24、miR-320、miR-29、miR-92a、miR-126、miR-199a、miR-208和miR-195在心肌梗死和其他心脏方面疾病中是很有前景的生物标记物[43]。
不仅如此, miRNA还用于基因治疗。根据2015年4月汤森路通的报告, 目前, 已有200多个miRNA药处在不同阶段的研发过程中。一些以miRNA为靶点的治疗药物已经进入临床试验, 如增强内源性miR-34表达的肿瘤抑制剂进入Ⅰ期临床试验, 用于肝炎治疗的miR-122抑制剂正在开展的Ⅱ期临床试验[44]。单个miRNA介导基因沉默能够作用于调节VSMC表型转化单个或多个信号通路中多个重要基因, 实现对疾病的多靶点调控。结合分子信号机制和表观遗传学, 由点到面、深入探究miRNA调控信号传导通路在VSMC表型转化中的作用, 对于血管重构这一复杂病理及相关疾病的早期诊断、发病机制、潜在靶点与创新药物研发, 具有积极意义。
| [1] | Coll-Bonfill N, Cruz-Thea BDL, Pisano MV, et al. Noncoding RNAs in smooth muscle cell homeostasis:implications in phenotypic switch and vascular disorders[J]. Pflug Arch Eur J Phy, 2016, 468: 1071–1087. DOI:10.1007/s00424-016-1821-x |
| [2] | Long X, Cowan SL, Miano JM. Mitogen-activated protein kinase 14 is a novel negative regulatory switch for the vascular smooth muscle cell contractile gene program[J]. Arterioscl Throm Vas, 2013, 33: 378–386. DOI:10.1161/ATVBAHA.112.300645 |
| [3] | Luo T, Cui S, Bian C, et al. Crosstalk between TGF-β/Smad3 and BMP/BMPR2 signaling pathways via miR-17-92 cluster in carotid artery restenosis[J]. Mol Cell Biochem, 2014, 389: 169–176. DOI:10.1007/s11010-013-1938-6 |
| [4] | Fan Z, Li C, Qin C, et al. Role of the PI3K/AKT pathway in modulating cytoskeleton rearrangements and phenotype switching in rat pulmonary arterial vascular smooth muscle cells[J]. DNA Cell Biol, 2014, 33: 12–19. DOI:10.1089/dna.2013.2022 |
| [5] | Joshi SR, Comer BS, Mclendon JM, et al. microRNA regulation of smooth muscle phenotype[J]. Mol Cell Pharmacol, 2012, 4: 1–16. |
| [6] | Yang Z, Zheng B, Zhang Y, et al. miR-155-dependent regulation of mammalian sterile 20-like kinase 2(MST2) coordinates inflammation, oxidative stress and proliferation in vascular smooth muscle cells[J]. Biochim Biophys Acta, 2015, 1852: 1477–1489. DOI:10.1016/j.bbadis.2015.04.012 |
| [7] | Yang LX, Liu G, Zhu GF, et al. microRNA-155 inhibits angiotensin Ⅱ-induced vascular smooth muscle cell proliferation[J]. J Renin Angiotensin Aldosterone Syst, 2014, 15: 109–116. DOI:10.1177/1470320313503693 |
| [8] | Li TJ, Chen YL, Gua CJ, et al. microRNA 181b promotes vascular smooth muscle cells proliferation through activation of PI3K and MAPK pathways[J]. Int J Clin Exp Pathol, 2015, 8: 10375–10384. |
| [9] | Liu X, Cheng Y, Chen X, et al. microRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2[J]. J Biol Chem, 2011, 286: 42371–42380. DOI:10.1074/jbc.M111.261065 |
| [10] | Choe N, Kwon JS, Kim JR, et al. The microRNA miR-132 targets Lrrfip1 to block vascular smooth muscle cell proliferation and neointimal hyperplasia[J]. Atherosclerosis, 2013, 229: 348–355. DOI:10.1016/j.atherosclerosis.2013.05.009 |
| [11] | Sun L, Bai Y, Zhao R, et al. Oncological miR-182-3p, a novel smooth muscle cell phenotype modulator, evidences from model rats and patients[J]. Arterioscl Throm Vas, 2016, 36: 1386–1397. DOI:10.1161/ATVBAHA.115.307412 |
| [12] | Torella D, Iaconetti C, Catalucci D, et al. microRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo[J]. Circ Res, 2011, 109: 880–893. DOI:10.1161/CIRCRESAHA.111.240150 |
| [13] | Hu W, Wang M, Yin H, et al. microRNA-1298 is regulated by DNA methylation and affects vascular smooth muscle cell function by targeting connexin 43[J]. Cardiovasc Res, 2015, 107: 534–545. DOI:10.1093/cvr/cvv160 |
| [14] | Wang S, Tang L, Zhou Q, et al. miR-185/P2Y6 axis inhibits angiotensin Ⅱ-induced human aortic vascular smooth muscle cell proliferation[J]. DNA Cell Biol, 2017, 36: 377–385. DOI:10.1089/dna.2016.3605 |
| [15] | Brock M, Samillan VJ, Trenkmann M, et al. AntagomiR directed against miR-20a restores functional BMPR2 signal-ling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension[J]. Eur Heart J, 2014, 35: 3203–3211. DOI:10.1093/eurheartj/ehs060 |
| [16] | Zhao N, Koenig SN, Trask AJ, et al. miR145 regulates TGFBR2 expression and matrix synthesis in vascular smooth muscle cells[J]. Circ Res, 2015, 116: 23–24. DOI:10.1161/CIRCRESAHA.115.303970 |
| [17] | Wu C. Molecular basis for antagonism between PDGF and the TGFβ family of signaling pathways by control of miR-24 expression[J]. EMBO J, 2010, 29: 559–573. DOI:10.1038/emboj.2009.370 |
| [18] | Cheng G, Wang X, Li Y, et al. let-7a-Transfected mesen-chymal stem cells ameliorate monocrotaline-induced pulmonary hypertension by suppressing pulmonary artery smooth muscle cell growth through STAT3-BMPR2 signaling[J]. Stem Cell Res, 2017, 8: 34. DOI:10.1186/s13287-017-0480-y |
| [19] | Huang K, Bao H, Yan ZQ, et al. microRNA-33 protects against neointimal hyperplasia induced by arterial mechanical stretch in the grafted vein[J]. Cardiovasc Res, 2017, 113: cvw257. |
| [20] | Xie B, Zhang C, Kang K, et al. miR-599 inhibits vascular smooth muscle cells proliferation and migration by targeting TGFB2[J]. PLoS One, 2015, 10: e0141512. DOI:10.1371/journal.pone.0141512 |
| [21] | Li S, Ran Y, Zhang D, et al. microRNA-138 plays a role in hypoxic pulmonary vascular remodelling by targeting Mst1[J]. Biochem J, 2013, 452: 281–291. DOI:10.1042/BJ20120680 |
| [22] | Choe N, Kwon JS, Yong SK, et al. The microRNA miR-34c inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by targeting stem cell factor[J]. Cell Signal, 2015, 27: 1056–1065. DOI:10.1016/j.cellsig.2014.12.022 |
| [23] | Cho JR, Lee CY, Lee J, et al. microRNA-761 inhibits angiotensin Ⅱ-induced vascular smooth muscle cell proliferation and migration by targeting mammalian target of rapamycin[J]. Clin Hemorheol Micro, 2015, 63: 45–56. |
| [24] | Hu R, Pan W, Fedulov AV, et al. microRNA-10a controls airway smooth muscle cell proliferation via direct targeting of the PI3 kinase pathway[J]. FASEB J, 2014, 28: 2347–2357. DOI:10.1096/fj.13-247247 |
| [25] | Dileepan M, Jude JA, Rao SP, et al. microRNA-708 regu-lates CD38 expression through signaling pathways JNK MAP kinase and PTEN/AKT in human airway smooth muscle cells[J]. Resp Res, 2014, 15: 1–12. DOI:10.1186/1465-9921-15-1 |
| [26] | Liang KW, Yin SC, Ting CT, et al. Berberine inhibits plate-let-derived growth factor-induced growth and migration partly through an AMPK-dependent pathway in vascular smooth muscle cells[J]. Eur J Pharmacol, 2008, 590: 343–354. DOI:10.1016/j.ejphar.2008.06.034 |
| [27] | Turczynska KM, Bhattachariya A, Sall J, et al. Stretch-sensitive down-regulation of the miR-144/451 cluster in vascular smooth muscle and its role in AMP-activated protein kinase signaling[J]. PLoS One, 2013, 8: e65135. DOI:10.1371/journal.pone.0065135 |
| [28] | Wang YS, Wang HY, Liao YC, et al. microRNA-195 regu-lates vascular smooth muscle cell phenotype and prevents neointimal formation[J]. Cardiovasc Res, 2012, 95: 517–526. DOI:10.1093/cvr/cvs223 |
| [29] | Chen H, Untiveros GM, Mckee LA, et al. micro-RNA-195 and -451 regulate the LKB1/AMPK signaling axis by targeting MO25[J]. PLoS One, 2012, 7: e41574. DOI:10.1371/journal.pone.0041574 |
| [30] | Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine[J]. Gene Dev, 2008, 22: 1276–1312. DOI:10.1101/gad.1653708 |
| [31] | Davis BN, Hilyard AC, Nguyen PH, et al. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype[J]. J Biol Chem, 2009, 284: 3728–3738. DOI:10.1074/jbc.M808788200 |
| [32] | Kim S, Kang H. miR-15b induced by platelet-derived growth factor signaling is required for vascular smooth muscle cell proliferation[J]. BMB Rep, 2013, 46: 550–554. DOI:10.5483/BMBRep.2013.46.11.057 |
| [33] | Talasila A, Yu H, Ackers-Johnson M, et al. Myocardin regulates vascular response to injury through miR-24/-29a and platelet-derived growth factor receptor-beta[J]. Arterioscl Throm Vas, 2013, 33: 2355–2365. DOI:10.1161/ATVBAHA.112.301000 |
| [34] | Qian Z, Zhang L, Chen J, et al. miR-328 targeting PIM-1 inhibits proliferation and migration of pulmonary arterial smooth muscle cells in PDGFBB signaling pathway[J]. Oncotarget, 2016, 7: 54998–55011. |
| [35] | Bandara V, Michael MZ, Gleadle JM. microRNA biogenesis in hypoxia[J]. MicroRNA, 2017, 6: 80–96. |
| [36] | Shan F, Li J, Huang QY. HIF-1 alpha-induced up-regulation of miR-9 contributes to phenotypic modulation in pulmonary artery smooth muscle cells during hypoxia[J]. J Cell Physiol, 2014, 229: 1511–1520. DOI:10.1002/jcp.v229.10 |
| [37] | Chen L, Li YS, Cui J, et al. miR-206 controls the phenotypic modulation of pulmonary arterial smooth muscle cells induced by serum from rats with hepatopulmonary syndrome by regulating the target gene, annexin A2[J]. Cell Physiol Biochem, 2014, 34: 1768–1779. DOI:10.1159/000366377 |
| [38] | Deng B, Du J, Hu R, et al. microRNA-103/107 is involved in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells by targeting HIF-1β[J]. Life Sci, 2016, 147: 117–124. DOI:10.1016/j.lfs.2016.01.043 |
| [39] | Li H, Zhao X, Shan H, et al. microRNAs in idiopathic pulmonary fibrosis:involvement in pathogenesis and potential use in diagnosis and therapeutics[J]. Acta Pharma Sin B, 2016, 6: 531–539. DOI:10.1016/j.apsb.2016.06.010 |
| [40] | An X, Sarmiento C, Tan T, et al. Regulation of multidrug resistance by microRNAs in anti-cancer therapy[J]. Acta Pharm Sin B, 2017, 7: 38–51. DOI:10.1016/j.apsb.2016.09.002 |
| [41] | Bai RN, Yang QN, Ruixi Xi, et al. miR-941 as a promising biomarker for acute coronary syndrome[J]. BMC Car-diovasc Disor, 2017, 17: 227. DOI:10.1186/s12872-017-0653-8 |
| [42] | Chen W, Li S. Circulating microRNA as a novel biomarker for pulmonary arterial hypertension due to congenital heart disease[J]. Pediatr Cardiol, 2017, 38: 86–94. DOI:10.1007/s00246-016-1487-3 |
| [43] | Kukreja RC, Yin C, Salloum FN. microRNAs:new players in cardiac injury and protection[J]. Mol Pharmacol, 2011, 80: 558–564. DOI:10.1124/mol.111.073528 |
| [44] | Rupaimoole R, Slack FJ. microRNA therapeutics:towards a new era for the management of cancer and other diseases[J]. Nat Rev Drug Discov, 2017, 16: 203–222. DOI:10.1038/nrd.2016.246 |
 2018, Vol. 53
2018, Vol. 53


