肝细胞肝癌(hepatocellular carcinoma, HCC)是最常见的肝癌亚型, 发病率占全球第五位, 严重威胁着人类健康[1]。肝细胞肝癌早期易转移, 术后易复发, 当前的治疗方法并不理想[2]。广谱多激酶抑制剂索拉非尼是FDA唯一批准的、用于治疗肝细胞肝癌的小分子化学药物, 然而该药物的药效并不令人满意, 平均只能延长患者3个月的存活期[3]。因此, 寻找理想的治疗肝细胞肝癌的新靶点, 并研发新的免疫疗法迫在眉睫, 近期有研究发现, 磷脂酰肌醇聚糖-3 (glypican-3, GPC3)是诊断和治疗肝细胞肝癌的理想靶点[4]。
GPC3是肝素硫酸多糖家族的一个膜蛋白, 其基因位于人类X染色体上(Xp26), 结构全长约900 kb, cDNA序列全长为2 263 bp, 编码580个氨基酸, 核心分子质量为70 kDa的膜蛋白[4, 5]。GPC3在HCC、结直肠癌和恶性黑素瘤等多种恶性肿瘤中高表达[6], 其中, HCC肿瘤细胞表面的GPC3表达率高达70%, 正常组织中均未检测到表达[7, 8]。
GPC3可能与HCC细胞的发生、发展有着密切的联系[9-12]。研究表明, GPC3的两个硫酸乙酰肝素(HS)侧链通过Wnt信号通路促进HCC细胞的增殖[13, 14], 同时, GPC3游离的HS侧链可以通过ERK和/或AKT信号蛋白激活相关的信号通路, 从而刺激周围的细胞生长[15]。此外, GPC3在HCC细胞中特异性高表达[16], 已成为HCC治疗和诊断的标记物, 研究者针对靶向GPC3的抗体进行了前期探索。其中以GC33为代表的药物进入了临床研究, GC33是一种特异性靶向GPC3的抗体, 该抗体表现出良好的安全性和耐受性, 同时由于GPC3在HCC细胞中的高表达, 该抗体也在恶性HCC患者上取得显著抗肿瘤疗效[17, 18]。
目前, 已报道靶向GPC3的抗体有全长抗体GC33、hGC33、YP7及单域抗体NH3, 以单链抗体作为“生物导弹”的相关研究目前还未开展[19]。本研究旨在利用噬菌体筛选技术获得靶向GPC3的高亲和力全人源单链抗体, 该抗体可有效避免人抗鼠抗反应(human anti-mouse antibody response, HAMA), 同时由于分子质量小, 使其具有良好的渗透性和内化能力[20]。其自身独特的性质可使其成为HCC新治疗性抗体和诊断试剂的优先选择。
材料与方法质粒、菌株、细胞及试剂 pET-22b-His载体由本实验室保存, E. coli Rosetta购自上海翊圣生物科技有限公司, HepG2、Huh-7和Bel-7402细胞由本实验室保存。质粒提取试剂盒、限制性内切酶Nco Ⅰ和Not Ⅰ均购自美国NEB公司; 羟氨苄青霉素(ampicillin, Amp)、盐酸四环素(tetracycline hydrochloride, Tet)、异丙基硫代半乳糖苷(isopropyl β-D-thiogalactoside, IPTG)、TMD显色液、BCA试剂盒、琼脂糖凝胶回收试剂盒、DNA marker、质粒提取试剂盒均购自上海生物工程股份有限公司; anti-His mouse monoclonal antibody、FITC goat anti mouse antibody、HRP goat anti mouse antibody、human glypican 3/GPC3/OCI-5 protein (His Tag)均购自北京义翘神州生物技术公司; HEPES购自美国GE公司; 全人源噬菌体单链抗体(single-chain variable fragment, scFv)抗体库为本课题组构建。
仪器 CM5芯片、Ni2+亲和柱、Biacore X100均购自美国GE公司; CO2恒温细胞培养箱、-70 ℃冰箱、细胞培养瓶、培养板、培养皿均购自Thermo公司; 冷冻离心机购自Beckman Coulter公司。
Anti-GPC3 scFv的筛选 本实验所用的人源抗体库的库容量约为1.2×1013 pfu·mL-1, 其包被所用的蛋白是市售全长GPC3蛋白。本实验用10 μg·mL-1全长GPC3蛋白在4 ℃包被过夜, 5%脱脂牛奶封闭2 h, 将噬菌体抗体库200 μL加入预先所包被的GPC3蛋白孔中, 37 ℃孵育2 h, 0.1% PBST震荡去除非特异性结合的噬菌体抗体, 加入Gly-HCl (pH 2.2)溶液100 μL进行洗脱, 震荡洗脱10 min, 立即用pH 8.5 Tris-HCl中和。感染10 mL大肠杆菌(OD600=0.5), 取感染后的菌液涂Tet+Amp平板, 计算菌落形成单位。剩余的菌液离心弃上清, 沉淀涂大型平板, 即为第1轮的sub-library。同上方法进行3轮的吸附-淘洗-洗脱-扩增, 每轮加入相同体积的噬菌体, 观察其是否富集。从第3轮和第4轮筛选的平板上挑选192个单克隆进行培养, 收集含有噬菌体上清进行ELISA初步筛选出能够与GPC3蛋白结合的阳性克隆。
Anti-GPC3 scFv阳性噬菌体克隆的鉴定 包被1 μg·mL-1市售全长GPC3蛋白, 用BSA溶液37 ℃共孵育2 h, 用0.05% PBST震荡去除非特异性结合的噬菌体抗体, 将收集的噬菌体上清37 ℃共孵育2 h, 将抗M13鼠源单克隆抗体作为一抗, 带有HRP的羊抗鼠抗体作为二抗, TMB显色后双波长法于酶标仪上测定OD450 nm和OD630 nm值, 并计算获取“OD450 nm-OD630 nm”值。
Anti-GPC3 scFv的构建和表达 用质粒提取试剂盒提取pET-22b-His质粒和pCantab-5E噬菌体质粒, 用Nco Ⅰ和Not Ⅰ进行双酶切, 纯化回收, 16 ℃过夜连接, 并转化到E. coli Rosetta (DE3)中, 在含Amp平板上37 ℃培养, 挑选单克隆进行测序, 进行鉴定。
将构建成功的工程菌株接种于平板上37 ℃过夜活化, 次日挑选单克隆于LB (100 μg·mL-1 Amp)液体培养基中。培养至OD值为0.6~1.0, 再以1:100的比例接种至发酵瓶中, 培养至对数生长期时, 加入终浓度0.5 mmol·L-1 IPTG, 于25 ℃诱导表达20 h。4 ℃、8 000 r·min-1离心15 min收集诱导表达的发酵液菌体, 用1/10初始发酵液体积的binding buffer (20 mmol·L-1 NaH2PO4-Na2HPO4, pH 7.4, 500 mmol·L-1 NaCl, 5 mmol·L-1咪唑)重悬菌体, 冰上温和搅拌10 min。加入终质量浓度1 mg·mL-1溶菌酶, 冰上温和搅拌30 min。再加入相应体积的5% triton-100温和搅拌10 min, 5 000 r·min-1离心30 min, 将2次离心的上清混合, 0.22 μm滤膜过滤。过滤后的样品用镍亲和柱层析纯化, 用不同浓度的咪唑溶液(20、50、100、250和500 mmol·L-1)进行梯度洗脱, 收集洗脱液。用15% SDS-PAGE分析各浓度洗脱液, 并用Western blot验证高纯度的洗脱样品(以抗His鼠源单克隆抗体为一抗, 带有HRP的羊抗鼠抗体作为二抗)。
ELISA法对anti-GPC3 scFv的活性初步检测 设置0.25~1 024 nmol·L-1的13个浓度梯度抗体, 以抗His鼠源单克隆抗体为一抗, 带有HRP的羊抗鼠抗体作为二抗, TMB显色后双波长法于酶标仪上测定OD450 nm和OD630 nm值, 计算出“OD450 nm-OD630 nm”值, 利用Graphpad软件进行KD值计算。
Biacore法测定anti-GPC3 scFv和GPC3间的亲和力常数先将市售的GPC3蛋白固定于CM5芯片上, 捕获单链抗体, 至响应值不低于2 000。用HBS-EP (pH 7.4)流动相缓冲液将单链抗体和GPC3蛋白稀释成不同的浓度梯度, 使单链抗体捕捉GPC3蛋白, 并测得结合常数Ka和解离常数Kd, 最后通过计算得到平衡解离常数KD=Kd/Ka。
Anti-GPC3 scFv与肿瘤细胞的结合能力的测定流式细胞技术检测anti-GPC3 scFv与GPC3阳性肝癌细胞(HepG2和Huh-7)及阴性细胞(Bel-7402)的结合率。设置0.25~1 024 nmol·L-1的13个1F7 scFv浓度梯度, 将抗His鼠源单克隆抗体作为一抗, 带有FITC的羊抗鼠抗体作为二抗, 从而验证抗体的靶向性。利用Flow job软件计算出其平均荧光强度, 用Graphpad软件进行KD值计算。从而验证其scFv与天然GPC3蛋白的结合能力和特异性。
Anti-GPC3 scFv与GPC3蛋白结合位点的预测 利用Molecular operating environment (MOE.2014)软件和SWISS-MODEL软件分别对1F7 scFv和GPC3蛋白进行建模并通过ZDOCK软件进行对接, 利用MOE软件进行分析获得1F7 scFv结合抗原表位。
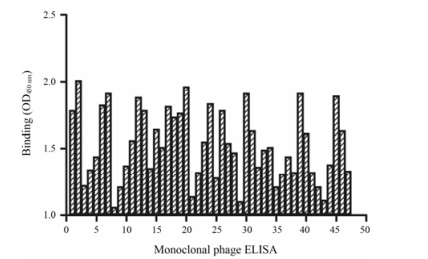
结果 1 anti-GPC3 scFv的筛选和鉴定通过4轮“吸附-淘洗-洗脱-扩增”后, 筛选的每轮输出率均有增加(表 1), 在第4轮的平板中挑取176个单克隆于96孔板中进行培养并表达含有anti-GPC3 scFv基因的噬菌体, 利用ELISA进行阳性单克隆的初步筛选。如图 1得到47个OD值在1.0以上的阳性克隆, 并进行测序分析, 对比发现共筛选出了4个富集的序列分别命名为1F7、1D7、1D4和1B10。通过IMGT抗体分析软件对4种scFv序列与全人源化抗体的同源性高达96.5%~98.2%, 并获得了4种富集的scFv序列。
| Table 1 Four rounds screen for anti-GPC3 scFv. Input and output are tested through dilution titer method. In addition, enrichment is the quotient of n and n-1 round of recovery rate. Single chain antibodies were effectively enriched |
|
Figure 1 The identification of positive clones. The figure is the summary of absorption data on 47 positive phage clones |
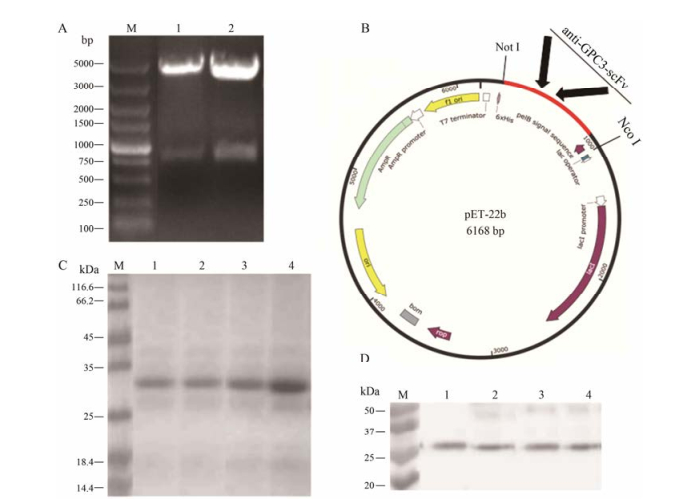
构建scFv需用双酶切获取scFv的基因, 图 2A中scFv的基因片段大小约为750 bp, 与带有His标签的载体连接, 构建为重组质粒(图 2B), 转化到大肠杆菌E. coli Rosetta中进行表达, 其表达产量可达到10 mg·L-1, SDS-PAGE显示4种构建的scFv其纯度较高, 无杂蛋白, 其分子质量约为28 kDa, 符合预期(图 2C)。Western blot显示蛋白的分子质量正确, 同时无蛋白降解(图 2D)。
|
Figure 2 The construction and expression of anti-GPC3 scFv. (A) Electrophoresis map of anti-GPC3 scFv phage plasmid. (B) The schematic map of anti-GPC3 scFv with His tag recombinant plasmid. (C) and (D) SDS-PAGE and Western blot analysis of four different anti-GPC3 scFv expressed by E. coli Rosetta. Lane 1: 1D7 scFv; Lane 2: 1D4 scFv; Lane 3: 1F7 scFv; Lane 4: 1B10 scFv. GPC3: Glypican-3; scFv: Single-chain variable fragment |
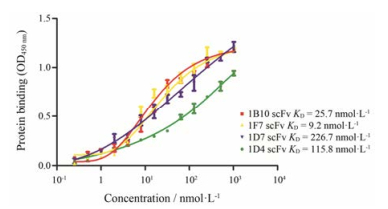
亲和力是治疗性抗体靶向肿瘤细胞最重要的性质之一, 因此先用ELISA法对4种anti-GPC3 scFv进行了初步检测, 利用Graphpad 5计算后, 如图 3, 1F7、1D7、1D4和1B10 4种anti-GPC3 scFv的KD值分别可达到9.2、226.7、115.8和25.7 nmol·L-1, 其中1F7 scFv的亲和力最高。
|
Figure 3 Preliminary detection of affinity of four anti-GPC3 scFv with ELISA method. This method used commercial human full length GPC3 protein |
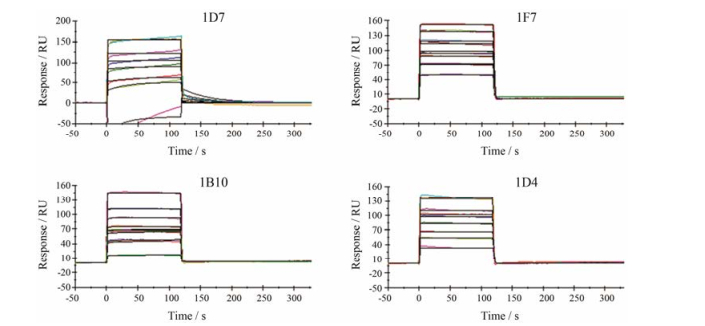
ELISA法只能够对scFv的亲和力进行初步测定。为了获取scFv准确的亲和力常数, 采用SPR技术对scFv的亲和力进行了测定, 结果显示(图 4), 其1F7的KD值最高, 可达到4.037 nmol·L-1, 而1D7、1D4和1B10 3种anti-GPC3 scFv的KD值分别为208、231和10.97 nmol·L-1。
|
Figure 4 Affinity and binding capacity analysis. Affinity of anti-GPC3 scFv for binding to commercial human full length GPC3 protein was measured by SPR. Experimental results for the real-time binding of GPC3 to immobilized anti-GPC3 scFv showed that the association rate increased with increasing concentrations of the GPC3 protein |
采用2株表达GPC3的HCC细胞和不表达GPC3的阴性细胞株Bel-7402来测定单链抗体对HCC细胞的结合能力。结果如图 5A显示, 64、128、256和512 nmol·L-1 1F7 scFv对Huh-7的结合率可分别达到31.9%、42.7%、67.7%和78.8%, 对HepG2的结合率可分别达到40.5%、54.6%、77.9%和87.4%, 但512 nmol·L-1 1F7 scFv对BEL-7402的结合率仅为6.14%, 1F7 scFv表现出良好的靶向性。此外, 利用Graphpad计算后, 1F7 scFv对GPC3阳性细胞Huh-7和HepG2表面的天然GPC3蛋白KD分别为39.8和10.2 nmol·L-1 (图 5B)。综上所述, 1F7 scFv是一种能够特异性、高亲和力靶向GPC3蛋白的单链抗体。
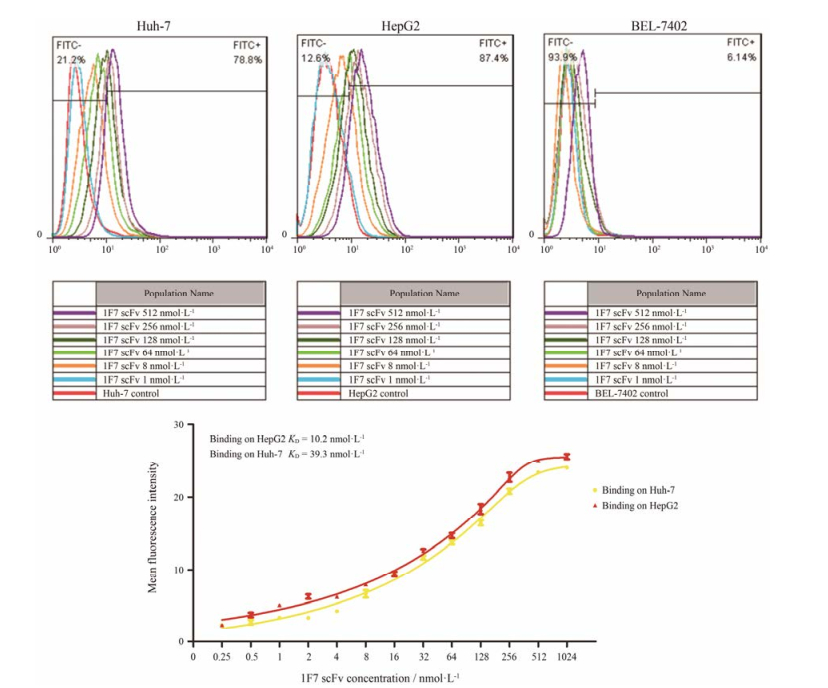
|
Figure 5 Affinity and binding capacity analysis. (A) FACS analysis of cell based on different concentration of 1F7 scFv binding on HepG2 (GPC3+), Huh-7 (GPC3+) and Bel-7402 (GPC3-) cells. (B) Detection of affinity of 1F7 scFv for nature GPC3 protein with flow cytometry |
通过生物信息学技术对GPC3与1F7 scFv进行同源模建、分子对接, 成功模拟GPC3与1F7 scFv对接的三维模型(图 6), 从理论上预测抗原抗体的结合位点(表 2), 为后续的活性实验奠定理论基础。

|
Figure 6 The prediction of interaction site between GPC3 and 1F7 scFv. (A) Complex of GPC3 and 1F7 scFv binding predicted by ZDOCK server. (B) Residues interaction in GPC3 and 1F7 scFv binding site |
| Table 2 Prediction on interactions between GPC3 and 1F7 scFv by ZDOCK |
目前已报道靶向GPC3的抗体有GC33、hGC33、YP7和NH3。GC33和hGC33已进入临床Ⅱ期, 用于治疗恶性肝细胞肝癌, YP7和NH3则处于临床前研究[11, 19, 20]。GC33通过诱导抗体依赖性细胞毒性作用(ADCC), 可抑制HCC肿瘤细胞在裸鼠皮下的生长[21]。hGC33是人源化的GC33, 其已应用于肝癌治疗, 但hGC33在Ⅱ期临床试验中并没有表现出显著的临床疗效[22]。YP7和NH3则是由美国NIH研发的2种不同类型的靶向GPC3单克隆抗体。YP7的识别位点与hGC33重叠, 都能识别GPC3的C-末端抗原[23]。NH3则为全人源的重链可变区抗体(heavy chain available antibody, VHH), 可有效地抑制肿瘤细胞的增殖[24, 25]。相较于GC33、hGC33和YP7, 单链抗体(scFv)分子质量小, 免疫原性低, 拥有良好的组织渗透能力, 更易进入实体瘤周围的微环境甚至实体瘤内部, 其血液循环和全身的廓清速度较快, 半衰期短, 肾脏蓄积少。此外, 其可与高效“弹头”药物构成免疫偶联物, 其良好的内化能力能更好地使药物进入细胞内发挥疗效。目前针对GPC3 scFv的ADC或免疫毒素药物研究目前还未开展, 新型免疫治疗药物将有助于解决HCC的治疗难题。
单链抗体作为“生物导弹”, 携带相关的毒素蛋白或相关的免疫细胞的激活受体等已逐步应用于临床研究, 高亲和力和良好的靶细胞表面蛋白结合能力是单链抗体发挥靶向功能的基础。ELISA及SPR技术均是对抗体亲和力测定的通用技术, 其结果均显示1F7 scFv的亲和力可达到纳摩尔级, 较1B10、1D4和1D7 scFv的亲和力要高出10~100倍。在细胞结合实验中, 1F7 scFv表现出对GPC3阳性肿瘤细胞表面的天然GPC3蛋白具有良好的结合能力, 但对GPC3阴性肿瘤细胞株无结合能力, 各项数据表明, 1F7 scFv对市售重组GPC3蛋白和天然GPC3蛋白均表现出良好的特异性和结合能力, 具备作为“生物导弹”的潜质。
本研究通过噬菌体库筛选得到4个富集scFv的序列, 结果表明1F7 scFv为理想的anti-GPC3 scFv, 该anti-GPC3 scFv可作为良好的载体, 为下一步的免疫疗法和肿瘤的影像诊断奠定基础。1F7 scFv能否有效抑制HCC的增殖, 及抑制肿瘤增殖的相关机制还需要进一步探索。
| [1] | El-Serag HB, Rudolph KL. Hepatocellular carcinoma:epidemiology and molecular carcinogenesis[J]. Gastroenterology, 2007, 132: 2557–2576. DOI:10.1053/j.gastro.2007.04.061 |
| [2] | Ng IO, Lai EC, Fan ST, et al. Prognostic significance of pathologic features of hepatocellular carcinoma. A multivariate analysis of 278 patients[J]. Cancer, 1995, 76: 2443–2448. DOI:10.1002/(ISSN)1097-0142 |
| [3] | Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma[J]. N Engl J Med, 2008, 359: 378–390. DOI:10.1056/NEJMoa0708857 |
| [4] | Shirakawa H, Kuronuma T, Nishimura Y, et al. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer[J]. Int J Oncol, 2009, 34: 649–656. |
| [5] | Ho M, Kim H. Glypican-3:a new target for cancer immunotherapy[J]. Eur J Cancer, 2011, 47: 333–338. DOI:10.1016/j.ejca.2010.10.024 |
| [6] | Li W, Guo L, Rathi P, et al. Redirecting T cells to glypican-3 with 4-1BB zeta chimeric antigen receptors results in Th1 polarization and potent antitumor activity[J]. Hum Gene Ther, 2017, 28: 437–448. DOI:10.1089/hum.2016.025 |
| [7] | Yamauchi N, Watanabe A, Hishinuma M, et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma[J]. Mod Pathol, 2005, 18: 1591–1598. DOI:10.1038/modpathol.3800436 |
| [8] | Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma:biological significance and temporospatial distribution[J]. Cancer Res, 1997, 57: 5179–5184. |
| [9] | Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma[J]. Cancer Sci, 2009, 100: 1403–1407. DOI:10.1111/cas.2009.100.issue-8 |
| [10] | Wei G, Ho M. The role of glypican-3 in regulating Wnt in hepatocellular carcinomas[J]. Cancer Rep, 2011, 1: 14–19. |
| [11] | Allegretta M, Filmus J. Therapeutic potential of targeting glypican-3 in hepatocellular carcinoma[J]. Anticancer Agents Med Chem, 2011, 11: 543–548. DOI:10.2174/187152011796011109 |
| [12] | Chen M, Li G, Yan J, et al. Reevaluation of glypican-3 as a serological marker for hepatocellular carcinoma[J]. Clin Chim Acta, 2013, 423: 105–111. DOI:10.1016/j.cca.2013.04.026 |
| [13] | Zittermann SI, Capurro MI, Shi W, et al. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo[J]. Int J Cancer, 2010, 126: 1291–1301. |
| [14] | Gao W, Kim H, Ho M. Human monoclonal antibody targeting the heparan sulfate chains of glypican-3 inhibits HGF-mediated migration and motility of hepatocellular carcinoma cells[J]. PLoS One, 2015, 10: e0137664. DOI:10.1371/journal.pone.0137664 |
| [15] | Wu Y, Liu H, Weng H, et al. Glypican-3 promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway[J]. Int J Oncol, 2015, 46: 1275–1285. |
| [16] | Suzuki S, Yoshikawa T, Hirosawa T, et al. Glypican-3 could be an effective target for immunotherapy combined with chemotherapy against ovarian clear cell carcinoma[J]. Cancer Sci, 2011, 102: 1622–1629. DOI:10.1111/cas.2011.102.issue-9 |
| [17] | Zhu AX, Gold PJ, El-Khoueiry AB, et al. First-in-man phase Ⅰ study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma[J]. Clin Cancer Res, 2013, 19: 920–928. DOI:10.1158/1078-0432.CCR-12-2616 |
| [18] | Ikeda M, Ohkawa S, Okusaka T, et al. Japanese phase Ⅰ study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma[J]. Cancer Sci, 2014, 105: 455–462. DOI:10.1111/cas.2014.105.issue-4 |
| [19] | Ishiguro T, Sugimoto M, Kinoshita Y, et al. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer[J]. Cancer Res, 2008, 68: 9832–9838. DOI:10.1158/0008-5472.CAN-08-1973 |
| [20] | Winter G, Griffiths AD, Hawkins RE, et al. Making antibodies by phage display technology[J]. Annu Rev Immunol, 1994, 12: 433–4556. DOI:10.1146/annurev.iy.12.040194.002245 |
| [21] | Takai H, Kato A, Ishiguro T, et al. Optimization of tissue processing for immunohistochemistry for the detection of human glypican-3[J]. Acta Histochem, 2010, 112: 240–250. DOI:10.1016/j.acthis.2008.11.025 |
| [22] | Aboualfa GK, Yen CJ, Carrasquillo JA, et al. Phase Ib study of RO5137382/GC33 in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC) (NCT00976170)[J]. J Clin Oncol, 2014, 32: 4100. |
| [23] | Gao W, Kim H, Feng M, et al. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy[J]. Hepatology, 2014, 60: 576–587. DOI:10.1002/hep.v60.2 |
| [24] | Feng M, Gao W, Wang R, et al. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma[J]. Proc Natl Acad Sci U S A, 2013, 110: E1083–E1091. DOI:10.1073/pnas.1217868110 |
| [25] | Gao W, Tang Z, Zhang YF, et al. Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signaling and protein synthesis[J]. Nat Commun, 2015, 6: 6536. DOI:10.1038/ncomms7536 |
 2017, Vol. 52
2017, Vol. 52


