据国际糖尿病联盟最新统计, 糖尿病目前已成为全球影响人数最为广泛的慢性疾病, 2017年共有4.25亿人罹患糖尿病, 到2035年患病人数将达到5.92亿人次[1]。我国糖尿病患病呈井喷态势, 成人患病率从2002年的2.6%上升到2016年的11.6%;潜在患者人数高达50.1%。其中2型糖尿病虽表现为简单血糖异常, 但发病机制异常复杂[2, 3]; 罹患2型糖尿病患者与健康人相比, 出现心血管疾病的风险要高出2~4倍。目前临床一线用药—生物碱天然产物山羊豆碱衍生的二甲双胍[4], 是一个多靶点降糖药物, 适宜于更广的患者人群。陆续上市的单靶点药物, 如促胰岛素分泌剂[5-7]、α-葡萄糖苷酶抑制剂[8]、PPARα-激动剂噻唑烷二酮类[9]、GLP-1受体激动剂[10]、新上市的DPP-4抑制剂[11]与SGLT-2抑制剂[12], 在临床发挥重要的作用, 仍难以满足发病机制异常复杂的庞大患者人群; 同时, 这些药物还存在不同程度不良反应, 限制了其使用范围[13]。2型糖尿病作为一种影响终生的疾病, 仍需更多有效的治疗和控制手段。
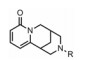
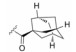
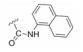
金雀花碱(cytisine, 1, 结构见图 1), 又名野靛碱或乌乐碱, 是从豆科槐属植物苦豆子中提取的一种生物碱单体。临床用0.15%的金雀花碱水溶液肌肉或静脉注射, 抢救因手术和各种创伤引起的反射性呼吸暂停、休克和新生儿窒息等[14], 其安全性已得到普遍证实。另外, 金雀花碱还具有抗心率失常、抗溃疡以及抗肿瘤等多种生物活性[15]。本课题组长期致力于从生物碱天然产物中寻找创新药物, 并首次发现具有独特桥环骨架的金雀花碱具有微弱的降糖活性。鉴于其独特的化学结构以及良好的成药性特征, 本文以金雀花碱为先导化合物, 以细胞糖消耗为活性导向, 分别在其12N-位上引入不同类型的取代基, 如烷基、苄基、酰基、磺酰基以及苯胺甲酰基等, 着重考察12N-位氮原子上不同取代基对降糖活性的影响, 总结构效关系。同时对代表性化合物开展体内药代动力学与初步作用机制研究。
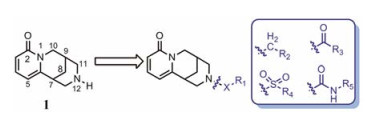
|
Figure 1 Structures of cytisinie and strategies of the structural modifications |
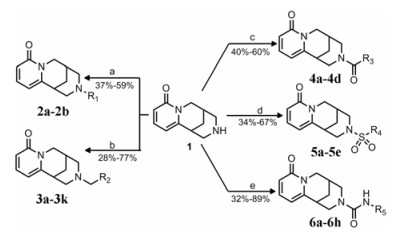
目标化合物的合成如合成路线1所示。以市售的金雀花碱1为原料, 二氯甲烷为反应溶剂, 三乙胺为缚酸剂, 分别滴加不同取代基的碘代烷、溴苄、酰氯、磺酰氯或异氰酸酯, 制得目标化合物12N–烷基取代2a~2b (收率介于37%~59%)、12N-苄基3a~3k (28%~77%)、12N-苯甲酰基4a~4d (40%~60%)、12N-芳香磺酰基5a~5e (34%~57%)以及12N-苯胺甲酰基6a~6h (32%~89%)。所有最终产物均经快速柱色谱方法分离纯化, 使其收率均有所降低。本研究共设计合成金雀花碱衍生物30个, 其中21个化合物未见文献报道, 分别为3b、3d、3e、3g、3i、3j、4a~4d、5b~5e、6a~6d和6f~6h。所有化合物结构均经1H NMR、13C NMR和HR-MS确证。
|
Scheme1 Synthetic routes to target compounds. Reagents and conditions: a) –10 ℃, NaH, alkyl iodides, THF, r.t. or 50 ℃; b) TEA, benzyl bromide, CH2Cl2, r.t.; c) TEA, benzoyl chloride, CH2Cl2, r.t.; d) TEA, benzenesulfonyl chloride, CH2Cl2, r.t.; e) TEA, isocyanate, CH2Cl2, rt. |
选取同样来自生物碱天然产物的二甲双胍作为阳性对照药, 所有新合成的目标化合物结构与在肌肉细胞L6的糖消耗活性结果见表 1。活性结果表明, 12N上引入苄基(3a~3k)的活性优于烷基(2a~2b), 苄基对位吸电子取代优于邻位吸电子取代, 其中化合物3d和3g表现出显著的降糖作用。12N上引入不同的磺酰基, 所得12N-磺酰基衍生物5a~5e的活性均未见明显提高; 12N上引入酰基(4a~4d)或者酰胺基(6a~6g)时, 衍生物如4a和6g的活性有所提高, 尤其化合物6h的活性明显增强, 提示苯环3位引入卤素氯原子有利于提升降糖活性。
| Table 1 Hypoglycemic activity of cytisine derivatives in L6 cells. The concentration of target compound is 25 μmol·L-1, and metformin is 5 mmol·L-1 |
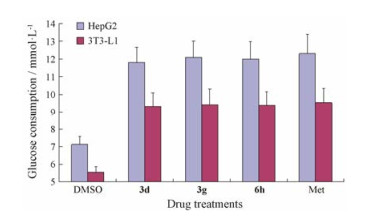
选取活性较好的3个代表性化合物3d、3g和6h分别在肝脏细胞HepG2和脂肪细胞3T3中进一步验证其体外降糖活性, 结果见图 2。结果表明化合物3d、3g和6h在25 μmol·L-1浓度下的糖消耗与二甲双胍在5 mmol·L-1的活性相当, 提示3个代表性化合物均表现出了良好的体外降糖活性。
|
Figure 2 Hypoglycemic activity of compounds 3d, 3g and 6h in HepG2 and 3T3 Cell (3d, 3g and 6h 25 μmol·L-1, respectively; Met 5 mmol·L-1) |
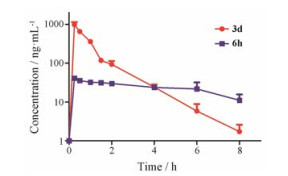
选取不同结构类型的代表性化合物, 12N-苄基取代3d和12N-酰基取代6h进行SD大鼠体内主要药代参数的测定。选用雄性SD大鼠两组(n = 3), 分别口服给予25 mg·kg-1待测化合物, 给药后按规定时间点(0、0.25、0.5、1、1.5、2、4、6、8 h)分别进行颈静脉插管取血测定, 结果见表 2和图 3。结果表明化合物3d表现出较好的口服药代动力学性质。化合物6h的主要药代参数Cmax与AUC均低于相应苄基取代3d, 这可能是由于化合物6h中的酰胺键在体内容易水解断裂所致。
| Table 2 Pharmacokinetic profiles of representative compounds after oral administration (25 mg·kg-1) |
|
Figure 3 Mean plasma concentration versus time curve of 3d and 6h after oral administration at 25 mg·kg-1 to mice (n = 3), respectively |
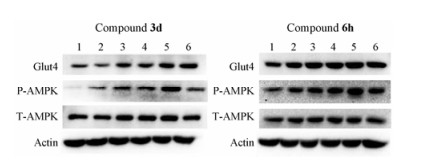
为了探讨此类桥环天然产物发挥降糖活性的作用机制, 选取代表性化合物3d和6h进行进一步研究。鉴于葡萄糖转运蛋白Glut4是在葡萄糖消耗过程中细胞摄取葡萄糖的关键蛋白, AMPK是促进葡萄糖有氧氧化的关键激酶, 本研究开展了激活Glut4和AMPK的活性评价研究。从图 4可知, 化合物3d和6h均可上调葡萄糖转运蛋白Glut4表达水平及AMPK磷酸化水平, 并呈现一定的剂量依赖性, 而总的AMPK水平无明显变化。该结果提示, 化合物3d和6h可能是通过上调Glut4表达以及激活AMPK激酶从而增加细胞糖消耗量。
|
Figure 4 Compounds 3d and 6h show potency on Glut4 and AMPK activation. 1: DMSO; 2–5: Drug concentration of 5, 10, 25 and 50 μmol·L-1 respectively; 6: Met 5 mmol·L-1 |
本研究以生物碱天然产物—金雀花碱为先导物, 共设计合成30个具有独特桥环骨架的金雀花碱衍生物, 其中21个是未见文献报道的全新化合物, 评价了其体外糖消耗活性。初步降糖构效关系表明, 12-位氮原子上引入适当的取代基有利于降糖活性的提高, 如引入三氟甲基苄基3d、碘苄基3g、甲氧基苯甲酰基4a以及氯苯胺甲酰6h金雀花碱衍生物均表现出显著的降糖作用。代表性化合物3d、3g和6h的体外降糖活性在不同的细胞系如肝脏细胞HepG2和脂肪细胞3T3中得到进一步验证。其中化合物3d不仅表现良好的降糖活性, 还拥有良好的药代动力学参数, 值得进一步研究。初步作用机制显示, 化合物3d增加细胞糖消耗量可能是通过上调了Glut4的表达以及激活了AMPK的活性。本研究结果为此类化合物的进一步结构修饰与优化提供了有益的科学数据, 有关深入的作用机制研究正在进行中。
实验部分熔点用梅特勒MP90熔点仪系统测定, 未经校正; 1H NMR和13C NMR用Bruker Avance Ⅲ 400、500、600核磁共振仪测定, 溶剂为DMSO-d6或CDCl3, TMS为内标; HR-MS用Autospec Ultima-TOF质谱测定仪测定; Flash柱分离纯化用Combiflash Rf200快速制备液相; 荧光检测用ZF-20D暗箱式紫外分析仪; 薄层色谱(TLC)采用E-Merck公司预铺硅胶铝箔卷; 试剂均为分析纯。
1 化学合成 1.1 12N-甲基金雀花碱 (2a)将金雀花碱400 mg (2.1 mmol)置于100 mL茄型瓶中, 加入四氢呋喃20 mL溶解, 加入氢化钠100 mg (4.2 mmol), 室温搅拌30 min, 然后加入碘甲烷355 mg (2.5 mmol), 室温搅拌, 反应过夜, 减压蒸干反应溶剂, 残留物加二氯甲烷溶解, 过滤, 硅胶拌样, 以二氯甲烷和甲醇为流动相, 经Flash柱色谱纯化, 得目标产物白色固体2a, 收率37%, 熔点: 139~141 ℃。1H NMR (500 MHz, DMSO-d6) δ 7.40~7.27 (m, 1H), 6.20 (d, J = 8.8 Hz, 1H), 6.07 (d, J = 6.4 Hz, 1H), 3.77 (d, J = 15.2 Hz, 1H), 3.68 (dd, J = 15.1, 6.3 Hz, 1H), 3.00 (s, 1H), 2.83 (d, J = 10.1 Hz, 1H), 2.73 (d, J = 9.6 Hz, 1H), 2.37 (s, 1H), 2.16 (d, J = 10.3 Hz, 1H), 2.11 (d, J = 10.6 Hz, 1H), 2.04 (s, 3H), 1.78 (d, J = 11.8 Hz, 1H), 1.65 (d, J = 12.1 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 162.8, 152.8, 139.5, 116.0, 104.5, 62.9, 62.4, 50.3, 46.5, 35.1, 27.9, 25.3; HR-MS: Calcd. for C12H16ON2 [M+H]+: 205.133 5, Found: 205.132 9。
1.2 12N-丙基金雀花碱 (2b)将金雀花碱400 mg (2.1 mmol)置于100 mL茄型瓶中, 加入四氢呋喃20 mL溶解, 加入氢化钠100 mg (4.2 mmol), 室温搅拌30 min, 然后加入碘丙烷425 mg (2.5 mmol), 加入至50 ℃反应过夜, 蒸干反应溶剂, 残留物加二氯甲烷溶解, 过滤, 硅胶拌样, 以二氯甲烷和甲醇为流动相, 经Flash柱色谱纯化, 得目标产物白色固体2b。收率59%, 熔点: > 200 ℃。1H NMR (500 MHz, DMSO-d6) δ 9.59 (s, 1H), 7.44 (t, J = 7.7 Hz, 1H), 6.39 (d, J = 8.9 Hz, 1H), 6.29 (d, J = 6.3 Hz, 1H), 3.96 (d, J = 15.7 Hz, 1H), 3.83 (dd, J = 15.7, 6.9 Hz, 1H), 3.54 (d, J = 12.2 Hz, 1H), 3.41 (s, 2H), 3.34 (dd, J = 16.3, 6.4 Hz, 1H), 3.22 (t, J = 10.2 Hz, 1H), 3.01~2.91 (m, 2H), 2.73 (s, 1H), 1.98 (d, J = 12.9 Hz, 1H), 1.87 (d, J = 12.8 Hz, 1H), 1.69~1.52 (m, 2H), 0.83 (t, J = 7.3 Hz, 3H); 13C NMR (126 MHz, DMSO-d6) δ 162.9, 148.4, 140.6, 117.1, 108.2, 59.3, 56.9, 56.6, 48.9, 32.6, 26.3, 23.1, 17.0, 11.5; HR-MS: Calcd. for C14H20ON2 [M+H]+: 233.164 8, Found: 233.164 0。
1.3 12N-取代苄基金雀花碱 (3a~3k)将金雀花碱400 mg (2.1 mmol)置于100 mL茄型瓶中, 加入二氯甲烷20 mL溶解, 搅拌下依次加入三乙胺0.51 g (6.3 mmol)和取代溴苄(2.1 mmol), 室温搅拌3 h, TLC监测, 反应完毕后, 反应液加入二氯甲烷20 mL, 然后依次以水(50 mL×3)和饱和食盐水(50 mL×3)洗涤, 无水硫酸钠干燥。过滤, 减压浓缩, 以二氯甲烷和甲醇为流动相, 经Flash柱色谱纯化, 得目标产物3a~3k。
1.3.1 12N-苄基金雀花碱 (3a)白色固体, 收率51%, 熔点: 148~150 ℃。1H NMR (500 MHz, CDCl3) δ 7.28 (dd, J = 7.6, 5.5 Hz, 1H), 7.21~7.14 (m, 3H), 7.03~6.96 (m, 2H), 6.49 (dd, J = 9.0, 1.1 Hz, 1H), 5.92 (d, J = 6.8 Hz, 1H), 4.11 (d, J = 15.3 Hz, 1H), 3.89 (dd, J = 15.3, 6.6 Hz, 1H), 3.47~3.38 (m, 2H), 2.94 (d, J = 10.0 Hz, 2H), 2.85 (dd, J = 10.6, 1.1 Hz, 1H), 2.43 (d, J = 2.4 Hz, 1H), 2.37 (d, J = 11.0 Hz, 1H), 2.31 (d, J = 10.5 Hz, 1H), 1.91 (d, J = 12.7 Hz, 1H), 1.79 (d, J = 12.7 Hz, 1H); 13C NMR (101MHz, CDCl3) δ 163.7, 151.3, 138.6 (2), 128.4, 128.2 (3), 127.1, 116.6, 104.8, 62.0 (2), 59.9, 49.9, 35.4, 28.1, 25.9; HR-MS: Calcd. for C18H21ON2 [M+H]+: 281.164 8, Found: 281.164 7。
1.3.2 12N-o-氰基苄基金雀花碱 (3b)白色固体, 收率78%, 熔点: 114~116 ℃。1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 7.6 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.30~7.25 (m, 2H), 7.08 (d, J = 7.8 Hz, 1H), 6.50 (d, J = 8.9 Hz, 1H), 5.93 (d, J = 6.7 Hz, 1H), 4.09 (d, J = 15.3 Hz, 1H), 3.89 (dd, J = 15.3, 6.5 Hz, 1H), 3.69~3.61 (m, 2H), 2.98 (s, 1H), 2.93 (d, J = 10.7 Hz, 1H), 2.86 (d, J = 10.4 Hz, 1H), 2.50 (t, J = 10.8 Hz, 3H), 1.94 (d, J = 12.7 Hz, 1H), 1.84 (d, J = 12.8 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 163.6, 151.0, 142.1, 138.6, 132.7 (2), 129.1, 127.6, 117.5, 116.8, 112.2, 104.8, 60.3, 59.9, 59.4, 49.9, 35.5, 28.1, 25.8; HR-MS: Calcd. for C19H20ON3 [M+H]+ 306.160 1, Found: 306.159 9。
1.3.3 12N-p-甲氧苄基金雀花碱 (3c)白色固体, 收率77%, 熔点: 101~103 ℃。1H NMR (400 MHz, CDCl3) δ 7.27 (t, J = 7.8 Hz, 1H), 6.91 (d, J = 8.0 Hz, 2H), 6.73 (d, J = 8.3 Hz, 2H), 6.48 (d, J = 8.9 Hz, 1H), 5.91 (d, J = 6.6 Hz, 1H), 4.08 (d, J = 15.3 Hz, 1H), 3.88 (dd, J = 15.3, 6.5 Hz, 1H), 3.76 (s, 3H), 3.40~3.31 (m, 2H), 2.92 (s, 2H), 2.84 (d, J = 10.2 Hz, 1H), 2.42 (s, 1H), 2.31 (t, J = 12.4 Hz, 2H), 1.90 (d, J = 12.4 Hz, 1H), 1.78 (d, J = 12.5 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 163.6, 158.6, 151.5, 138.6 (2), 129.4, 116.5, 113.6 (3), 104.7, 61.4, 59.9 (2), 55.2, 50.0, 35.5, 28.1, 26.0; HR-MS: Calcd. for C19H23O2N2 [M+H]+ 311.175 4, Found: 311.175 1。
1.3.4 12N-p-三氟甲基苄基金雀花碱盐酸盐 (3d)白色固体, 收率41%, 熔点: 103~105 ℃。1H NMR (500 MHz, DMSO-d6) δ 10.35 (s, 1H), 7.84 (s, 4H), 7.47 (dd, J = 8.7, 7.2 Hz, 1H), 6.47 (d, J = 8.9 Hz, 1H), 6.33 (d, J = 6.8 Hz, 1H), 4.47 (d, J = 11.7 Hz, 1H), 4.39 (d, J = 11.8 Hz, 1H), 4.01 (d, J = 15.7 Hz, 1H), 3.86 (dd, J = 15.7, 7.0 Hz, 1H), 3.50 (d, J = 11.7 Hz, 1H), 3.43 (s, 1H), 3.33 (s, 1H), 3.24~3.14 (m, 1H), 2.77 (s, 1H), 1.94 (d, J = 12.9 Hz, 1H), 1.87 (d, J = 12.8 Hz, 1H), 1.23 (s, 1H); 13C NMR (126 MHz, DMSO-d6) δ 162.7 (2), 148.1, 140.4, 133.3 (2), 126.0 (2), 125.6, 123.4, 117.0, 107.8, 59.5, 56.5, 56.4, 48.6, 32.4, 26.2, 22.8; HR-MS: Calcd. for C19H20ON2F3·HCl [M-HCl+ H]+ 349.152 2, Found: 349.152 0。
1.3.5 12N-2', 6'-二氟苄基金雀花碱 (3e)白色固体, 收率54%, 熔点: 158~160 ℃。1H NMR (500 MHz, CDCl3) δ 7.27 (dd, J = 6.5, 2.5 Hz, 1H), 7.25~7.18 (m, 1H), 6.89~6.82 (m, 2H), 6.44 (dd, J = 9.0, 1.3 Hz, 1H), 5.96 (dd, J = 6.8, 1.2 Hz, 1H), 4.00 (d, J = 15.3 Hz, 1H), 3.87 (dd, J = 15.2, 6.9 Hz, 1H), 3.68~3.61 (m, 2H), 2.92 (t, J = 11.7 Hz, 2H), 2.43~2.36 (m, 2H), 1.82 (d, J = 12.8 Hz, 1H), 1.66 (d, J = 12.7 Hz, 1H), 1.25 (s, 2H); 13C NMR (126 MHz, DMSO-d6) δ 162.6 (2), 160.8, 152.4, 139.2, 130.5, 115.7 (2), 111.9, 111.7, 104.1, 59.4, 58.8, 50.0, 48.3, 34.8, 27.6, 25.1; HR-MS: Calcd. for C18H19ON2F2 [M+H]+ 317.146 0, Found: 317.145 7。
1.3.6 12N-2', 6'-二氯苄基金雀花碱 (3f)白色固体, 收率41%, 熔点: 138~140 ℃。1H NMR (500 MHz, DMSO-d6) δ 7.21~7.18 (m, 3H), 7.06 (dd, J = 8.4, 7.6 Hz, 1H), 6.47~6.38 (m, 1H), 5.88 (dd, J = 6.8, 0.8 Hz, 1H), 3.71 (d, J = 12.5 Hz, 1H), 3.59 (d, J = 12.5 Hz, 1H), 2.93 (d, J = 1.7 Hz, 1H), 2.87~2.82 (m, 2H), 2.61 (dd, J = 10.7, 1.6 Hz, 1H), 2.54 (d, J = 10.9 Hz, 1H), 2.38 (s, 1H), 1.91~1.86 (m, 1H), 1.82~1.78 (m, 1H), 1.25 (s, 2H); 13C NMR (126 MHz, DMSO-d6) δ 162.6, 152.3, 138.8, 136.4 (2), 133.7, 130.2, 128.8 (2), 115.6, 103.8, 60.2, 59.4, 55.8, 50.0, 35.0, 27.7, 25.4; HR-MS: Calcd. for C18H19ON2Cl2 [M+H]+ 349.086 9, Found: 349.087 2。
1.3.7 12N-p-碘苄基金雀花碱盐酸盐 (3g)白色固体, 收率32%, 熔点: 160 ℃。(dec.) 1H NMR (500 MHz, DMSO-d6) δ 9.96 (s, 1H), 7.82 (d, J = 7.6 Hz, 2H), 7.46~7.30 (m, 3H), 6.38 (d, J = 8.9 Hz, 1H), 6.24 (d, J = 6.6 Hz, 1H), 4.42~4.15 (m, 2H), 3.95 (d, J = 15.7 Hz, 1H), 3.82 (dd, J = 15.6, 6.9 Hz, 1H), 3.51~3.38 (m, 2H), 3.33~3.13 (m, 2H), 2.75 (s, 1H), 1.91 (d, J = 12.4 Hz, 1H), 1.85 (d, J = 12.2 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 162.7, 148.0, 140.2, 138.0 (3), 134.6, 129.2, 117.2, 107.4, 97.5, 59.7, 56.4, 56.2, 48.5, 32.3, 26.2, 22.9; HR-MS: Calcd. for C18H19ON2I·HCl [M-HCl+H]+ 407.061 5, Found: 407.060 0。
1.3.8 12N-p-氟苄基金雀花碱 (3h)白色固体, 收率64%, 熔点: 159~161 ℃。1H NMR (500 MHz, DMSO-d6) δ 7.34 (dd, J = 8.9, 6.9 Hz, 1H), 7.04~6.97 (m, 4H), 6.26 (d, J = 9.0 Hz, 1H), 6.03 (d, J = 6.8 Hz, 1H), 3.83 (d, J = 15.3 Hz, 1H), 3.70 (dd, J = 15.3, 6.5 Hz, 1H), 3.43 (d, J = 13.8 Hz, 1H), 3.37 (d, J = 9.5 Hz, 1H), 3.00 (s, 1H), 2.87 (d, J = 10.6 Hz, 1H), 2.73 (d, J = 10.4 Hz, 1H), 2.39 (s, 1H), 2.31~2.23 (m, 2H), 1.83 (d, J = 12.6 Hz, 1H), 1.70 (d, J = 12.6 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 162.6, 160.6, 152.5, 139.2, 134.7, 130.2, 130.1, 115.7, 115.3, 115.1, 104.3, 60.7, 60.0, 59.8, 50.1, 35.0, 27.8, 25.6; HR-MS: Calcd. for C18H19ON2F [M+H]+ 299.155 4, Found: 299.154 4。
1.3.9 12N-p-溴苄基金雀花碱 (3i)白色固体, 收率43%, 熔点: 100~102 ℃。1H NMR (500 MHz, DMSO-d6) δ 7.40~7.32 (m, 3H), 6.91 (d, J = 8.3 Hz, 2H), 6.26 (dd, J = 9.0, 1.2 Hz, 1H), 6.03 (d, J = 6.8 Hz, 1H), 3.84 (d, J = 15.3 Hz, 1H), 3.69 (dd, J = 15.3, 6.4 Hz, 1H), 3.42 (d, J = 14.1 Hz, 1H), 3.36 (d, J = 2.8 Hz, 1H), 3.00 (s, 1H), 2.87 (d, J = 10.6 Hz, 1H), 2.73~2.69 (m, 1H), 2.38 (d, J = 2.3 Hz, 1H), 2.33~2.23 (m, 2H), 1.83 (d, J = 12.6 Hz, 1H), 1.70 (d, J = 12.7 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 162.8, 152.6, 139.4, 138.4, 131.6 (2), 130.8 (2), 120.4, 115.9, 104.5, 60.9, 60.1 (2), 50.3, 35.1, 28.0, 25.8; HR-MS: Calcd. for C18H19ON2Br [M+H]+ 359.075 4, Found: 359.074 2。
1.3.10 12N-p-氯苄基金雀花碱 (3j)白色固体, 收率28%, 熔点: 120~122 ℃。1H NMR (500 MHz, DMSO-d6) δ 7.34 (dd, J = 8.9, 6.9 Hz, 1H), 7.24 (d, J = 8.3 Hz, 2H), 6.97 (d, J = 8.3 Hz, 2H), 6.26 (dd, J = 9.0, 1.2 Hz, 1H), 6.03 (d, J = 6.8 Hz, 1H), 3.84 (d, J = 15.3 Hz, 1H), 3.70 (dd, J = 15.3, 6.5 Hz, 1H), 3.44 (d, J = 14.1 Hz, 1H), 3.38 (s, 1H), 3.00 (s, 1H), 2.87 (d, J = 10.6 Hz, 1H), 2.73~2.69 (m, 1H), 2.38 (d, J = 2.2 Hz, 1H), 2.33~2.23 (m, 2H), 1.83 (d, J = 12.6 Hz, 1H), 1.70 (d, J = 12.7 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 162.6, 152.4, 139.2, 137.8, 131.7, 130.2 (2), 128.4 (2), 115.7, 104.3, 60.6, 59.9 (2), 50.1, 35.0, 27.9, 25.6; HR-MS: Calcd. for C18H20ON2Cl [M+H]+ 315.125 9, Found: 315.125 8。
1.3.11 12N-p-硝基苄基金雀花碱 (3k)白色固体, 收率32%, 熔点: 129~131 ℃。1H NMR (500 MHz, DMSO-d6) δ 8.04 (d, J = 8.6 Hz, 2H), 7.37 (dd, J = 9.0, 6.9 Hz, 1H), 7.21 (d, J = 8.6 Hz, 2H), 6.30 (dd, J = 9.0, 1.2 Hz, 1H), 6.04 (dd, J = 6.8, 1.0 Hz, 1H), 3.89 (d, J = 15.3 Hz, 1H), 3.71 (dd, J = 15.2, 7.1 Hz, 1H), 3.61 (d, J = 15.0 Hz, 1H), 3.52 (d, J = 15.0 Hz, 1H), 3.02 (s, 1H), 2.94~2.86 (m, 1H), 2.73~2.67 (m, 1H), 2.43~2.37 (m, 2H), 2.32 (dd, J = 10.7, 1.8 Hz, 1H), 1.85 (d, J = 12.7 Hz, 1H), 1.73 (d, J = 12.7 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 162.8, 152.5, 147.6, 147.1, 139.4, 129.5, 123.8, 116.0, 104.5, 60.7, 60.2, 56.6, 50.3, 35.1, 28.1, 27.0, 25.7, 19.2; HR-MS: Calcd. for C18H19O3N3 [M+H]+ 326.149 9, Found: 326.148 8。
1.4 12N-取代苯甲酰基金雀花碱 (4a~4d)将金雀花碱400 mg (2.1 mmol)置于100 mL茄型瓶中, 加入二氯甲烷20 mL溶解, 搅拌下依次加入三乙胺0.51 g (6.3 mmol)和取代苯甲酰氯(2.5 mmol), 室温搅拌3 h, TLC监测, 反应完毕后, 反应液加入二氯甲烷20 mL, 然后依次以水(50 mL×1), 5% NaOH水溶液(50 mL×1)和饱和食盐水(50 mL×3)洗涤, 无水硫酸钠干燥。过滤, 减压浓缩, 以二氯甲烷和甲醇为流动相, 经Flash柱色谱纯化, 得目标产物4a~d。
1.4.1 12N-p-甲氧基苯甲酰基金雀花碱 (4a)白色固体, 收率59%, 熔点: > 200 ℃。1H NMR (500 MHz, DMSO-d6) δ 7.32 (t, J = 7.6 Hz, 1H), 6.85 (s, 4H), 6.30 (d, J = 9.0 Hz, 1H), 6.03 (s, 1H), 4.25 (m, 2H), 3.75 (s, 3H), 3.64 (s, 2H), 3.09 (s, 2H), 2.46 (s, 1H), 1.95 (m, 2H), 1.23 (s, 1H); 13C NMR (126 MHz, DMSO-d6) δ 170.2, 162.6, 160.4, 150.0, 139.4, 128.7 (3), 128.1, 116.5, 113.9, 105.3, 55.7 (3), 49.0, 34.6, 27.5, 25.7; HR-MS: Calcd. for C19H20O3N2 [M+H]+ 325.154 7, Found: 325.154 8。
1.4.2 12N-p-金刚烷基苯甲酰基金雀花碱 (4b)白色固体, 收率54%, 熔点: > 200 ℃。1H NMR (500 MHz, DMSO-d6) δ 7.32 (t, J = 7.9 Hz, 1H), 6.21 (d, J = 8.0 Hz, 2H), 4.55 (d, J = 12.9 Hz, 1H), 4.42 (d, J = 12.1 Hz, 1H), 3.89 (d, J = 15.5 Hz, 1H), 3.61 (dd, J = 15.5, 6.2 Hz, 1H), 3.15 (d, J = 11.7 Hz, 2H), 2.90 (d, J = 12.9 Hz, 1H), 2.45 (s, 1H), 1.95~1.84 (m, 5H), 1.63 (m, 12H); 13C NMR (151 MHz, DMSO-d6) δ 175.9, 162.5, 150.0, 139.2, 116.4, 105.4, 51.9, 50.3, 48.8, 41.6, 38.7 (3), 36.4 (3), 34.7, 28.3 (3), 27.6, 26.0; HR-MS: Calcd. for C22H28O2N2 [M+H]+ 353.222 4, Found: 353.221 0。
1.4.3 12N-o-三氟甲氧基苯甲酰基金雀花碱 (4c)白色固体, 收率40%, 熔点: > 200 ℃。1H NMR (500 MHz, DMSO-d6) δ 7.61~7.28 (m, 4H), 7.20 (t, J = 7.5 Hz, 1H), 6.34 (m, 1H), 6.27~6.14 (m, 1H), 4.60 (m, 1H), 3.66 (m, 2H), 3.41 (m, 2H), 3.23 (d, J = 11.8 Hz, 1H), 3.09 (m, 1H), 2.55 (s, 1H), 1.99 (m, 2H); 13C NMR (126 MHz, DMSO-d6) δ 165.3, 162.6, 149.6, 143.9, 139.5, 131.6, 129.5, 128.5, 127.6, 121.1, 116.7, 105.6, 105.3, 53.6, 49.1, 47.7, 34.4, 27.4, 25.3; HR-MS: Calcd. for C19H17O3N2F3 [M+H]+ 379.126 4, Found: 379.125 0。
1.4.4 12N-2', 4'-二氟苯甲酰基金雀花碱 (4d)白色固体, 收率58%, 熔点: > 200 ℃。1H NMR (600 MHz, DMSO-d6) δ 7.36~7.01 (m, 3H), 6.41~6.18 (m, 2H), 5.91 (d, J = 5.9 Hz, 1H), 4.68 (d, J = 12.4 Hz, 1H), 4.52 (d, J = 12.0 Hz, 1H), 3.75~3.67 (m, 1H), 3.55~3.43 (m, 1H), 3.23 (s, 1H), 3.08~3.02 (m, 1H), 2.55 (s, 1H), 2.35 (s, 1H), 2.02~1.92 (m, 2H); 13C NMR (126 MHz, DMSO-d6) δ 164.5, 162.7, 150.1, 149.7, 139.5, 130.2, 116.9, 116.6, 112.5, 105.4, 105.2, 104.9, 54.3, 49.2, 48.0, 34.7, 27.6, 25.6; HR-MS: Calcd. for C18H16O2N2F2 [M+H]+ 331.125 3, Found: 331.124 1。
1.5 12N-取代磺酰基金雀花碱 (5a~5e)将金雀花碱400 mg (2.1 mmol)置于100 mL茄型瓶中, 加入二氯甲烷20 mL溶解, 搅拌下依次加入三乙胺0.51 g (6.3 mmol)和取代磺酰氯, 室温搅拌3 h, TLC监测, 反应完毕后, 反应液加入二氯甲烷20 mL, 然后依次以水(50 mL), 5% NaOH水溶液(50 mL×2)和饱和食盐水(50 mL×2)洗涤, 无水硫酸钠干燥。过滤, 减压浓缩, 以二氯甲烷和甲醇为流动相, 经Flash柱色谱纯化, 得目标产物5a~5e。
1.5.1 12N-p-甲基苯磺酰基金雀花碱 (5a)白色固体, 收率67%, 熔点: > 200 ℃。1H NMR (500 MHz, CDCl3) δ 7.48 (d, J = 8.1 Hz, 2H), 7.28~7.23 (m, 3H), 6.46 (d, J = 8.9 Hz, 1H), 5.99 (d, J = 6.7 Hz, 1H), 3.97 (d, J = 15.6 Hz, 1H), 3.88 (dd, J = 15.6, 6.5 Hz, 1H), 3.77 (t, J = 9.9 Hz, 2H), 3.06 (s, 1H), 2.78~2.69 (m, 2H), 2.53 (s, 1H), 2.42 (s, 3H), 1.93 (d, J = 13.1 Hz, 1H), 1.73 (d, J = 13.1 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 163.3, 148.5, 143.9, 138.8, 133.5, 129.8 (2), 127.4 (2), 117.7, 105.4, 52.6, 51.9, 48.9, 34.2, 27.1, 25.2, 21.6; HR-MS: Calcd. for C18H20O3N2S [M+H]+ 345.126 7, Found: 345.126 7。
1.5.2 12N-m-乙酰基苯磺酰基金雀花碱 (5b)白色固体, 收率52%, 熔点: 178~180 ℃。1H NMR (400 MHz, CDCl3) δ 8.21~8.09 (m, 2H), 7.73 (d, J = 7.6 Hz, 1H), 7.58 (t, J = 7.7 Hz, 1H), 7.29~7.20 (m, 1H), 6.38 (d, J = 9.0 Hz, 1H), 5.98 (d, J = 6.7 Hz, 1H), 3.87 (m, 4H), 3.07 (s, 1H), 2.86 (t, J = 11.8 Hz, 2H), 2.64 (s, 3H), 2.55 (s, 1H), 1.95 (d, J = 12.9 Hz, 1H), 1.77 (d, J = 13.1 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 196.3, 163.1, 148.2, 138.7, 137.9 (2), 132.4, 131.0, 129.8, 126.8, 117.8, 105.2, 52.7, 51.9, 48.8, 34.1, 27.0, 26.7, 25.0; HR-MS: Calcd. for C19H20O4N2S [M+H]+ 373.121 7, Found: 373.121 7。
1.5.3 12N-p-甲磺酰基苯磺酰基金雀花碱 (5c)白色固体, 收率42%, 熔点: > 200 ℃。1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 7.8 Hz, 2H), 7.71 (d, J = 7.7 Hz, 2H), 7.27 (s, 1H), 6.40 (d, J = 8.8 Hz, 1H), 6.02 (d, J = 6.3 Hz, 1H), 3.92~3.68 (m, 4H), 3.19 (s, 3H), 3.11~2.97 (m, 3H), 2.52 (s, 1H), 1.96 (d, J = 12.5 Hz, 1H), 1.82 (d, J = 12.8 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 163.0, 148.2, 144.4, 143.0, 138.9, 128.6 (2), 127.6 (2), 117.9, 105.4, 52.8, 51.8, 48.8, 44.4, 34.1, 26.9, 25.0; HR-MS: Calcd. for C18H20O5N2S2 [M+H]+ 409.088 7, Found: 409.088 9。
1.5.4 12N-p-甲氧基苯磺酰基金雀花碱 (5d)白色固体, 收率46%, 熔点: > 200 ℃。1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 8.8 Hz, 2H), 7.30~7.25 (m, 1H), 6.92 (d, J = 8.8 Hz, 2H), 6.46 (d, J = 9.0 Hz, 1H), 5.98 (d, J = 6.7 Hz, 1H), 4.00~3.84 (m, 5H), 3.76 (t, J = 13.2 Hz, 2H), 3.06 (s, 1H), 2.76 (m, 2H), 2.53 (s, 1H), 1.93 (d, J = 13.1 Hz, 1H), 1.74 (d, J = 13.1 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 163.2 (2), 148.5, 138.8, 129.5 (2), 128.0, 117.7, 114.3 (2), 105.3, 55.7, 52.6, 51.8, 48.9, 34.2, 27.0, 25.1; HR-MS: Calcd. for C18H20O4N2S [M+ H]+ 360.161 7, Found: 360.161 7。
1.5.5 12N-p-噻吩基磺酰基金雀花碱 (5e)白色固体, 收率34%, 熔点: > 200 ℃。1H NMR (500 MHz, DMSO-d6) δ 8.05 (d, J= 4.8 Hz, 1H), 7.61~7.55 (m, 1H), 7.38 (t, J = 7.8 Hz, 1H), 7.28 (d, J = 3.7 Hz, 1H), 6.27 (d, J = 9.0 Hz, 1H), 6.19 (d, J = 6.7 Hz, 1H), 3.83 (d, J = 15.5 Hz, 1H), 3.75 (dd, J = 15.5, 6.2 Hz, 1H), 3.68 (d, J = 10.9 Hz, 1H), 3.56 (d, J = 10.7 Hz, 1H), 3.21 (s, 1H), 2.65 (d, J = 10.9 Hz, 1H), 2.62~2.53 (m, 2H), 1.84 (d, J= 12.7 Hz, 1H), 1.73 (d, J = 12.7 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 162.8, 150.3, 139.7, 135.6, 134.7, 133.7, 129.0, 116.9, 105.2, 53.5, 52.6, 49.4, 33.7, 26.9, 24.4; HR-MS: Calcd. for C15H16O3N2S2[M+H]+ 337.067 5, Found: 337.067 3。
1.6 12N-取代苯胺甲酰基金雀花碱 (6a~6h)将金雀花碱400 mg (2.1 mmol)置于100 mL茄型瓶中, 加入二氯甲烷20 mL溶解, 然后缓慢滴加异氰酸酯(2.5 mmol), 室温搅拌1 h后, 加入三乙胺0.17 g (2.1mmol), TLC监测, 反应完毕后, 反应液加入二氯甲烷20 mL, 然后依次以水(50 mL×2)和饱和食盐水(50 mL×2)洗涤, 无水硫酸钠干燥。过滤, 减压浓缩, 以二氯甲烷和甲醇为流动相, 经Flash柱色谱纯化, 得目标产物6a~6h。
1.6.1 12N-m-甲基苯胺甲酰基金雀花碱 (6a)白色固体, 收率45%, 熔点: 177~179 ℃。1H NMR (500 MHz, DMSO-d6) δ 8.28 (s, 1H), 7.34~7.29 (m, 1H), 7.09~7.01 (m, 3H), 6.71 (d, J = 5.7 Hz, 1H), 6.19 (d, J = 9.0 Hz, 1H), 6.15 (d, J = 6.7 Hz, 1H), 4.26 (d, J = 12.8 Hz, 1H), 4.11 (d, J = 11.6 Hz, 1H), 4.05 (d, J = 15.5 Hz, 1H), 3.69 (dd, J = 15.4, 6.4 Hz, 1H), 3.18~3.12 (m, 1H), 3.06 (t, J = 12.9 Hz, 2H), 2.46 (s, 1H), 2.20 (s, 3H), 1.91 (s, 2H); 13C NMR (151 MHz, DMSO-d6) δ 162.7, 155.7, 150.7, 140.6, 139.3, 137.7, 128.5, 123.0, 121.00, 117.5, 116.2, 105.1, 51.2, 50.3, 49.0, 34.4, 27.4, 25.6, 21.6; HR-MS: Calcd. for C19H21O2N3 [M+H]+ 324.170 6, Found: 324.169 6。
1.6.2 12N-萘胺甲酰基金雀花碱 (6b)白色固体, 收率32%, 熔点: 117~119 ℃。1H NMR (500 MHz, DMSO-d6) δ 8.49 (s, 1H), 7.85 (d, J = 8.1 Hz, 1H), 7.68 (d, J = 8.1 Hz, 1H), 7.50~7.27 (m, 5H), 7.13 (d, J = 7.2 Hz, 1H), 6.38 (d, J = 8.9 Hz, 1H), 6.25 (d, J = 6.7 Hz, 1H), 4.41 (d, J = 12.9 Hz, 1H), 4.26 (d, J = 12.6 Hz, 1H), 4.12 (d, J = 15.5 Hz, 1H), 3.77 (dd, J = 15.5, 6.3 Hz, 1H), 3.18 (m, 2H), 2.89 (s, 1H), 2.73 (s, 1H), 1.98 (s, 2H); 13C NMR (126 MHz, DMSO-d6) δ 162.8, 156.2, 150.8, 139.6, 136.0, 134.1, 130.2, 128.1, 126.1, 125.8 (2), 125.5, 124.1, 123.4, 116.2, 105.7, 51.3, 49.9, 49.2, 34.7, 27.5, 25.8; HR-MS: Calcd. for C22H21O2N3 [M+ H]+ 360.170 7, Found: 360.169 4。
1.6.3 12N-3', 5'-二氯苯胺甲酰基金雀花碱 (6c)白色固体, 收率57%, 熔点: > 200 ℃。1H NMR (500 MHz, DMSO-d6) δ 8.70 (s, 1H), 7.37 (d, J = 1.8 Hz, 2H), 7.31 (dd, J = 8.9, 6.9 Hz, 1H), 7.08 (s, 1H), 6.16 (t, J = 8.4 Hz, 2H), 4.24 (d, J = 13.0 Hz, 1H), 4.10 (d, J = 12.3 Hz, 1H), 4.05 (d, J = 15.5 Hz, 1H), 3.68 (dd, J = 15.4, 6.3 Hz, 1H), 3.16 (d, J = 11.7 Hz, 2H), 3.10 (d, J= 15.6 Hz, 1H), 2.47 (s, 1H), 1.93 (s, 2H); 13C NMR (126 MHz, DMSO-d6) δ 162.8, 155.2, 150.5, 143.5, 139.5, 134.2 (2), 121.3, 117.9 (2), 116.5, 105.4, 51.5, 50.4, 49.1, 34.5, 27.5, 25.7; HR-MS: Calcd. for C18H17O2N3Cl2[M+H]+ 378.077 1, Found: 378.075 7.
1.6.4 12N-2', 6'-二异丙基苯胺甲酰基金雀花碱 (6d)白色固体, 收率49%, 熔点: > 200 ℃。1H NMR (500 MHz, DMSO-d6) δ 7.58 (s, 1H), 7.34 (dd, J = 8.8, 7.0 Hz, 1H), 7.14 (t, J = 7.6 Hz, 1H), 7.01 (d, J = 7.7 Hz, 2H), 6.25 (d, J = 8.9 Hz, 1H), 6.13 (d, J = 6.7 Hz, 1H), 4.33 (d, J = 12.9 Hz, 1H), 4.22 (d, J = 11.9 Hz, 1H), 3.92 (d, J= 15.6 Hz, 1H), 3.76 (dd, J = 15.5, 6.5 Hz, 1H), 3.09 (t, J = 14.6 Hz, 3H), 2.78~2.64 (m, 2H), 2.44 (s, 1H), 1.92 (s, 2H), 1.06 (d, J = 6.8 Hz, 3H), 1.00 (d, J = 6.7 Hz, 3H), 0.91 (dd, J = 15.1, 6.7 Hz, 6H); 13C NMR (126 MHz, DMSO-d6) δ 162.9, 156.1, 150.9, 147.6, 147.4, 139.2, 134.4, 127.4, 123.1 (2), 116.5, 105.2, 50.7, 49.9, 49.3, 34.7, 28.3 (2), 27.5, 25.9, 24.3, 24.2, 24.1, 23.8; HR-MS: Calcd. for C24H31O2N3[M+H]+ 394.248 9, Found: 394.247 5。
1.6.5 12N-p-氟苯胺甲酰基金雀花碱 (6e)白色固体, 收率87%, 熔点: 184~186 ℃。1H NMR (500 MHz, DMSO-d6) δ 8.39 (s, 1H), 7.31 (dd, J = 9.0, 6.9 Hz, 1H), 7.27~7.21 (m, 2H), 7.01 (t, J = 8.9 Hz, 2H), 6.21~6.13 (m, 2H), 4.26 (d, J= 12.9 Hz, 1H), 4.11 (d, J = 12.7 Hz, 1H), 4.05 (d, J = 15.5 Hz, 1H), 3.69 (dd, J = 15.4, 6.4 Hz, 1H), 3.15~3.03 (m, 3H), 2.46 (s, 1H), 1.91 (s, 2H); 13C NMR (151 MHz, DMSO-d6) δ 162.6, 158.7, 157.1, 155.7, 150.6, 139.3, 137.0, 122.2, 116.2, 115.2, 115.0, 105.1, 51.2, 50.3, 48.9, 34.4, 27.4, 25.6; HR-MS: Calcd. for C18H18O2N3F [M+H]+ 328.145 6, Found: 328.146 7。
1.6.6 12N-2'-甲基-5'-氯苯胺甲酰基金雀花碱 (6f)白色固体, 收率78%, 熔点: > 200 ℃。1H NMR (600 MHz, DMSO-d6) δ 8.97 (s, 1H), 7.32 (dd, J = 8.8, 7.0 Hz, 1H), 7.14 (s, 2H), 6.81 (s, 1H), 6.20 (dd, J= 39.8, 7.8 Hz, 2H), 5.18 (s, 1H), 4.84 (d, J = 8.2 Hz, 1H), 4.15 (d, J= 15.4 Hz, 1H), 3.67 (dd, J = 15.4, 6.3 Hz, 1H), 3.38 (d, J = 12.8 Hz, 1H), 3.29~3.20 (m, 2H), 2.56 (s, 1H), 2.04 (d, J = 12.7 Hz, 1H), 1.93 (d, J = 12.6 Hz, 1H), 1.80 (s, 3H); 13C NMR (151 MHz, DMSO-d6) δ 182.1, 162.7, 149.7, 141.5, 139.2, 135.5, 131.8, 129.9, 128.6, 126.5, 116.6, 105.4, 54.5, 53.7, 48.5, 35.1, 28.3, 25.9, 17.4; HR-MS: Calcd. for C19H20ON3ClS [M+H]+ 374.108 8, Found: 374.109 1。
1.6.7 12N-p-三氟甲基苯胺甲酰基金雀花碱 (6g)白色固体, 收率79%, 熔点: > 200 ℃。1H NMR (500 MHz, DMSO-d6) δ 8.75 (s, 1H), 7.53 (d, J = 8.6 Hz, 2H), 7.48 (d, J = 8.6 Hz, 2H), 7.33~7.28 (m, 1H), 6.17 (t, J = 7.9 Hz, 2H), 4.29 (d, J = 12.9 Hz, 1H), 4.14 (d, J = 12.2 Hz, 1H), 4.07 (d, J = 15.5 Hz, 1H), 3.69 (dd, J = 15.4, 6.4 Hz, 1H), 3.17~3.08 (m, 3H), 2.48 (s, 1H), 1.93 (s, 2H); 13C NMR (126 MHz, DMSO-d6) δ 162.8, 155.4, 150.6, 144.8, 139.5 (2), 126.1 (2), 119.7 (3), 116.4, 105.3, 51.5, 50.5, 49.1, 34.5, 27.5, 25.7; HR-MS: Calcd. for C19H18O2N3F3 [M+H]+ 378.142 4, Found: 378.142 2。
1.6.8 12N-m-氯苯胺甲酰基金雀花碱 (6h)白色固体, 收率89%, 熔点: > 200 ℃。1H NMR (500 MHz, DMSO-d6) δ 8.54 (s, 1H), 7.41 (s, 1H), 7.34~7.28 (m, 1H), 7.23~7.17 (m, 2H), 6.97~6.90 (m, 1H), 6.17 (dd, J = 11.9, 8.0 Hz, 2H), 4.26 (d, J = 12.9 Hz, 1H), 4.14~4.03 (m, 2H), 3.69 (dd, J = 15.4, 6.4 Hz, 1H), 3.18~3.06 (m, 3H), 2.47 (s, 1H), 1.92 (s, 2H); 13C NMR (126 MHz, DMSO-d6) δ 162.8, 155.5, 150.7, 142.5, 139.5, 133.2, 130.5, 122.0, 119.6, 118.5, 116.4, 105.3, 51.4, 50.4, 49.2, 34.5, 27.52, 25.74; HR-MS: Calcd. for C18H18O2N3Cl[M+H]+ 344.116 0, Found: 344.115 7。
2 生物学实验 2.1 体外降糖活性测定将稳定传代的肌肉细胞L6、肝脏细胞HepG2或脂肪细胞3T3 (MEMα + 10% FBS + 1% P/S), 按照每毫升2.0×105个细胞铺96孔板, 每个样品5个复孔。置于细胞培养箱中, 培养24 h, 换无血清培养基饥饿8 h, 然后, 用含有一定终浓度的无血清培养基换液继续处理24 h (即药物处理24 h), 用葡萄糖氧化酶法(葡萄糖检测试剂盒, 北京九强生物科技有限公司)检测培养基上清中葡萄糖剩余量。细胞糖消耗量即为培养基总糖量减去培养基中剩余葡萄糖量。
2.2 初步药代动力学评价取健康雄性SD大鼠, 每组3只(雄性, 体重200~250 g), 分别口服给药25 mg·kg-1, 给药体积为10 mL·kg-1。给药溶液为给药前1 h内配置。给药后按规定时间点(0、0.25、0.5、1、1.5、2、4、6和8 h)经颈静脉插管取全血约0.3 mL, 置含有EDTA-K3为抗凝剂的离心管中, 全血样品采出后离心分离血浆。血浆样品经有机溶剂处理后采用液相色谱串联质谱法测定, 并计算药动学参数。样品分析期间, 血浆样品收集后置于-20 ℃冰箱保存。
2.3 降糖机制初步探究将稳定传代的L6细胞, 按照每毫升1.0×105个细胞铺6孔板, 培养48 h后至汇合度达到90%以上, 换2% FBS诱导培养基, 继续诱导分化培养4天, 每两天换液一次。然后换无血清培养基饥饿过夜, 第二天加药处理, 24 h后收样。Western blot分析Glut4及p-AMPK表达水平的变化。
致谢: 核磁共振氢谱和碳谱由中国医学科学院药物研究所分析测试中心测定, 高分辨质谱由中国医学科学院医药生物技术研究所分析测试中心测定。| [1] | Pelletier C, Dai S, KC Roberts, et al. Report summary[J]. Chronic Dis Inj Can, 2012, 33: 53–54. |
| [2] | Sabatino A, Regolisti G, Cosola C, et al. Intestinal microbiota in type 2 diabetes and chronic kidney disease[J]. Curr Diab Rep, 2017, 17: 16. DOI:10.1007/s11892-017-0841-z |
| [3] | Xia C, Rao X, Zhong J. Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation[J]. J Diabetes Res, 2017, 2017: 6494795. |
| [4] | Song R. Mechanism of metformin:a tale of two sites[J]. Diabetes Care, 2016, 39: 187–189. DOI:10.2337/dci15-0013 |
| [5] | Tang WL, Wang YM, Du WM, et al. Analysis of adverse drug reaction of antidiabetic agents according to documents within forty-two years in China[J]. Chin J New Drugs Clin Remed (中国新药与临床杂志), 2002, 21: 753–756. |
| [6] | Bolen S, Feldman L, Vassy J, et al. Systematic review:comparative effectiveness and safety of oral medications for type 2 diabetes mellitus[J]. Ann Intern Med, 2007, 147: 386–399. DOI:10.7326/0003-4819-147-6-200709180-00178 |
| [7] | Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus:a cohort study[J]. Ann Intern Med, 2012, 157: 601–610. DOI:10.7326/0003-4819-157-9-201211060-00003 |
| [8] | Coniff RF, Shapiro JA, Seaton TB. Long-term efficacy and safety of acarbose in the treatment of obese subjects with non-insulin-dependent diabetes mellitus[J]. Arch Intern Med, 1994, 154: 2442–2448. DOI:10.1001/archinte.1994.00420210080009 |
| [9] | Nissen SE, Wolski K. Rosiglitazone revisited:an updated meta-analysis of risk for myocardial infarction and cardiovas cular mortality[J]. Arch Intern Med, 2010, 170: 1191–1201. |
| [10] | Bettge K, Kahle M, Abd El, et al. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists:A systematic analysis of published clinical trials[J]. Diabetes Obes Metab, 2017, 19: 336–347. DOI:10.1111/dom.12824 |
| [11] | Mascolo A, Rafaniello C, Sportiello L, et al. Dipeptidyl peptidase (DPP)-4 inhibitor-induced arthritis/arthralgia:a review of clinical cases[J]. Drug Safety, 2016, 39: 401. DOI:10.1007/s40264-016-0399-8 |
| [12] | Redford C, Doherty L, Smith J, et al. SGLT2 inhibitors and the risk of diabetic ketoacidosis[J]. Pract Diabetes, 2015, 32: 263–264a. DOI:10.1002/pdi.2015.32.issue-7 |
| [13] | Lan JQ, Zhu CJ. Recent advances in pharmacological intervention for prediabetes[J]. Acta Pharm Sin (药学学报), 2015, 50: 1565–1572. |
| [14] | Zheng Z. Oral atomized liquid with nicotine replacing cytosine and preparation method thereof: CN, 078913[P]. 2014-12-24. |
| [15] | Canu Boido C, Sparatore F. Synthesis and preliminary pharmacological evaluation of some cytisine derivatives[J]. Farmaco, 1999, 54: 438–451. DOI:10.1016/S0014-827X(99)00049-X |
 2018, Vol. 53
2018, Vol. 53