2. 山西大学 化学化工学院, 山西 太原 030006;
3. 中国医学科学院、北京协和医学院药物研究所, 北京 100050
2. College of Chemistry and Chemical Engineering, Shanxi University, Taiyuan 030006, China;
3. Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
在过去的一个多世纪, 疾病的治疗明显提高了人类的平均预期寿命。然而, 与衰老相关的认知功能减退已成为老年人健康的最大威胁之一[1]。衰老是生命过程中的必经阶段, 是一种自然规律, 由经典信号通路和转录因子等共同调节, 其中中枢神经系统是重要的机能调节系统, 也是受衰老影响最大的系统之一。
在衰老过程中, 学习记忆功能减退是中枢神经系统的一个突出表现。目前已在多种动物模型中证实胆碱功能减退与衰老性认知功能降低有关, 且越来越多的证据表明单胺类神经递质去甲肾上腺素(norepinephrine, NE)、多巴胺(dopamine, DA)和5-羟色胺(serotonin, 5-HT)也参与学习记忆调控。
神经元通过释放神经递质来调节目标神经元细胞功能和突触传递性能。调节神经元的细胞体分布在脑干、中脑和基底前脑的特定位置, 通过突触传递方式广泛传播化学信号, 从而影响多个脑区细胞的兴奋性和突触可塑性。其中, NE主要源于蓝斑(locus coeruleus, LC), DA源于中脑被盖腹区和黑质, 5-HT源于脑干缝际核。这些神经递质对记忆的调节发挥着重要作用, 它们既可以影响工作记忆(指短期记忆的储存和处理), 又可以影响情绪记忆(长期记忆, 指外界条件刺激形成的记忆)。目前, 各个脑区和多种神经递质共同调节学习记忆的观点受到研究者的广泛认同。
然而, 伴随衰老过程学习记忆能力的减退, 中枢单胺类神经递质发生着复杂的变化, 本文综述了衰老过程中相关脑区单胺类神经递质的改变情况、调节记忆的机制和导致递质异常的病理因素, 以便更好地理解单胺类神经递质对衰老性学习记忆减退的影响, 并为研究改善学习记忆的药物提供靶点信息。
1 去甲肾上腺素脑内大多数NE由LC释放, LC是脑干神经调控中心, 向中枢神经系统的海马、杏仁核、下丘脑、小脑和皮质等多个区域投射, 同时LC也接收来自下丘脑、杏仁核和前额皮质等的投射[2]。NE是学习记忆必需的神经递质, 可以调节神经系统可塑性, 被认为是有效的记忆调节器。海马和前额皮质(prefrontal cortex, PFC)部位NE主要参与工作记忆的调节; 杏仁核部位NE主要参与情绪记忆的调节。
1.1 去甲肾上腺素与学习记忆功能NE调节学习记忆功能与脑区NE水平及不同受体结合有关。研究发现, 在新物体识别实验中, 青年大鼠(40~45日龄)无法区分新旧物体, 而成年大鼠(60+日龄)能够很好区分; 并且发现与未参加实验的成年大鼠相比, 参与实验的成年大鼠内侧颞叶中NE水平明显增高, 而是否参与实验对青年大鼠NE水平并无影响。在给予青年大鼠育亨宾使NE水平升高后, 则可以很好地识别新旧物体, 说明NE对学习记忆的影响与年龄有关, 并且提高NE水平可以改善学习记忆能力[3]。Barsegyan等[4]发现给SD大鼠杏仁核注射NE不仅可以提高情绪记忆, 还可以提高大鼠的空间记忆能力; 并且Groch等[5]发现, 增加慢波睡眠早期杏仁核和海马部位NE水平促进记忆巩固。Hammerschmidt等[6]研究发现, 在APP/PS1突变小鼠体内由于NE水平降低, 而导致认知功能损伤, 当给予NE前体药物L-二羟苯基丝氨酸后可以适当提高小鼠的空间记忆功能。然而, 长期持续性给予正常大鼠NE再摄取抑制剂, 即长期使NE水平升高, 会损伤大鼠空间学习记忆能力[7]。综上所述, 适当水平的NE可以提高学习记忆功能, 而NE水平过高或者过低都会产生相反的作用。这可能是因为NE通过激动不同的受体而发挥不同作用。适当水平的NE通过适度激活杏仁核α1、β肾上腺素受体[8]、海马β1受体[9]及PFC中α2A受体[10], 可提高情绪记忆和工作记忆; 而NE水平过高会抑制海马、PFC中突触前α2A受体, 与低亲和力的α1受体结合[11, 12], 从而损伤记忆[13]。
已有大量研究表明, NE具有调节突触可塑性、诱导长时程增强(long-term potentiation, LTP)而提高认知功能的作用。早有研究报道, NE与β受体结合, 可以激活细胞外调节蛋白激酶(ERK), 从而调节早期和晚期LTP形成[14]; 通过激活蛋白激酶(PKA)和钙调蛋白激酶(CaMKII)通路, 调节谷氨酸受体1 (GluR1) Ser845和Ser831位点的磷酸化水平, 从而调节α-氨基-3-羟基-5-甲基-异恶唑丙酸(AMPA)受体功能和LTP, 影响学习记忆功能[15]。近期研究发现, 海马CA1区域NE激活β受体使DNA甲基化、组蛋白乙酰化和磷酸化而调节基因表达和转录, 参与表观遗传机制, 进而增加LTP持久性[16]。NE可以诱导即刻早期基因Fos的表达[17], 增加脑源性神经营养因子(BDNF)水平[9], 促进海马突触可塑性相关蛋白(Arc)表达[18], 诱导LTP提高记忆功能。Carriba等[19]研究表明NE可以通过调节N-甲基-D-天冬氨酸(NMDA)受体激活环磷酸腺苷元反应元件结合蛋白(CREB)进而诱导LTP。Wang等[20]研究发现, PFC中NE通过激活突触前α2A受体, 抑制环磷酸腺苷−超极化激活环核苷酸门控阳离子通道(cAMP-HCN)信号通路, 诱导LTP增强工作记忆。此外, 研究证明NE可以降低γ-氨基丁酸(γ-aminobutyric acid, GABA)神经递质的抑制作用, 调节下丘脑−杏仁核突触可塑性, 从而诱导LTP[21]。由上可见, NE可通过调节AMPA受体功能, 激活ERK、CREB, 增加Arc和BDNF水平, 抑制cAMP-HCN通路、降低GABA神经元反馈抑制作用而诱导LTP效应, 提高学习记忆功能。
1.2 衰老性去甲肾上腺素改变越来越多的研究表明, 在自然衰老动物模型[22]、药物诱导的衰老动物模型[23]、TgCRND8转基因小鼠痴呆模型[24]和阿尔茨海默病(Alzheimer’s disease, AD)患者[25]中学习记忆能力减退与NE减少相关。这可能是由于NE水平降低影响LTP效应。Luo等[22]发现给自然衰老大鼠注射NE可修复LTP损伤, 改善大鼠记忆能力。在AD患者中发现, NE通过增加甲酰肽-2受体(FPR2)水平而促进神经胶质细胞摄取β淀粉样蛋白(Aβ), 诱导产生Aβ降解酶, 降低Aβ水平; 同时激活原肌球蛋白相关激酶B (TrkB), 抵抗Aβ毒性, 进而抵抗Aβ诱导的LTP损伤, 增强学习记忆功能[26, 27]。可见, NE在衰老认知过程中发挥重要的调节作用。
衰老性NE水平降低可能由多种因素诱发。①早有研究证明伴随衰老过程单胺氧化酶(MAO)含量增加; MAO抑制剂盐酸司立吉林可通过减少单胺氧化脱氨反应, 增加突触间隙NE和DA含量, 进而有效提高老年啮齿类动物的学习能力[28]。② Mei等[24]近期研究表明, 内源性甲醛(formaldehyde, FA)可能是造成与衰老相关的NE减少和学习记忆减退的重要因素。实验发现, 衰老相关的DNA脱甲基作用使得海马中FA累积, 从而使衰老大鼠海马内NE量减少; 同样在体外FA可以使NE迅速灭活, 并且向健康成年大鼠海马内注入FA后可以明显降低NE水平。③ Mustapic等[29]研究发现, 早期AD患者血浆中多巴胺β羟化酶(DBH, 合成NE的限速酶)活性降低, 这可能是中枢NE水平降低的另一个原因。此外, NE由DA转化而来, 脑内DA水平的减少也是NE水平降低的潜在原因。
2 多巴胺DA主要源于中脑腹侧被盖区, 向PFC、海马、杏仁核、前扣带皮层、内嗅皮层、颞叶和基底前脑等多个脑区投射。DA一直以来被认为是与认知、情感相关的重要神经递质。近年来, 越来越多的研究证明纹状体、PFC和海马部位的DA在控制记忆的神经动力学信号和记忆提取的精确性中发挥着重要作用[30]。
2.1 多巴胺与学习记忆功能纹状体DA在调节学习记忆过程中发挥着重要的作用。大量研究表明, 在工作记忆更新过程中, 纹状体DA释放增加, 这是高级认知功能形成的基础[31]。应用6-羟基多巴胺(6-OHDA)损耗纹状体DA, 导致穿孔突触数量过度增加、神经元凋亡而损伤认知功能[32]。同时, 纹状体DA减少是导致帕金森病(Parkinson’s disease, PD)患者空间学习记忆功能降低的重要因素[33]。应用他莫昔芬增加Aβ致痴呆模型小鼠纹状体中DA水平, 可明显改善小鼠空间记忆和情景记忆[34]。纹状体DA发挥增强学习记忆功能主要是通过激活多巴胺D1受体[35]或D2受体[36, 37]。研究证明, 纹状体多巴胺调节工作记忆的另一个机制可能是向PFC投射, 影响PFC功能[38]。
DA在调节学习记忆相关的突触可塑性中发挥重要作用, 并且突触可塑性与DA水平呈倒U型相关[39]。一定量的DA可以增强突触可塑性, 诱导LTP, 增强认知功能。研究发现海马中DA激活D1受体诱导BDNF表达, 调节记忆的编码和储存[40]; DA与D1/D5受体结合后激活cAMP-PKA通路, 一方面可促进NMDA受体[41]和AMPA受体磷酸化, 增强突触可塑性, 诱导LTP; 另一方面可促进DA和cAMP调节的磷蛋白(DARPP-32)磷酸化, 抑制蛋白磷酸酶1 (PP-1), 激活CREB诱导LTP, 增强学习记忆功能[37, 42]。然而, DA水平过低或过高都会抑制突触可塑性, 损伤认知功能。DA水平降低时, 激活突触后D2受体, 抑制突触可塑性, 损伤记忆功能; DA水平过高时, 激活D1受体, 降低NMDA受体活性, 使细胞内Ca2+水平达不到触发LTP的阈值而诱导长时程抑制(LTD), 损伤学习记忆功能[39]。由上可见, DA可通过调节NMDA受体、AMPA受体及cAMP-PKA/ CREB/BDNF通路, 诱导LTP效应, 提高学习记忆功能。
此外, 研究发现果蝇蘑菇体(mushroom body, MB)中特定DA神经元参与味觉记忆形成[43], 并且DA对记忆具有双向调节作用, 即激活D1受体增强记忆获得, 激活位于蘑菇体上的DA受体(DAMD受体)产生记忆遗忘[44]。
2.2 衰老性多巴胺改变研究发现, 在自然衰老、AD患者、AD动物模型和PD患者的纹状体、海马、PFC、基底核等多个脑区DA含量降低, 导致LTP损伤, 认知功能减退[45, 46]。研究证明, DA合成有助于调节年轻人学习记忆相关的网络活动, 而衰老性DA水平降低将影响学习记忆相关网络活动, 导致学习记忆功能减退[47]。有研究发现, DA影响脑区血氧水平, 这可能是衰老性认知功能降低的重要机制之一:在老年人的皮层下部位(海马、纹状体和杏仁核)检测到DA含量降低使血氧水平升高; 而在皮层部位DA含量降低使血氧水平降低[48]。研究发现, D1/D5受体激动剂SKF38393可以通过激活Src家族酪氨酸激酶通路改善Aβ诱导的LTP损伤, 并且多巴胺能药物治疗PD和AD患者的认知障碍效果明显[33, 49]。可见DA水平降低是衰老及衰老相关疾病记忆功能减退的重要原因。
衰老性DA水平降低可能由多种因素诱发。①研究发现, 在老年人脑腹侧被盖区和黑质中DA神经元减少, 从而使DA释放减少[50]。②酪氨酸羟化酶(TH)是脑内合成DA的限速酶, 可催化DA合成。Moreno-Castilla等[51]研究发现, 在Aβ转基因小鼠脑内Aβ沉积, 诱导皮质TH减少, 使DA合成减少; Dickerson等[52]发现, 与年轻大鼠相比, 衰老大鼠脑中神经调节因子受体ErbB4表达水平降低, 从而使TH水平降低, 导致衰老相关的DA水平降低。③探索基因多态性有助于理解衰老性DA水平降低的原因。研究发现, 正常衰老和AD患者增加PFC儿茶酚胺氧甲基转移酶(COMT)和DBH基因编码酶突变, 可降低这两种酶的活性, 进而降低细胞外DA水平, 影响工作记忆[53, 54]。
3 5-羟色胺5-羟色胺(5-HT)广泛分布在脑内多个区域, 在活动节律、食物摄取、性行为、情绪状态和学习记忆等方面发挥着重要的调节作用。中缝核中的5-HT神经元向海马、额叶、颞叶皮层和鼻中隔等结构发射与认知功能相关的信号[55], 并且通过激动特定的受体而调节多巴胺能、胆碱能、γ-氨基丁酸能和谷氨酸能神经系统共同调节学习、短时程和长时程记忆。
3.1 5-羟色胺与学习记忆功能适当增加脑内5-HT水平能改善学习记忆功能, 而5-HT水平降低可导致学习记忆损伤。研究发现, 应用不可逆性色氨酸羟化酶抑制剂PCPA或色氨酸损耗技术降低脑中5-HT水平, 可明显损伤大鼠的学习记忆能力[56, 57]。Fernandez等[55]研究发现, 选择性损伤SertCre小鼠中缝核5-HT神经元, 使5-HT合成释放减少, 导致空间认知功能降低; Pet1基因敲除小鼠(脑内5-HT水平降低80%)在新物体识别实验中表现出明显的空间记忆损伤, 然而脑部注射5-HT后, 可明显改善记忆损伤。Kuo等[58]研究发现, 长期给予5-HT再摄取抑制剂氟西泮可以促进经颅直流电刺激诱导LTP, 增强认知功能。临床研究发现, 抗抑郁治疗可通过提高抑郁症患者脑内5-HT水平, 改善患者的认知功能[59]。
5-HT激活不同的受体, 调节突触可塑性, 进而影响学习记忆。与学习记忆密切相关的5-HT受体主要包括: 5-HT1A/1B/1D、5-HT2A/2B/2C、5-HT3、5-HT4、5-HT5、5-HT6和5-HT7受体。5-HT激活海马CA1区5-HT1A受体介导抑制兴奋性突触后电位, 这对于诱导LTP发挥重要作用[55]。并且发现在5-HT1A受体敲除小鼠的腹侧海马中BDNF、TrkB和丝裂原激活的蛋白激酶(MAPK)水平降低[60], 可能是诱导LTP的另一个机制。在成年大鼠视觉皮层, 5-HT激活5-HT2受体, 促进三磷酸肌醇(IP3)与其受体结合, 增加Ca2+水平, 增强突触可塑性[61]。5-HT激活5-HT4受体可以提高BDNF、CREB、β-连环蛋白和蛋白激酶(Akt)水平, 增加蛋氨酸脑啡肽(MEK)活性, 促进Arc表达, 增强突触可塑性[62, 63]。5-HT激活5-HT7受体, 从而增强CA3~CA1区AMPA受体介导的突触可塑性[64]。相反, 5-HT激活5-HT6受体调节细胞周期蛋白依赖性激酶(Cdk5), 使mTOR过表达进而损伤记忆功能[65]; 并且分选微管连接蛋白14 (SNX14)抑制性调节5-HT6受体介导的cAMP-PKA通路, 进而抑制LTP[66]。
5-HT可以通过调节多巴胺能、胆碱能、GABA能和谷氨酸能神经传导而调节学习记忆功能。大量研究证明5-HT激活5-HT1A受体, 可以增加狨猴皮层DA水平, 增强DA能神经传导[67]; 激活5-HT4受体, 促进Ach释放, 增强胆碱能作用[68]; 激活5-HT7受体增加海马CA1区GABA神经递质的释放, 增强兴奋性GABA能传导[69], 进而增强学习记忆功能。然而, 激活5-HT6受体可以增强皮层抑制性GABA能神经传导, 抑制胆碱能传导, 下调皮质−纹状体谷氨酸能神经传导[70, 71], 而对记忆产生抑制作用。由上可见, 在哺乳动物中, 5-HT通过激活不同的受体并影响其他神经传递系统, 从而对学习记忆发挥直接或间接的调节作用。
此外, 5-HT可以调控果蝇和秀丽隐杆线虫的学习记忆能力。研究发现秀丽隐杆线虫ADF感觉神经元释放的5-HT与5-HT4/7受体结合, 激活胰岛素信号通路, 增强温度感知记忆[72]。果蝇背中侧的配对神经元向MB投射5-HT射线而调节果蝇嗅觉学习记忆功能和抗昏迷记忆。应用等辐射分析法和遗传学实验研究发现, 5-HT激活MB中5-HT1A受体促进短期记忆和长期记忆的巩固; 激活椭球体中5-HT2受体可以调节MB活性促进学习记忆的巩固; 激活5-HT7受体, 可以通过影响5-HT2回路而间接调节MB功能[73, 74]。
3.2 衰老性5-羟色胺改变伴随衰老过程, 脑区多个部位5-HT水平明显降低。研究证明, 正常衰老大鼠皮层、海马和下丘脑等部位5-HT水平明显降低[75], 并且与衰老性学习记忆功能降低[76, 77]有关。此外, 在AD和老年抑郁症(geriatric depression, GD)患者脑中也发现同样的改变[78]。5-HT水平降低会影响学习记忆相关的突触可塑性。S-100β是突触合成所需的营养因子, 由星形胶质细胞释放, 在神经突的延伸、细胞微管、树状稳定性和生长相关蛋白43 (GAP-43)的调节中发挥着重要作用。5-HT损耗可通过影响S-100β, 造成大脑形态学和认知能力的永久改变[79]。长期食用色氨酸丰富的食物可以提高衰老脑中枢5-HT水平, 降低AD模型大鼠脑中Aβ沉积, 改善学习记忆功能[80, 81]。给予5-HT再摄取抑制剂可以抑制AD鼠Aβ生成, 并改善GD症患者认知功能[82]。
衰老性5-HT水平降低可能由多种因素诱发。①衰老过程伴随着白介素6 (interleukin-6, IL-6)等炎性因子增加, 导致色氨酸羟化酶(TPH)活性降低[75], 5-HT合成减少, 进而损伤学习记忆[78]。②衰老小鼠脑内BDNF信号通路衰退, 导致5-HT投射减少, 进而影响学习记忆[83]。③正常衰老和AD患者PFC中COMT突变增加、MAO-A活性增加等也是衰老性5-HT水平降低的重要原因。
4 结语学习和记忆是人类日常活动和社会交际的核心能力。尽管公共医疗卫生的改善已经延长了人类寿命, 然而, 无论是正常衰老还是衰老性疾病如AD、PD及GD伴随的学习记忆减退依然严重困扰着老年人。本课题组前期致力于研究衰老和相关学习记忆减退的机制及防治措施, 取得了一些研究成果[84, 85]。大量证据表明衰老性学习记忆减退与脑内神经递质的改变密切相关, 因此研究衰老过程中神经递质的变化及其调节学习记忆的机制具有重要意义, 可为本课题组开展进一步研究奠定基础。
综上所述, 适当地提高衰老脑区单胺类神经递质水平, 可以有效改善认知功能。如图 1所示, NE、DA和5-HT与特定脑区受体结合, 通过调节受体和相关信号通路功能、调节相关酶的活性和蛋白表达水平, 并通过影响其他神经递质及参与表观遗传调控等方式, 调节突触可塑性, 影响学习记忆功能。
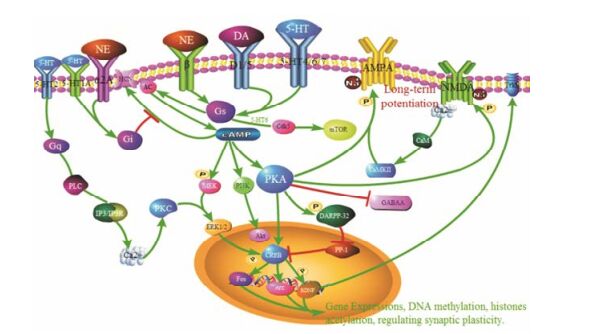
|
Figure 1 Monoaminergic signaling mechanisms of learning and memory |
| [1] | Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline[J]. Nature, 2010, 464: 529–535. DOI:10.1038/nature08983 |
| [2] | Mammarella N, Di Domenico A, Palumbo R, et al. Noradrenergic modulation of emotional memory in aging[J]. Ageing Res Rev, 2016, 27: 61–66. DOI:10.1016/j.arr.2016.03.004 |
| [3] | Nirogi R, Abraham R, Jayarajan P, et al. Difference in the norepinephrine levels of experimental and non-experimental rats with age in the object recognition task[J]. Brain Res, 2012, 1453: 40–45. DOI:10.1016/j.brainres.2012.03.013 |
| [4] | Barsegyan A, McGaugh JL, Roozendaal B. Noradrenergic activation of the basolateral amygdala modulates the consolidation of object-in-context recognition memory[J]. Front Behav Neurosci, 2014, 90: 576–583. |
| [5] | Groch S, Wilhelm I, Diekelmann S, et al. Contribution of norepinephrine to emotional memory consolidation during sleep[J]. Psychoneuroendocrinology, 2011, 36: 1342–1350. DOI:10.1016/j.psyneuen.2011.03.006 |
| [6] | Hammerschmidt T, Kummer MP, Terwel D, et al. Selective loss of noradrenaline exacerbates early cognitive dysfunction and synaptic deficits in APP/PS1 mice[J]. Biol Psychiatry, 2013, 73: 454–463. DOI:10.1016/j.biopsych.2012.06.013 |
| [7] | Walling SG, Milway JS, Ingram M, et al. The effects of prolonged administration of norepinephrine reuptake inhibitors on long-term potentiation in dentate gyrus, and on tests of spatial and object recognition memory in rats[J]. Neurobiol Learn Mem, 2016, 128: 92–102. DOI:10.1016/j.nlm.2015.12.013 |
| [8] | Jacklin EC, Boughner E, Kent K, et al. Memory of a drug lapse:role of noradrenaline[J]. Neuropharmacology, 2015, 99: 98–105. DOI:10.1016/j.neuropharm.2015.07.020 |
| [9] | Mellocarpes PB, Vargas LD, Gayer MC, et al. Hippocampal noradrenergic activation is necessary for object recognition memory consolidation and can promote BDNF increase and memory persistence[J]. Neurobiol Learn Mem, 2016, 127: 84–92. DOI:10.1016/j.nlm.2015.11.014 |
| [10] | Wang M, Ramos BP, Paspalas CD, et al. Al-pha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex[J]. Cell, 2007, 129: 397–410. DOI:10.1016/j.cell.2007.03.015 |
| [11] | Arnsten AF, Murphy B, Merchant K. The selective dopamine D4 receptor antagonist, PNU-101387G, prevents stress-induced cognitive deficits in monkeys[J]. Neu-ropsychopharmacology, 2000, 23: 405–410. DOI:10.1016/S0893-133X(00)00133-0 |
| [12] | Lv J, Zhan SY, Li GX, et al. α1-Adrenoceptors in the hippocampal dentate gyrus involved in learning-dependent long-term potentiation during active-avoidance learning in rats[J]. Neuroreport, 2016, 27: 1211–1216. DOI:10.1097/WNR.0000000000000679 |
| [13] | Bouret S, Sara SJ. Network reset:a simplified overarching theory of locus coeruleus noradrenaline function[J]. Trends Neurosci, 2005, 28: 574–582. DOI:10.1016/j.tins.2005.09.002 |
| [14] | Winder DG, Martin KC, Muzzio IA, et al. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors[J]. Neuron, 1999, 24: 715–726. DOI:10.1016/S0896-6273(00)81124-1 |
| [15] | Peng Y, Li PP, Li L, et al. Progress of clinical trials in Alzheimer's disease drugs[J]. Acta Pharm Sin (药学学报), 2016, 51: 1185–1195. |
| [16] | Maity S, Jarome TJ, Blair J, et al. Noradrenaline goes nuclear:epigenetic modifications during long-lasting synaptic potentiation triggered by activation of β-adrenergic receptors[J]. J Physiol, 2016, 594: 863–881. DOI:10.1113/tjp.2016.594.issue-4 |
| [17] | Murchison CF, Schutsky K, Jin SH, et al. Norepinephrine and β1-adrenergic signaling facilitate activation of hippocampal CA1 pyramidal neurons during contextual memory retrieval[J]. Neurosci, 2011, 181: 109–116. DOI:10.1016/j.neuroscience.2011.02.049 |
| [18] | McReynolds JR, Donowho K, Abdi A, et al. Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions[J]. Neurobiol Learn Mem, 2010, 93: 312–321. DOI:10.1016/j.nlm.2009.11.005 |
| [19] | Carriba P, Pardo L, Parra-Damas A, et al. ATP and noradrenaline activate CREB in astrocytes via noncanonical Ca2+ and cyclic AMP independent pathways[J]. Glia, 2012, 60: 1330–1344. DOI:10.1002/glia.v60.9 |
| [20] | Wang M, Gamo NJ, Yang Y, et al. Neuronal basis of age-related working memory decline[J]. Nature, 2011, 476: 210–223. DOI:10.1038/nature10243 |
| [21] | Tully K, Bolshakov VY. Emotional enhancement of memory:how norepinephrine enables synaptic plasticity[J]. Mol Brain, 2010, 3: 15–23. DOI:10.1186/1756-6606-3-15 |
| [22] | Luo Y, Zhou J, Li MX, et al. Reversal of aging-related emotional memory deficits by norepinephrine via regulating the stability of surface AMPA receptors[J]. Aging Cell, 2015, 14: 170–179. DOI:10.1111/acel.2015.14.issue-2 |
| [23] | Yang W, Yu J, Zhao L, et al. Polysaccharides from Flammulina velutipes improve scopolamine-induced impairment of learning and memory of rats[J]. J Funct Foods, 2015, 18: 411–422. DOI:10.1016/j.jff.2015.08.003 |
| [24] | Mei Y, Jiang C, Wan Y, et al. Aging-associated formalde-hyde-induced norepinephrine deficiency contributes to age-related memory decline[J]. Aging Cell, 2015, 14: 659–668. DOI:10.1111/acel.2015.14.issue-4 |
| [25] | Chalermpalanupap T, Kinkead B, Hu WT, et al. Targeting norepinephrine in mild cognitive impairment and Alzheimer's disease[J]. Alzheimers Res Ther, 2013, 5: 21–29. DOI:10.1186/alzrt175 |
| [26] | Kong Y, Ruan L, Qian L, et al. Norepinephrine promotes microglia to uptake and degrade amyloid β peptide through upregulation of mouse formyl peptide receptor 2 and induction of insulin-degrading enzyme[J]. J Neurosci, 2010, 30: 11848–11857. DOI:10.1523/JNEUROSCI.2985-10.2010 |
| [27] | Liu X, Ye K, Weinshenker D. Norepinephrine protects against amyloid-β toxicity via TrkB[J]. J Alzheimers Dis, 2015, 44: 251–260. |
| [28] | de Lima MN, Laranja DC, Caldana F, et al. Reversal of age-related deficits in object recognition memory in rats with L-deprenyl[J]. Exp Gerontol, 2005, 40: 506–511. DOI:10.1016/j.exger.2005.03.004 |
| [29] | Mustapic M, Presecki P, Pivac N, et al. Geno-type-independent decrease in plasma dopamine β-hydroxylase activity in Alzheimer's disease[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2013, 44: 94–99. DOI:10.1016/j.pnpbp.2013.02.002 |
| [30] | Apitz T, Bunzeck N. Dopamine controls the neural dynamics of memory signals and retrieval accuracy[J]. Neuropsychopharmacology, 2013, 38: 2409–2417. DOI:10.1038/npp.2013.141 |
| [31] | Backman L, Nyberg L, Soveri A, et al. Effects of working-memory training on striatal dopamine release[J]. Science, 2011, 333: 718–719. DOI:10.1126/science.1204978 |
| [32] | Anaya-Martinez V, Gutierrez-Valdez AL, Ordoñez-Librado JL, et al. The presence of perforated synapses in the striatum after dopamine depletion, is this a sign of maladaptive brain plasticity?[J]. Microscopy (Oxf), 2014, 63: 427–435. |
| [33] | Thurm F, Schuck NW, Fauser M, et al. Dopamine modulation of spatial navigation memory in Parkinson's disease[J]. Neurobiol Aging, 2016, 38: 93–103. DOI:10.1016/j.neurobiolaging.2015.10.019 |
| [34] | Pandey D, Banerjee S, Basu M, et al. Memory enhancement by tamoxifen on amyloidosis mouse model[J]. Horm Behav, 2016, 79: 70–73. DOI:10.1016/j.yhbeh.2015.09.004 |
| [35] | Higa KK, Young JW, Ji B, et al. Striatal dopamine D1 receptor suppression impairs reward-associative learning[J]. Behav Brain Res, 2017, 323: 100–110. DOI:10.1016/j.bbr.2017.01.041 |
| [36] | Bäckman L, Nyberg L, Soveri A, et al. Effects of working-memory training on striatal dopamine release[J]. Science, 2011, 333: 718. DOI:10.1126/science.1204978 |
| [37] | Srivastava P, Dhuriya YK, Gupta R, et al. Protective effect of curcumin by modulating BDNF/DARPP32/CREB in arsenic-induced alterations in dopaminergic signaling in rat corpus striatum[J]. Mol Neurobiol, 2016. DOI:10.1007/s12035-016-0288-2 |
| [38] | Landau SM, Lal R, O'Neil JP, et al. Striatal dopamine and working memory[J]. Cereb Cortex, 2009, 19: 445–454. DOI:10.1093/cercor/bhn095 |
| [39] | Thirugnanasambandam N, Grundey J, Paulus W, et al. Dose-dependent nonlinear effect of L-DOPA on paired associative stimulation-induced neuroplasticity in humans[J]. J Neurosci, 2011, 31: 5294–5299. DOI:10.1523/JNEUROSCI.6258-10.2011 |
| [40] | Li B, Arime Y, Hall FS, et al. Impaired spatial working memory and decreased frontal cortex BDNF protein level in dopamine transporter knockout mice[J]. Eur J Pharmacol, 2010, 628: 104–107. DOI:10.1016/j.ejphar.2009.11.036 |
| [41] | Floresbarrera E, Thomases DR, Heng LJ, et al. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic PKA and D1 dopamine receptor signaling[J]. Biol Psychiatry, 2014, 75: 508–516. DOI:10.1016/j.biopsych.2013.07.033 |
| [42] | Jay TM. Dopamine:a potential substrate for synaptic plasticity and memory mechanisms[J]. Prog Neurobiol, 2003, 69: 375–390. DOI:10.1016/S0301-0082(03)00085-6 |
| [43] | Masek P, Worden K, Aso Y, et al. A dopamine-modulated neural circuit regulating aversive taste memory in drosophila[J]. Curr Biol, 2015, 25: 1535–1541. DOI:10.1016/j.cub.2015.04.027 |
| [44] | Berry JA, Cervantes-Sandoval I, Nicholas EP, et al. Dopa-mine is required for learning and forgetting in drosophila[J]. Neuron, 2012, 74: 530–542. DOI:10.1016/j.neuron.2012.04.007 |
| [45] | Backman L, Lindenberger U, Li SC, et al. Linking cognitive aging to alterations in dopamine neurotransmitter functioning:recent data and future avenues[J]. Neurosci Biobehav Rev, 2010, 34: 670–677. DOI:10.1016/j.neubiorev.2009.12.008 |
| [46] | Papenberg G, Bäckman L, Nagel IE, et al. COMT polymorphism and memory dedifferentiation in old age[J]. Psychol Aging, 2014, 29: 374–383. DOI:10.1037/a0033225 |
| [47] | Braskie MN, Landau SM, Wilcox CE, et al. Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults[J]. Hum Brain Mapp, 2011, 32: 947–961. DOI:10.1002/hbm.v32.6 |
| [48] | Guitart-Masip M, Salami A, Garrett D, et al. BOLD variability is related to dopaminergic neurotransmission and cognitive aging[J]. Cereb Cortex, 2016, 26: 2074–2083. DOI:10.1093/cercor/bhv029 |
| [49] | Koch G, Di Lorenzo F, Bonnì S, et al. Dopaminergic modulation of cortical plasticity in Alzheimer's disease patients[J]. Neuropsychopharmacology, 2014, 39: 2654–2661. DOI:10.1038/npp.2014.119 |
| [50] | Chowdhury R, Guitart-Masip M, Bunzeck N, et al. Dopa-mine modulates episodic memory persistence in old age[J]. J Neurosci, 2012, 32: 14193–14204. DOI:10.1523/JNEUROSCI.1278-12.2012 |
| [51] | Moreno-Castilla P, Rodriguez-Duran LF, Guzman-Ramos K, et al. Dopaminergic neurotransmission dysfunction induced by amyloid-β transforms cortical long-term potentiation into long-term depression and produces memory impairment[J]. Neurobiol Aging, 2016, 41: 187–199. DOI:10.1016/j.neurobiolaging.2016.02.021 |
| [52] | Dickerson JW, Hemmerle AM, Numan S, et al. Decreased expression of ErbB4 and tyrosine hydroxylase mRNA and protein in the ventral midbrain of aged rats[J]. Neuroscience, 2011, 163: 482–489. |
| [53] | Greenwood PM, Lin MK, Sundararajan R, et al. Healthy aging increases the cognitive effects of two genes that influ-ence extracellular dopamine[J]. Psychol Aging, 2014, 29: 363–373. DOI:10.1037/a0036109 |
| [54] | Lukiw WJ, Rogaev EI. Genetics of aggression in Alz-heimer's disease (AD)[J]. Front Aging Neurosci, 2017, 9: 87–96. |
| [55] | Fernandez SP, Muzerelle A, Scotto-Lomassese S, et al. Constitutive and acquired serotonin deficiency alters memory and hippocampal synaptic plasticity[J]. Neuropsychopharmacology, 2017, 42: 512–523. DOI:10.1038/npp.2016.134 |
| [56] | Du JK, Jensen JB, Sanchez C, et al. Vortioxetine dose-dependently reverses 5-HT depletion-induced deficits in spatial working and object recognition memory:a potential role for 5-HT1A receptor agonism and 5-HT3 receptor antagonism[J]. Eur Neuropsychopharmacol, 2014, 24: 160–171. DOI:10.1016/j.euroneuro.2013.07.001 |
| [57] | Jenkins TA, Elliott JJ, Ardis TC, et al. Tryptophan depletion impairs object-recognition memory in the rat:reversal by risperidone[J]. Behav Brain Res, 2010, 208: 479–483. DOI:10.1016/j.bbr.2009.12.030 |
| [58] | Kuo HI, Paulus W, Batsikadze G, et al. Chronic enhancement of serotonin facilitates excitatory transcranial direct current stimulation-induced neuroplasticity[J]. Neuropsychopharmacology, 2015, 41: 1223–1230. |
| [59] | Castellano S, Ventimiglia A, Salomone S, et al. Selective serotonin reuptake inhibitors and serotonin and noradrenaline reuptake inhibitors improve cognitive function in partial responders depressed patients:results from a prospective observational cohort study[J]. CNS Neurol Disord Drug Targets, 2016, 15: 1290–1298. DOI:10.2174/1871527315666161003170312 |
| [60] | Wu YC, Hill RA, Klug M, et al. Sex-specific and region-specific changes in BDNF-TrkB signalling in the hippo-campus of 5-HT1A receptor and BDNF single and double mutant mice[J]. Brain Res, 2012, 1452: 10–17. DOI:10.1016/j.brainres.2012.03.011 |
| [61] | Park SW, Jang HJ, Cho KH, et al. Developmental switch of the serotonergic role in the induction of synaptic long-term potentiation in the rat visual cortex[J]. Korean J Physiol Pharmacol, 2012, 16: 65–70. DOI:10.4196/kjpp.2012.16.1.65 |
| [62] | Pascualbrazo J, Castro E, Díaz A, et al. Modulation of neuroplasticity pathways and antidepressant-like behavioural responses following the short-term (3 and 7 days) administration of the 5-HT4 receptor agonist RS67333[J]. Int J Neuropsychopharmacol, 2012, 15: 631–643. DOI:10.1017/S1461145711000782 |
| [63] | Eriksson TM, Delagrange P, Spedding M, et al. Emotional memory impairments in a genetic rat model of depression:involvement of 5-HT/MEK/Arc signaling in restoration[J]. Mol Psychiatry, 2012, 17: 173–184. DOI:10.1038/mp.2010.131 |
| [64] | Costa L, Trovato C, Musumeci SA, et al. 5-HT1A and 5-HT7 receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission[J]. Hippocampus, 2012, 22: 790–801. DOI:10.1002/hipo.v22.4 |
| [65] | Dayer AG, Jacobshagen M, Chaumont-Dubel S, et al. 5-HT6 receptor:a new player controlling the development of neural circuits[J]. ACS Chem Neurosci, 2015, 6: 951–960. DOI:10.1021/cn500326z |
| [66] | Ha CM, Park D, Kim Y, et al. SNX14 is a bifunctional negative regulator for neuronal 5-HT6 receptor signaling[J]. J Cell Sci, 2015, 128: 1848–1861. DOI:10.1242/jcs.169581 |
| [67] | Baba S, Murai T, Nakako T, et al. The serotonin 5-HT1A receptor agonist tandospirone improves executive function in common marmosets[J]. Behav Brain Res, 2015, 287: 120–126. DOI:10.1016/j.bbr.2015.03.025 |
| [68] | Segu L, Lecomte MJ, Wolff M, et al. Hyperfunction of muscarinic receptor maintains long-term memory in 5-HT4 receptor knock-out mice[J]. PLoS One, 2010, 5: e9529. DOI:10.1371/journal.pone.0009529 |
| [69] | Tokarski K, Kusek M, Hess G. 5-HT7 receptors modulate GABAergic transmission in rat hippocampal CA1 area[J]. J Physiol Pharmacol, 2011, 62: 535–540. |
| [70] | de Bruin NM, Prickaerts J, van Loevezijn A, et al. Two novel 5-HT6 receptor antagonists ameliorate scopolamine-induced memory deficits in the object recognition and object location tasks in Wistar rats[J]. Neurobiol Learn Mem, 2011, 96: 392–402. DOI:10.1016/j.nlm.2011.06.015 |
| [71] | Tassone A, Madeo G, Schirinzi T, et al. Activation of 5-HT6 receptors inhibits corticostriatal glutamatergic transmission[J]. Neuropharmacology, 2011, 61: 632–637. DOI:10.1016/j.neuropharm.2011.05.004 |
| [72] | Li Y, Zhao Y, Huang X, et al. Serotonin control of thermo-taxis memory behavior in nematode caenorhabditis elegans[J]. PLoS One, 2013, 8: e77779. DOI:10.1371/journal.pone.0077779 |
| [73] | Johnson O, Becnel J, Nichols CD. Serotonin receptor activity is necessary for olfactory learning and memory in drosophila melanogaster[J]. Neuroscience, 2011, 192: 372–381. DOI:10.1016/j.neuroscience.2011.06.058 |
| [74] | Lee PT, Lin HW, Chang YH, et al. Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in drosophila[J]. Proc Natl Acad Sci U S A, 2011, 108: 13794–13799. DOI:10.1073/pnas.1019483108 |
| [75] | Haider S, Saleem S, Perveen T, et al. Age-related learning and memory deficits in rats:role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system[J]. Age (Dordr), 2014, 36: 9653. DOI:10.1007/s11357-014-9653-0 |
| [76] | Oliveira L, Graeff FG, Pereira SR, et al. Correlations among central serotonergic parameters and age-related emotional and cognitive changes assessed through the elevated T-maze and the morris water maze[J]. Age (Dordr), 2010, 32: 187–196. DOI:10.1007/s11357-009-9123-2 |
| [77] | Saleem S, Tabassum S, Ahmed S, et al. Senescence related alteration in hippocampal biogenic amines produces neuro-psychological deficits in rats[J]. Pak J Pharm Sci, 2014, 27: 837–845. |
| [78] | Charlton RA, Lamar M, Zhang A, et al. Associations between pro-inflammatory cytokines, learning, and memory in late-life depression and healthy aging[J]. Int J Geriatr Psychiatry, 2017. DOI:10.1002/gps.4686 |
| [79] | Shapiro LA, Bialowasmcgoey LA, Whitakerazmitia PM. Effects of S100β on serotonergic plasticity and neuroinflammation in the hippocampus in down syndrome and Alzheimer's disease:studies in an S100β overexpressing mouse model[J]. Cardiovasc Psychiatry Neurol, 2010, 2010: 153657. |
| [80] | Musumeci G, Castrogiovanni P, Castorina S, et al. Changes in serotonin (5-HT) and brain-derived neurotrophic factor (BDFN) expression in frontal cortex and hippocampus of aged rat treated with high tryptophan diet[J]. Brain Res Bull, 2015, 119: 12–18. DOI:10.1016/j.brainresbull.2015.09.010 |
| [81] | Noristani HN, Verkhratsky A, Rodríguez JJ. High tryp-tophan diet reduces CA1 intraneuronal β-amyloid in the triple transgenic mouse model of Alzheimer's disease[J]. Aging Cell, 2012, 11: 810–822. DOI:10.1111/j.1474-9726.2012.00845.x |
| [82] | Butzlaff M, Ponimaskin E. The role of serotonin receptors in Alzheimer's disease[J]. Opera Med Physiol, 2016, 2: 77–86. |
| [83] | Rodriguez JJ, Noristani HN, Verkhratsky A. The sero-tonergic system in ageing and Alzheimer's disease[J]. Prog Neurobiol, 2012, 99: 15–41. DOI:10.1016/j.pneurobio.2012.06.010 |
| [84] | Duan DD, Gao L, Wang KX, et al. Baicalein prolongs the lifespan of Drosophila melanogaster through antioxidation activity[J]. Acta Pharm Sin (药学学报), 2016, 51: 1401–1406. |
| [85] | Duan DD, Wang KX, Zhou YZ, et al. Baicalein exerts beneficial effects in D-galactose induced aging rats through attenuation of inflammation and metabolic dysfunction[J]. Rejuvenation Res, 2017. DOI:10.1089/rej.2017.1919 |
 2017, Vol. 52
2017, Vol. 52


