2. 北京大学药学院化学生物学系, 北京 100191
2. Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China
一般认为, 药物在生物体内能够发挥各种药理作用, 本质在于药物经给药部位进入生物体血液循环(肠道直接作用与外用药除外), 一定浓度的药物分子与靶点分子产生特异性或非特异性结合进而引起相应的生物效应。对大多数药物而言, 药理作用的强弱和持续时间的长短, 与药物分子在靶点部位的浓度呈正比。然而目前直接测定药物分子在靶点部位的浓度较为困难。药物分子进入人体后, 可经血液运送至作用部位(靶点部位)。血液中的游离药物通过扩散进入细胞外液, 到达细胞膜或进而扩散至细胞内与靶点结合。血液中的药物浓度与细胞外液及细胞内的药物浓度间可形成可逆平衡, 故血液中的药物浓度可间接地反映药物在靶点部位的浓度[1]。因此, 血药浓度在一定程度上决定了药物效应的发挥。
虽然目前普遍认为中药含有多种化学成分, 各成分含量通常较低, 而其入血成分(包括中药化学成分原形及其代谢产物)的血药浓度更低。但是究竟中药体内成分血药浓度有多低?在这样的浓度下, 单一成分发挥药效的可能性有多大?还没有充分的依据和认识。西药成分单一, 血药浓度与效应间关系明确; 而作为复杂体系的中药, 其入血成分浓度与药效间的关系较难阐明。为此, 本文拟通过检索和分析临床常用中西药血药浓度的相关文献, 将常规剂量下中药体内化合物最大血药浓度和西药最小有效血药浓度进行比较, 为深入探讨中药药效物质及其作用机制提供思路和参考。本文首先采用中国学术期刊全文数据库、Pubmed和Scifinder对相关文献进行检索。中文检索词分别为:“血药浓度”, 二次检索词为“西药”、“中药”, 通过主题词、关键词、摘要和全文等多个字段进行检索查找。根据西药血药浓度的中文文献检索结果, 对相应西药继续进行英文文献追踪。中药血药浓度文献检索的英文检索词分别为:“pharmacokinetics”, 二次检索词为“tratidional Chinese medicine”, 通过“research topic”进行检索查找, 并将文献类型限制为“Journal”。为防止检索结果与已检索出的中文文献重复将语言限制为“English”。同时, 本文所列西药均为收载于《临床常用药物手册》[2]中的临床常用西药, 且文献中要有明确的有效血药浓度下限。中药最大血药浓度的文献需标明确切的给药量, 且在临床常用剂量下开展的研究, 需以中药单味药、提取物(以提取物入药的中药, 文中提及折合生药量, 并根据此折合剂量判断是否为临床常用剂量)或复方入药, 排除以单体成分直接给药情况。对上述检索结果, 筛选出相关文献。最后, 记录西药药品名称、最小有效血药浓度、中药名称、样品类型、给药方式、剂量、化合物、化合物类型、最大血药浓度和文献来源。
1 临床常用中西药血药浓度的文献资料来源临床常用西药最小有效血药浓度数据来源: ① 临床药代动力学研究文献报道。如Koup等[3]建立了氨茶碱治疗呼吸系统疾病危重患者的指导原则及其临床药代动力学研究方法。静脉给予72例患者氨茶碱(负荷剂量5.6 mg·kg-1, 维持剂量0.9、0.68、0.45 mg·kg-1·h-1), 采用高效液相色谱法测定血清茶碱含量。结果发现, 72%研究对象(52例)在血药浓度为8~20 mg·L-1时起效, 只有2例静脉滴注氨茶碱后, 在有效血药浓度5~25 mg·L-1范围之外起效。② 血药浓度监测文献报道。血药浓度监测是以药代动力学原理为指导, 分析测定药物在血液中的浓度, 用以评价疗效或确定给药方案, 使给药方案个体化, 以提高药物治疗水平, 达到临床安全、有效和合理用药的目的, 通常用于治疗窗窄、毒性强、服药周期长和服药后个体差异大的药物。在此类文献中通常会标明所研究西药的最低有效血药浓度。如Xiao等[4]用放射免疫分析法对229例患者体内地高辛血药浓度进行了监测。结果发现, 治疗血药浓度范围(0.5~2 ng·mL-1)内有152例(占66.2%)。由此可知, 地高辛吸收个体差异大, 治疗指数低, 应及时监测血药浓度。③ 临床用药相关专著。如《现代临床药物学》[5]、《Pediatric Dosage Handbook》[6]。西药有效血药浓度是通过对大量临床资料进行统计而确定, 而中药作为复杂体系, 药效物质基础较难明确, 目前尚未见开展此方面研究的报道。中药血药浓度信息多通过对人血浆或动物血浆中目标成分的药代动力学研究得到最大血药浓度。虽然动物血浆中的中药成分的最大血药浓度值不能等同于人体内血药浓度情况, 然而有文献认为基础代谢率、热卡、肝肾功能、血药浓度、药-时曲线下面积、肌酐、血液循环等与体表面积基本成正比[7]。如Zhou等[8]采用HPLC法研究了大鼠灌胃给予11.5 mg·kg-1 (相当于人口服给药200 mg)氨茶碱后血浆药代动力学, 结果发现氨茶碱Cmax为74.349 ± 7.599 μg·mL-1, AUC0-∞为457.664 ± 61.173 μg·mL-1·h。Li[9]对20名志愿者口服200 mg氨茶碱缓释片后血浆药代动力学进行了研究, 结果发现氨茶碱Cmax为2.862 ± 0.374 μg·mL-1, AUC0-∞为43.323 ± 7.362 μg·mL-1·h。比较上述实验结果, 相同剂量下氨茶碱在大鼠血浆中最大血药浓度大于人血浆。与血药浓度与体表面积成正比的理论一致。故在此理论下, 动物用药剂量与人用药剂量进行折合换算, 换算后剂量下的动物血药浓度在一定程度上也可以反映人体内的情况。
因此, 本文除了总结中药给药后化合物在人体内的血药浓度外, 也加入了动物体内血药浓度。如Liu等[10]开展了生脉注射液中ginsenoside-Rg1的药代动力学研究。采用LC-ESI-MS/MS法对10名健康志愿者(男女比例1:1, 年龄20~30岁, 身体质量指数为19~25 kg·m-2)静脉注射60 mL (常规剂量)生脉注射液后0、5、15和30 min, 1、2、3、4、6、8、12和24 h血浆中的ginsenoside-Rg1含量进行测定。采用WinNonlin® software对数据进行分析, 得到了相应药动学参数(AUC、Cmax、Tmax等)。此外, Liu等[11]研究了痛经模型大鼠灌胃香附四物汤后4种主要活性成分小檗碱、原阿片碱、四氢黄连碱和延胡索乙素的药代动力学。采用LC-MS/MS测定了痛经模型大鼠(220~250 g)给予香附四物汤3.78 g·kg-1 (相当于人0.6 g·kg-1, 常规剂量)后0、5、15、30、60、120、240、360、480和1 920 min时血浆中指标成分的含量, 经WinNonlin® software分析, 得到了相应药动学参数(AUC、Cmax和Tmax等)。
2 中西药血药浓度概况查阅文献总结得到了73种西药在人体内的最小有效血药浓度及40种中药(单味或复方)给药后体内211种化合物的最大血药浓度, 其中以人血浆为研究对象的体内化合物共42种(包括原形成分25种, 代谢产物18种); 以大鼠血浆为研究对象的体内化合物共169种。中药研究部分涉及的人或动物用药剂量均与临床常用剂量相符, 未见明显超出临床常用剂量的情况。具体数据见表 1、2。
| Table 1 The minimum effective blood concentrations of 73 Western medicine chemicals. Document types: (1) Pharmacokinetic studies; (2) Therapeutic drug monitoring studies; (3) Clinical monograph |
| Table 2 The maximum blood concentration of in vivo constituents of traditional Chinese medicine in human/animal plasma. iv: Intravenous injection; ip: Peritoneal injection; ig: Intragastric administration; po: Oral administration |
73种西药最小有效血药浓度和211种中药体内化合物(代谢产物)最大血药浓度的分布情况见表 3。其中西药人体最小有效血药浓度值最低的为氟奋乃静(0.13 ng·mL-1), 最小有效血药浓度值最高的为水杨酸类药物(100 000 ng·mL-1); 中药体内化合物在人血浆中, 最大血药浓度值最小的为直肠给予广痛消气雾剂后人血浆中盐酸小檗碱(0.31 ng·mL-1), 最大的为静脉给药黄芪总皂苷注射液后人血浆中的astragaloside Ⅳ (5 190 ng·mL-1); 中药体内化合物在动物血浆中, 最大血药浓度值最小的为灌胃茵陈术附汤后大鼠血浆中的ononin (0.34 ng·mL-1), 最大的为灌胃生脉散后大鼠血浆中的5, 8-dihydroxy-1, 4-naphthoquinone (9 965.75 ng·mL-1)。
| Table 3 The distribution of blood concentrations of Western medicines chemicals and Chinese medicines |
就整体而言, 血药浓度小于100 ng·mL-1的中药化学成分(代谢产物)共143种, 占总统计所得成分数量的68%, 西药共17种, 占总统计所得的23%;血药浓度处于100~1 000 ng·mL-1区间内的中药化学成分(代谢产物)共48种, 占总统计所得成分数量的23%, 西药共25种, 占总统计所得的34%;血药浓度处于1 000~100 000 ng·mL-1区间内的中药化学成分(代谢产物)共20种, 占总统计所得成分数量的9%, 西药共31种, 占总统计所得的42%。见表 3和图 1~3。

|
Figure 1 The distribution of 31 Western medicines and 20 Chinese medicines blood concentration in the interval of 1 000-10 000 ng·mL-1 |

|
Figure 2 The distribution of blood concentrations of 25 Western medicines and 48 in vivo constituents of Chinese medicines (including 2 metabolites) in the interval of 100-1 000 ng·mL-1 |
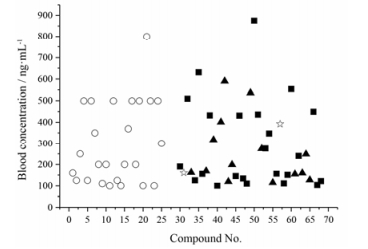
|
Figure 3 The distribution of blood concentrations of 17 Western medicines and 143 in vivo constituents of Chinese medicines (including 16 metabolites) in the interval of 0-100 ng·mL-1 |
由此可知, 中西药血药浓度分布总体趋势:随着血药浓度由低到高, 西药数量呈由少到多的变化, 中药体内化合物(原形/代谢产物)数量则由多到少。即西药最小血药浓度多分布在较高的浓度区间(1 000~100 000 ng·mL-1); 中药体内化合物(原形/代谢产物)最大血药浓度较集中分布在低浓度区间(<100 ng·mL-1); 高浓度区间中药体内化合物分布尤为少, 且所调查的中药化学成分最大血药浓度值未见超出西药最小有效血药浓度值范围, 最大血药浓度最高值是西药最小有效血药浓度最高值的1/10。本文所列的中药体内化合物最大血药浓度, 均是在人或动物给药剂量与临床常用剂量相符的情况下测定得到的。根据本文总结的数据可知, 中药体内化学成分的最大血药浓度比西药最小有效血药浓度低, 且二者之间差距较大, 推测中药起效时体内各个化合物(代谢产物)实际血药浓度可能并未达到单独起作用所需的血药浓度。
4 展望与思考本文通过比较总结临床常用西药血药浓度(最小有效血药浓度)及中药体内化合物血药浓度(最大血药浓度), 发现中药绝大多数体内化合物的最大血药浓度远远低于西药最小有效血药浓度。
根据本文分析的结果, 关于“中药药效物质如何产生药效作用”思考如下:目前中药多成分多靶点的协同作用的理论是对中药作用机制的一个公认的重要解释[69, 70], 但是由本文分析的结果可见, 中药体内(大多数)单一化学成分的浓度远低于西药最小有效血药浓度, 这能激动相关靶点而引起相应药理效应吗?“多成分的协同作用”也许提示不需要达到一定的血药浓度, 亦或许所需要的浓度可明显低于西药的最低有效浓度?然而本文总结发现, 有90%以上的中药体内成分的最大血药浓度低于1 000 ng·mL-1, 却有42%西药的最小有效血药浓度处于10 000~100 000 ng·mL-1, 即至少有90%中药体内成分的血药浓度低于血药浓度较高的西药的最小有效血药浓度的1/10~1/1 000。同时, 由于血液中的药物浓度可间接地反映药物在靶点部位的浓度, 因此, 在这样低浓度下单一的中药体内成分激动相应靶点引起药理效应的可能性, 值得进一步思考和研究。或许中药成分发挥药效作用还存在其他机制?作者最近报道[71], 中药土茯苓主要成分花旗松素灌胃给药后在大鼠体内共鉴定出191个代谢产物, 活性预测结果显示其中60个代谢产物有5个相同的靶点(nucleoside diphosphate kinase等); 预测有41个代谢产物或通过作用于靶点glycogen synthase kinase-3 beta发挥抗肿瘤活性, 其中有6个代谢产物已有抗肿瘤活性的文献报道。上述研究结果表明, 中药的原形成分及其代谢产物有可能作用于相同的靶点以共同发挥药理活性。“中药最大血药浓度低于西药最小有效血药浓度”提示, 这种叠加作用会不会是中药发挥药理作用的重要机制之一[72]?总之, 本文对常用中西药血药浓度情况的综述虽然不能涵盖所有情况, 但在一定程度上反映了中西药体内情况的显著差异, 这为今后如何定义中药药效物质基础的概念提供新的思考, 为研究中药药理作用机制提供新的思路。
| [1] | Huang ZM, Yang JR. Clinical Pharmacology (临床药理学)[M]. Beijing: Military Medical Science Press, 2009: 18. |
| [2] | Xu XD. Handbook of Clinical Drugs (临床常用药物手册)[M]. Beijing: People's Military Medical Press, 2011: 62. |
| [3] | Koup JR, Schentag JJ, Vance JW, et al. System for clinical pharmacokinetic monitoring of theophylline therapy[J]. Am J Hosp Pharm, 1976, 33: 949–956. |
| [4] | Xiao ZR, Li AG. Analysis of therapeutic drug monitoring results of digoxin in 229 cases[J]. Guangdong Pharm J (广东药学), 2003, 13: 25–26. |
| [5] | Tang G, Li DK. Modern Clinical Pharmacology (现代临床药理学)[M]. Beijing: Chemical Industry Press, 2003: 593. |
| [6] | Taketomo CK, Hodding JH, Kraus DM. Pediatric Dosage Handbook[M]. 10th ed. Ohio: Lexicomp, 20023: 1305. |
| [7] | Li JT. Clinical Pharmacology (临床药理学)[M]. 3rd ed. Beijing: People's Medical Publishing House, 2007: 1971. |
| [8] | Zhou MY, Chen QC, Fu YZ, et al. Study on pharmacokinetics of aminophylline in rat[J]. Chem Bioeng (化学与生物工程), 2011, 28: 72–74. |
| [9] | Li ZS. Pharmacokinetics of Aminophylline and Its Influencing Factors on Human (氨茶碱的人体药动学及其影响因素研究)[D]. Shandong:Shangdong University, 2010:6. |
| [10] | Liu Y, Xu SJ, Wu ZF, et al. Determination of ginsenosideRg1 in human plasma and its application to pharmacokinetic studies following intravenous administration of 'Shenmai' injection[J]. Phytother Res, 2009, 23: 65–71. DOI:10.1002/ptr.v23:1 |
| [11] | Liu P, Li W, Li ZH, et al. Comparisons of pharmacokinetic and tissue distribution profile of four major bioactive components after oral administration of Xiang-Fu-Si-Wu decoction effective fraction in normal and dysmenorrheal symptom rats[J]. J Ethnopharmacol, 2014, 154: 696–703. DOI:10.1016/j.jep.2014.04.044 |
| [12] | Guo HX, Zhang HS, Liu RX, et al. Determination of nifedipine plasma concentration of mothers and fetuses in pregnancy induced hypertension[J]. Chin J Clin Pharmacol (中国临床药理学杂志), 2004, 20: 420–422. |
| [13] | Tian GQ, Ma WX, Gao SN, et al. Effects of clonazepam serum concentrations on dosage, pharmacodynamics and ADR in sleep disorder patients[J]. Chin Pharm J (中国药学杂志), 2006, 41: 1671–1673. |
| [14] | Mou Y, Sun SJ. Study on the effective blood concentration of cyclosporin A after renal transplantation[J]. Chin Pharm (中国药业), 2004, 13: 65. |
| [15] | Zhang XK, Wu CY, Wu AQ, et al. The relationship between clinical response and serum levels with clomipramine[J]. J Clin Psychol Med (临床精神医学杂志), 2000, 10: 257–259. |
| [16] | Liu HM, Lin ZG, Ren JJ, et al. Preliminary exploration on reference range of serum quetiapine fumarate concentration[J]. World Clin Drugs (世界临床药物), 2012, 33: 612–615. |
| [17] | Li JH, Zhong H, Shen WM. Relationship between serum concentration and clinical efficacy of aripiprazole in the treatment of patients with schizophrenia[J]. Chin J Clin Pharmacol Ther (中国临床药理学与治疗学), 2011, 16: 76–78. |
| [18] | Zhao JP, Li HD, Peng WX, et al. The influential factors on pharmacokinetics of clozapine and relationship between clinical efficacy and clozapine plasma concentration in schizophrenia[J]. Chin Psychiatry (中华精神科杂志), 1996, 29: 131–134. |
| [19] | Chen GH, Yan SQ. A preliminary study on the relationship between plasma concentration and therapeutic effect of amiodarone[J]. Chin J Hosp Pharm (中国医院药学杂志), 1989, 9: 8–10. |
| [20] | Li D, Xiang L. Individualized drug administration and clinical monitoring of amikacin[J]. Chin J Hosp Pharm (中国医院药学杂志), 2003, 23: 441–442. |
| [21] | Shen J, Zhou YN, Liu YF, et al. Clinical study of the safety in elderly patients with lower respiratory infections and therapeutic drug monitoring of teicoplanin[J]. Chin J Clin Pharm (中国临床药学杂志), 2009, 18: 129–131. |
| [22] | Zhang J, Huang SP, Wu D. Analysis on plasma concentration monitoring of rifampicin and isoniazid in tuberculosis patients[J]. Chin Pharm (中国药房), 2010, 21: 2842–2844. |
| [23] | Song ZB, Liao WP, Yi YH, et al. The influence of lamotrigine on EEG at therapeutic serum concentration[J]. Acad J Guangzhou Med Coll (广州医学院学报), 2003, 31: 16–18. |
| [24] | Yamashita T, Matsuoka A, Funatsu A, et al. The antithrombotic effect of human activated protein C on He-Ne laser-induced thrombosis in rat mesenteric microvessels[J]. Thromb Res, 1994, 75: 33–40. DOI:10.1016/0049-3848(94)90137-6 |
| [25] | Feng WB, Xie M, Chen X, et al. A study of once-daily adult optimal dosage of carbamazepine for maintaining therapeutic serum concentration during night[J]. J Nanhuang Univ Med Ed (南华大学学报医学版), 2002, 30: 235–237. |
| [26] | Bates T, Siller G, Crathern BC, et al. Timing of prophylactic antibiotics in abdominal surgery:trial of a pre-operative versus an intra-operative first dose[J]. Br J Surg, 1989, 76: 52–56. DOI:10.1002/(ISSN)1365-2168 |
| [27] | Holdiness MR. Clinical pharmacokinetics of the antituber-culosis drugs[J]. Clin Pharmacokinet, 1984, 9: 511–544. DOI:10.2165/00003088-198409060-00003 |
| [28] | Vajda FJE, Drummer OH, Morris PM, et al. Gas chromatographic measurement of plasma levels of sodium valproate:tentative therapeutic range of a new anticonvulsant in the treatment of refractory epileptics[J]. Clin Exp Pharmacol Physiol, 1978, 5: 67–73. DOI:10.1111/cep.1978.5.issue-1 |
| [29] | Kang GF. Clinical Biochemistry and Biochemistry Test (临床生物化学和生物化学检验)[M]. 2nd ed. Beijing: People's Medical Publishing House, 1999: 225. |
| [30] | Xu MJ, Yin JG, Xie LY, et al. Pharmacokinetics and tolerance of toal astragalosides after intravenous infusion of astragalosides injection in healthy Chinese volunteers[J]. Phytomedicine, 2013, 20: 1105–1111. DOI:10.1016/j.phymed.2013.05.004 |
| [31] | Liu J, Wang JS, Kong LY, et al. Comparative pharmacokinetics of paeoniflorin in plasma of vascular dementia and normal rats orally administrated with Danggui-Shaoyao-San or pure paeoniflorin[J]. Fitoterapia, 2011, 82: 466–473. DOI:10.1016/j.fitote.2010.12.004 |
| [32] | Chen ZJ, Kong SS, Song FF, et al. Pharmacokinetic study of luteolin, apigenin, chrysoeriol and diosmetin after oral administration of Flos Chrysanthemi extract in rats[J]. Fitoterapia, 2012, 83: 1616–1622. DOI:10.1016/j.fitote.2012.09.011 |
| [33] | Lu CM, Lin LC, Tsai TH. Determination and pharmacokinetic study of gentiopicroside, geniposide, baicalin, and swertiamarin in Chinese herbal formulae after oral administration in rats by LC-MS/MS[J]. Molecules, 2014, 19: 21560–21578. DOI:10.3390/molecules191221560 |
| [34] | Duan KF, Yuan ZF, Guo W, et al. LC-MS/MS determination and pharmacokinetic study of five flavone components after solvent extraction/acid hydrolysis in rat plasma after oral administration of Verbena officinalis L[J]. J Ethnopharmacol, 2011, 135: 201–208. DOI:10.1016/j.jep.2011.01.002 |
| [35] | Qian XC, Zhang L, Tao Y, et al. Simultaneous determination of ten alkaloids of crude and wine-processed Rhizoma Coptidis aqueous extracts in rat plasma by UHPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study[J]. J Pharm Biomed Anal, 2015, 105: 64–73. DOI:10.1016/j.jpba.2014.11.049 |
| [36] | Yuan J, Wang Y, An R, et al. Simultaneous determination of six alkaloids and one monoterpene in rat plasma by liquid chromatography-tandem mass spectrometry and pharmacokinetic study after oral administration of a Chinese medicine Wuji Pill[J]. J Chromatogr B, 2012, 895-896: 154–161. DOI:10.1016/j.jchromb.2012.03.036 |
| [37] | Yin R, Chen JQ, Zhao YG, et al. Simultaneous determination of six alkaloid components in rat plasmaand its application to pharmacokinetic study of Danmu preparations by an ultra fast liquid chromatography-electrosprayionization-tandem mass spectrometry[J]. J Chromatogr B, 2015, 983-984: 10–17. DOI:10.1016/j.jchromb.2014.12.026 |
| [38] | Jung HR, Kim KJ, Ham SH, et al. Simultaneous determination of puerarin and its active metabolite in human plasma by UPLC-MS/MS:application to a pharmacokinetic study[J]. J Chromatogr B, 2014, 971: 64–67. DOI:10.1016/j.jchromb.2014.09.015 |
| [39] | Li YH, Huang Y, Wang Y, et al. Pharmacokinetic comparison of the vasorelaxant compound ferulic acid following the administration of Guanxin Ⅱ to healthy volunteers and patients with angina pectoris[J]. Exp Ther Med, 2013, 6: 1283–1289. |
| [40] | Wang YL, Ding CG, Wu CS, et al. HPLC-MS and HPLCMS/MS analysis of seven active constituents of Xiao-Xu-Ming decoction and application to a pharmacokinetic study after oral administration to rat[J]. Acta Pharm Sin B, 2012, 2: 188–197. DOI:10.1016/j.apsb.2012.01.003 |
| [41] | Liu MH, Li PL, Zeng X, et al. Identification and pharmacokinetics of multiple potential bioactive constituents after oral administration of radix astragali on cyclophosphamideinduced immunosuppression in Balb/c mice[J]. Int J Mol Sci, 2015, 16: 5047–5071. DOI:10.3390/ijms16035047 |
| [42] | Shaw L, Lin LC, Tsai T, et al. HPLC-MS/MS analysis of a traditional Chinese medical formulation of Bu-Yang-Huan-WuTang and its pharmacokinetics after oral administration to rats[J]. PLoS One, 2012, 7: e43848. DOI:10.1371/journal.pone.0043848 |
| [43] | Wang R, Zhu L, Deng ZH, et al. Pharmacokinetics study of Guangtongxiao Foam Aerosols in human plasma[J]. Chin J Exp Tradit Med Form (中国实验方剂学杂志), 2013, 19: 182–186. |
| [44] | Xie YG, Mu HJ, Li Z, et al. Supression of chronic central pain by superoxide dismutase in rats with spinal cord injury:inhibition of the NMDA receptor implicated[J]. Exp Ther Med, 2014, 8: 1137–1141. |
| [45] | Wei H, Miao HJ, Yun YL, et al. Validation of an LC-MS/MS method for quantitative analysis of the 5 bioactive components of Wuzhi capsule in human plasma samples[J]. Ther Drug Monit, 2014, 36: 781–788. DOI:10.1097/FTD.0000000000000079 |
| [46] | Liu XY, Wei CM, Zhang R, et al. Pharmacokinetic study of icariside Ⅱ after a single dose administration of YiGu capsule in healthy Chinese volunteers[J]. Drug Res, 2013, 63: 457–461. DOI:10.1055/s-00023610 |
| [47] | Wang CZ, Kim KE, Du GJ, et al. Ultra-performance liquid chromatography and time-of-flight mass spectrometry analysis of ginsenoside metabolites in human plasma[J]. Am J Chin Med, 2011, 39: 1161–1171. DOI:10.1142/S0192415X11009470 |
| [48] | Hu ZY, Yang JL, Cheng C, et al. Combinatorial metabolism notably affects human systemic exposure to ginsenosides from orally administered extract of Panax notoginseng Roots (Sanqi)[J]. Drug Metab Dispos, 2013, 41: 1457–1469. DOI:10.1124/dmd.113.051391 |
| [49] | Li GL, Tang ZS, Yang J, et al. Simultaneous determination of five components in rat plasma by UPLC-MS/MS and its application to a comparative pharmacokinetic study in Baihe Zhimu Tang and Zhimu extract[J]. Molecules, 2015, 20: 6700–6714. DOI:10.3390/molecules20046700 |
| [50] | Cai F, Xu W, Wei H, et al. Simultaneous determination of active xanthone glycosides, timosaponins and alkaloids in rat plasma after oral administration of Zi-Shen Pill extract for the pharmacokinetic study by liquid chromatography-tandem mass spectrometry[J]. J Chromatogr B, 2010, 878: 1845–1854. DOI:10.1016/j.jchromb.2010.05.024 |
| [51] | Lv CX, Li Q, Zhang YW, et al. A UFLC-MS/MS method with a switching ionization mode for simultaneous quantitation of polygalaxanthone Ⅲ, four ginsenosides and tumulosic acid in rat plasma:application to a comparative pharmacokinetic study in normal and Alzheimer's disease rats[J]. J Mass Spectrom, 2013, 48: 904–913. DOI:10.1002/jms.3230 |
| [52] | Zhang Q, Ma YM, Wang ZT, et al. Pharmacokinetics difference of multiple active constituents from decoction and maceration of Fuzi Xiexin Tang after oral administration in rat by UPLCMS/MS[J]. J Pharm Biomed Anal, 2014, 92: 35–46. DOI:10.1016/j.jpba.2013.12.038 |
| [53] | Zhu YH, Tong L, Zhou SP, et al. Simultaneous determination of active flavonoids and alkaloids of Tang-Min-Ling-Pill in rat plasma by liquid chromatography tandem mass spectrometry[J]. J Chromatogr B, 2012, 904: 51–58. DOI:10.1016/j.jchromb.2012.07.010 |
| [54] | He W, Liu GH, Cai H, et al. Integrated pharmacokinetics of five protoberberine-type alkaloids in normal and insomnic rats after single and multiple oral administration of Jiao-Tai-Wan[J]. J Ethnopharmacol, 2014, 154: 635–644. DOI:10.1016/j.jep.2014.04.040 |
| [55] | Wang Q, Jiang P, Ye FY, et al. Identification and pharmacokinetics of multiple constituents in rat plasma after oral administration of Yinchenzhufu decoction[J]. J Ethnopharmacol, 2014, 153: 714–724. DOI:10.1016/j.jep.2014.03.039 |
| [56] | Shi XQ, Tang YP, Zhu HX, et al. Pharmacokinetic comparison of seven major bio-active components in normal and blood deficiency rats after oral administration of Danggui Buxue decoction by UPLC-TQ/MS[J]. J Ethnopharmacol, 2014, 153: 169–177. DOI:10.1016/j.jep.2014.02.004 |
| [57] | Song YG, Su D, Lu TL, et al. Differential pharmacokinetics and the brain distribution of morphine and ephedrine constitutional isomers in rats after oral administration with Keke capsule using rapid-resolution LC-MS/MS[J]. J Sep Sci, 2014, 37: 352–359. DOI:10.1002/jssc.v37.4 |
| [58] | Xie WB, Qiu XJ, Huang X, et al. Comparison between the pharmacokinetics of meranzin hydrate in a rat model of chronic depression and in controls following the oral administration of Chaihu-Shugan-San[J]. Exp Ther Med, 2013, 6: 913–918. |
| [59] | Xu YP, Huang KX, Pan Y, et al. A rapid UFLC-MS/MS method for simultaneous determination of formononetin, cryptotanshinone, tanshinone ⅡA and emodin in rat plasma and its application to a pharmacokinetic study of Bu Shen Huo Xue formula[J]. J Chromatogr B, 2013, 932: 92–99. DOI:10.1016/j.jchromb.2013.06.011 |
| [60] | Zhang YJ, Qiang SP, Sun J, et al. Liquid chromatographyhydride generation-atomic fluorescence spectrometry determination of arsenic species in dog plasma and its application to a pharmacokinetic study after oral administration of Realgar and Niu Huang Jie Du Pian[J]. J Chromatogr B, 2013, 917-918: 93–99. DOI:10.1016/j.jchromb.2012.12.029 |
| [61] | Xu HR, Li Q, Yin YD, et al. Simultaneous determination of three alkaloids, four ginsenosides and limonin in the plasma of normal and headache rats after oral administration of WuZhu-Yu decoction by a novel ultra fast liquid chromatographytandem mass spectrometry method:application to a comparative pharmacokinetics and ethological study[J]. J Mass Spectrom, 2013, 48: 519–532. DOI:10.1002/jms.3183 |
| [62] | Sun Z, Zhao LS, Zuo LH, et al. A UHPLC-MS/MS method for simultaneous determination of six flavonoids, gallic acid and 5, 8-dihydroxy-1, 4-naphthoquinone in rat plasma and its application to a pharmacokinetic study of Cortex Juglandis Mandshuricae extract[J]. J Chromatogr B, 2014, 958: 55–62. DOI:10.1016/j.jchromb.2014.03.013 |
| [63] | Sun H, Wu FF, Zhang AH, et al. Pharmacokinetic study of schisandrin, schisandrol B, schisantherin A, deoxyschisandrin, and schisandrin B in rat plasma after oral administration of Shengmaisan formula by UPLC-MS[J]. J Sep Sci, 2013, 36: 485–491. DOI:10.1002/jssc.v36.3 |
| [64] | Wang BL, Hu JP, Sheng L, et al. Chemical-pharmacokineticpharmacodynamic fingerprints of Schisandra chinensis alcoholic extract[J]. Acta Pharm Sin (药学学报), 2013, 48: 734–740. |
| [65] | Qiao X, Ye M, Xiang C, et al. Analytical strategy to reveal the in vivo process of multi-component herbal medicine:a pharmacokinetic study of licorice using liquid chromatography coupled with triple quadrupole mass spectrometry[J]. J Chromatogr A, 2012, 1258: 84–93. DOI:10.1016/j.chroma.2012.08.041 |
| [66] | Cheng C, Liu XW, Du FF, et al. Sensitive assay for measurement of volatile borneol, isoborneol, and the metabolite camphor in rat pharmacokinetic study of Borneolum (Bingpian) and Borneolum syntheticum (synthetic Bingpian)[J]. Acta Pharmacol Sin, 2013, 34: 1337–1348. DOI:10.1038/aps.2013.86 |
| [67] | Yang B, Dong W, Zhang AH, et al. Ultra-performance liquid chromatography coupled with electrospray ionization/quadrupole-time-of-flight mass spectrometry for rapid analysis of constituents of Suanzaoren decoction[J]. J Sep Sci, 2011, 34: 3208–3215. DOI:10.1002/jssc.v34.22 |
| [68] | Liao CR, Chang S, Yin SL, et al. A HPLC-MS/MS method for the simultaneous quantitation of six alkaloids of Rhizoma Corydalis Decumbentis in rat plasma and its application to a pharmacokinetic study[J]. J Chromatogr B, 2014, 944: 101–106. DOI:10.1016/j.jchromb.2013.11.010 |
| [69] | Han YQ, Xu J, Gong SX, et al. Chemical constituents and mechanism of Corydalis Rhizoma based on HPLC-QTOF/MS and G protein-coupled receptor analysis[J]. Acta Pharm Sin (药学学报), 2016, 51: 1302–1308. |
| [70] | Han YQ, Xu J, Zhang XM, et al. Network pharmacologybased study on mechanism of Yuanhu Zhitong Dropping Pills in the treatment of primary dysmenorrhea[J]. Acta Pharm Sin (药学学报), 2016, 51: 380–387. |
| [71] | Yang P, Xu F, Li HF, et al. Detection of 191 taxifolin metabolites and their distribution in rats using HPLC-ESI-ITTOF-MS[J]. Molecules, 2016, 21: 1209. DOI:10.3390/molecules21091209 |
| [72] | Xu F, Yang DH, Shang MY, et al. Effective forms, additive effect, and toxicities scattering effect of pharmacodynamic substances of TCMs some reflections evoked by the study on the metabolic disposition of traditional Chinese medicines (TCM)[J]. World Sci Technol Modern Tradit Chin Med Mater Med (世界科学技术-中医药现代化), 2014, 16: 688–703. |
 2017, Vol. 52
2017, Vol. 52


