2. 首都医科大学附属北京友谊医院, 北京 100050
2. Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
大量研究证实, 缺氧/复氧(hypoxia/reoxygenation, H/R)通常造成活性氧簇(oxygen species, ROS)生成增加[1], 进而启动不可控的氧化应激损伤和随后的细胞凋亡[2]。这些改变伴随着细胞器的失活和多种疾病的发生[3-5]。随着空气污染物排放和吸烟者的大量增多, 许多肺部炎症(如慢性阻塞性肺病、哮喘和肺癌等)的发生率在发展中国家呈上升趋势[6]。因为这类疾病以反复发作和病程长为特点, 而且肺部的病理改变往往也是不可逆的, 从而缺乏有效的治疗手段。长期的缺氧/复氧和氧化应激损伤在这类疾病发病中扮演着重要的角色, 而这些疾病的发作同时又加重了组织的缺氧状态, 形成恶性循环[5]。
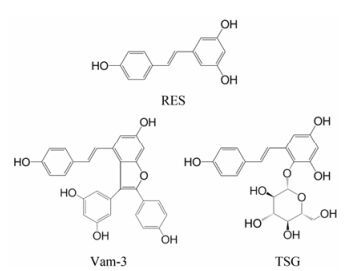
二苯乙烯苷(2, 3, 5, 4'-tetrahydroxystilbene-2-O-β-D-glycoside, TSG)是传统中药何首乌的主要活性成分, 与白藜芦醇(resveratrol, RES)相似, 属于多羟基茋类化合物(图 1)。因其2-位通过β-糖苷键与葡萄糖相结合, 故具有良好的水溶性[7]。何首乌因补益精血、乌须发、强筋骨和补肝肾的功效, 自古便广泛应用于各种药方[8]。现代研究也证实, TSG具有抗衰老[9, 10]、抗炎[11-13]、抗动脉粥样硬化[14, 15]、保肝[16]、抗氧化和自由基清除[17]等作用。这些研究指出, TSG在分子水平上可能主要通过调控PI3K/AKT、ROSiNOS、NF-κB和JNK/MAPK等通路, 及在整体动物上通过APP和SIRT等通路发挥作用。尤其近年来越来越多的文献[17]提出, TSG在抗氧化应激损伤方面或许具有独到作用。然而, 更具体和深入的机制仍有待阐明。对TSG保护作用机制的研究将有利于药物研发和临床应用。
本实验室曾报道了另一种白藜芦醇和TSG的同系物——Vam-3 (图 1)通过降低nSMase2水平和神经酰胺表达, 抑制香烟烟雾诱导的支气管上皮细胞(BEAS-2B)线粒体途径凋亡的作用[18]; 通过降低SIRT1与p53表达, 抑制硝普钠诱导的大鼠关节软骨细胞线粒体途径凋亡[19]。本研究将通过TSG的保护作用, 从另一个方面探讨线粒体途径在H/R损伤中的角色, 及MAPK、HIF-1α和p53信号通路在TSG抗氧化应激损伤中的作用。
|
Figure 1 Chemical structures of RES, Vam-3 and TSG |
材料 TSG (棕红色无定形粉末, 纯度≥98%)由中国医学科学院药物研究所姚春所实验室合成。支气管上皮细胞(BEAS-2B系)购于ATCC公司。乳腺癌细胞(MCF-7系及其GFP-Bax稳定转染的MCF-7/ GFP-Bax系)储存于本实验室。四甲基噻唑蓝(thiazolyl blue tetrazolium bromide, MTT)购于Sigma-Aldrich公司; 单克隆Bax、Bcl-2、细胞色素C、p38 MAPK、SAPK/JNK1/2、ERK1/2、p-p38 MAPK、p-SAPK/ JNK1/2、p-ERK1/2、HIF-1α、p53和p-p53 (Ser 15) 一抗购于Abcam公司; 单克隆β-actin、COX IV、GAPDH、caspase-3和caspase-9一抗购于Cell Signaling Technology公司; 线粒体分离试剂盒购于Thermo Fisher Scientific公司。丙二醛(malondialdehyde, MDA)和超氧化物歧化酶(superoxide dismutase, SOD)检测试剂盒购于南京建成生物科技研究所; DCFH-DA探针、JC-1探针购于上海碧云天生物技术有限公司; Hoechst 33258染料购于东仁化学科技(上海)有限公司; zVAD-fmk购于上海蓝木化工有限公司; FITC、HPR偶联二抗购于北京中杉金桥生物技术有限公司; ELISA试剂盒购于BioLegend公司; 其他试剂、溶剂购于本地公司。
细胞培养 BEAS-2B、MCF-7和MCF-7/GFP-Bax细胞培养于37 ℃、5% CO2中[含4 500 mg·L-1 D-葡萄糖的DMEM培养基, 10%胎牛血清(FBS)]。生长至约80%融合时1:2传代(BEAS-2B约每1.5天, MCF-7与MCF-7/GFP-Bax约每1天), 同时更换新鲜培养基。取对数生长期的BEAS-2B细胞进行实验, 96孔板和24孔板接种细胞数分别为1×104和5×104个/孔, 培养过夜。缺氧实验前1 h, 待测化合物组每孔加入不同浓度的TSG。模型组和空白对照组加入等量溶剂DMSO。
体外缺氧/复氧(H/R)损伤模型的建立 本实验参照目前采用最广泛的混合气体培养法建立体外H/R损伤模型[20], 即按95:5 (v/v)混合惰性气体N2和CO2, 然后通入一个装有实验细胞的密闭盒子, 使其内原有气体排空形成无氧环境, 同时更换培养基至低糖、低血清即为缺氧; 培养一定时间后再取出细胞, 更换为正常培养基进行正常培养即为复氧。缺氧时长为4 h (含1 000 mg·L-1 D-葡萄糖的DMEM培养基, 2.5% FBS), 复氧时长为6 h (含4 500 mg·L-1 D-葡萄糖的DMEM培养基, 10% FBS)。另设一组于37 ℃、5% CO2环境下正常伴随培养作为空白对照。
MTT检测 设置TSG终浓度为3.13~100μmol·L-1, 倍半稀释, 一共7个浓度。37 ℃、5% CO2孵育1 h, 之后进行H/R造模操作。注意在H/R处理全过程中, 所更换的培养基内也始终含有既定浓度的TSG或溶剂。完成预定的H/R处理后, 各孔加入0.5% MTT溶液40μL, 37 ℃、5% CO2孵育4 h。吸除上清, 加入DMSO 200 μL, 避光放置5 min, 室温振荡混匀, 测定OD570。细胞存活率(%)=(OD处理组-OD本底)/(OD空白对照组-OD本底)×100。
荧光探针DCFH-DA检测ROS 取BEAS-2B细胞接种于黑壁96孔板中, 培养过夜。TSG终浓度为12.5~100 μmol·L-1, 倍半稀释, 一共4个浓度。同法H/R处理后, 参照试剂盒说明书, 加入DCFH-DA荧光探针。37 ℃培养箱中避光孵育30 min, 用无血清培养基轻柔洗涤细胞3次, 荧光酶标仪检测(激发波长488 nm, 发射波长525 nm)。
试剂盒检测总SOD活性和MDA生成 细胞加药和H/R处理后, 参照试剂盒说明书操作, 收集细胞用于检测总SOD活性, 以及收集细胞上清检测MDA含量。
荧光显微镜观察MCF-7/GFP-Bax细胞核形态的改变及Bax至线粒体的转位 细胞加药和H/R处理后, PBS漂洗细胞2次, 每次3~5 min。缓慢加入预冷4%多聚甲醛, 室温固定15 min。PBS轻柔漂洗细胞2次。加入终质量浓度为10 μg·mL-1 Hoechst 33258染色液, 37 ℃避光孵育30 min。避光下, 吸除Hoechst 33258染色液, PBS漂洗细胞2次, 每次3~5 min, 加入新鲜无血清DMEM培养基, 荧光显微镜下立即观察。各实验组分别随机取3个视野, 于蓝色滤光片(激发波长358 nm, 发射波长461 nm)观察细胞核形态, 计算有不正常细胞核的细胞所占细胞总数百分比; 于蓝色滤光片(激发波长488 nm, 发射波长525 nm)动态观察并记录每个视野细胞总数和有绿色环状荧光点(GFP-Bax转位并聚集于线粒体)细胞数, 计算有环状荧光点细胞数占细胞总数百分比。
荧光探针JC-1检测线粒体膜电位(ΔφM) 细胞接种于黑壁96孔板中, TSG终浓度为12.5~100 μmol·L-1, 倍半稀释, 一共4个浓度。阴性对照组、空白对照组于37 ℃、5% CO2环境下正常伴随培养, 待测化合物组、模型组同法H/R处理。阴性对照组加入终浓度10 μmol·L-1解耦联试剂羰基氰化间氯苯腙(carbonyl cyanide m-chlorophenyl hydrazone, CCCP)处理细胞20 min后, 同其余各组一道弃去培养基, 按照说明书避光加入含JC-1探针(终质量浓度10 μg·mL-1)的新鲜培养基, 于37 ℃培养箱中避光孵育30 min后吸除上清液。PBS洗涤2次, 加入新鲜培养基, 荧光酶标仪检测, 同时使用倒置荧光显微镜观察(激发波长488 nm, 发射波长525 nm观察绿色单体形式; 激发波长540 nm, 发射波长590 nm观察红色聚合物形式)。
免疫荧光检测细胞色素C释放 取对数生长期的BEAS-2B细胞, 以细胞数5×105个/孔接种于多聚赖氨酸预包被的激光共聚焦专用玻底培养皿中, 培养过夜。同法H/R处理细胞后, PBS轻柔漂洗2次。后续操作均避光下进行。加入含有MitoTracker Red CM-XRos (终浓度为100 nmol·L-1)染料的新鲜无血清DMEM培养基, 37 ℃孵育30 min。吸出上清, PBS漂洗2次。用预冷4%多聚甲醛室温固定细胞20 min。PBS轻柔漂洗2次, 0.5% Triton X-100的PBS溶液室温通透细胞15 min, PBS漂洗2次。山羊血清室温封闭2 h。再用PBS漂洗2次, 加入抗细胞色素C单克隆一抗(1:200稀释), 置于免疫荧光湿盒内4 ℃孵育过夜。PBS漂洗3次, 加入FITC偶联的二抗, 室温孵育1 h。PBS漂洗3次, 加入含有DAPI (终质量浓度为10 μg·mL-1)染料的新鲜无血清DMEM培养基, 37 ℃孵育15 min。吸出上清, PBS轻柔漂洗2次。激光共聚焦显微镜立即观察, 记录结果。
细胞总蛋白及线粒体、胞浆蛋白的提取 取已进行相应处理的细胞, 弃去培养基, 预冷的PBS漂洗2遍。胰酶消化细胞, 离心弃去上清, 收集细胞沉淀。在细胞沉淀中加入含蛋白酶抑制剂和蛋白磷酸酶抑制剂的蛋白裂解液, 冰上作用40 min, 每10 min剧烈震荡一次。4 ℃、12 000 ×g离心20 min, 上清即为总蛋白成分。添加蛋白酶抑制剂和蛋白磷酸酶抑制剂至试剂A和试剂C, 按照线粒体分离试剂盒说明书操作, 之后差速离心提取细胞线粒体、胞浆蛋白成分。
Western blotting 10% SDS聚丙烯酰胺凝胶电泳分离蛋白, 然后转移到PVDF膜上。转移上蛋白的PVDF膜用TBST配制的5%脱脂奶粉室温封闭2 h TBST洗3遍。4 ℃下与TBST稀释的一抗孵育过夜。用TBST洗3遍, 然后在TBST稀释的辣根过氧化酶偶联的二抗中孵育2 h, 用TBST洗3遍, 在LAS-4000化学发光系统(Fujifilm, 日本)检测, ImageJ 1.8.0分析条带灰度。
统计学方法 每个实验平行3次, 每次设3个复孔。实验结果均以均值±标准差(x± s)表示, 用SPSS 17.0软件进行数据统计。组间差异采用one-way ANOVA进行统计学处理, P ≤0.05时认为有显著性差异。
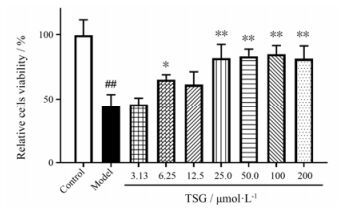
结果 1 TSG增加H/R处理后的BEAS-2B细胞的存活率经过H/R处理后, BEAS-2B细胞相对存活率显著下降(P<0.01)。而最低在6.25 μmol·L-1时便可观察到TSG的预处理显著提高BEAS-2B细胞相对存活率(P<0.05) (图 2)。
|
Figure 2 TSG significantly improved cells viability. The relative cells viability was determined by MTT assay. Mean intensity of control group was normalized as 100%. Results are presented as x± s from at least 3 independent experiments. ##P < 0.01 vs control; *P < 0.05, **P < 0.01 vs model |
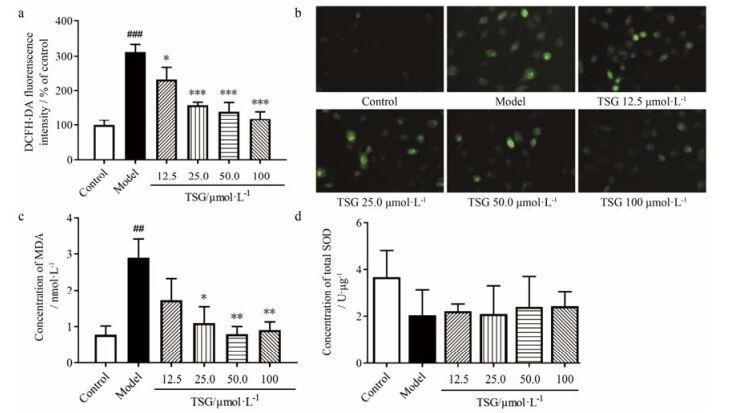
H/R处理显著增加BEAS-2B细胞ROS的产生。在给予TSG预处理后, BEAS-2B细胞的ROS产生被显著抑制(图 3a, b)。MDA是细胞脂质过氧化的生物标志物。ROS积累造成氧化失衡的一个重要体现就是细胞MDA生成的升高。在本实验中, H/R处理显著增加BEAS-2B细胞MDA浓度, 而TSG的预处理能显著抑制MDA浓度的升高(图 3c)。至于SOD, 其在抗氧化损伤中扮演着重要的角色, 然而在本实验中并未观察到空白对照组、H/R处理组和TSG处理组之间的显著性差异(图 3d)。
|
Figure 3 TSG inhibited the production of ROS and MDA in BEAS-2B cells. (a) The DCFH-DA fluorescence was read in a microplate reader. Mean intensity of control group was normalized as 100%. (b) Representative images of the cells stained with DCFH-DA probe were examined by using fluorescence microscope to detect the intensity of ROS fluorescence. (c) The concentration of MDA was assayed with a reagent kit. (d) Total SOD was extracted and the content was assayed with a reagent kit. Results are presented as x± s from at least 3 independent experiments. ##P < 0.01, ###P < 0.001 vs control; *P < 0.05, **P < 0.01, ***P < 0.001 vs model |
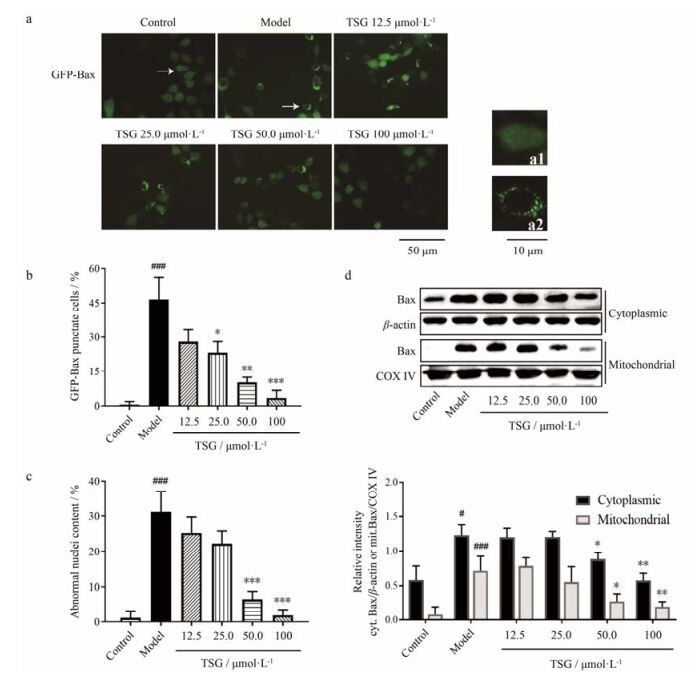
在正常培养的GFP-Bax-stable MCF-7细胞中, 与Bax蛋白偶联的绿色荧光弥散地分布在整个细胞中(图 4a1), 表明Bax均匀分布于胞浆。Hoechst 33258染料标记的细胞核呈正常、均一的形态。H/R处理10 h之后, 绿色荧光蛋白聚集成斑点, 形成环绕于细胞核的小环(图 4a2), 表明Bax蛋白在胞浆转位、积累于同一位置。H/R处理的细胞核在倒置荧光显微镜下可见染色质固缩、核边缘皱缩及碎片化等形态改变。在TSG预处理之后, 具有GFP-Bax荧光斑点和异常细胞核的细胞比例显著下降(图 4b, c), 表明TSG抑制了H/R处理的GFP-Bax-stable MCF-7细胞Bax从胞浆的转位以及细胞核损伤。
|
Figure 4 TSG inhibited the translocation of Bax and nuclear deformation. GFP-Bax-stable MCF-7 cells were pretreated with TSG for 2 h before challenged with 10-h H/R process. (a) The cells stained with Hoechst 33258 nuclear stain were examined by using fluorescence microscope to detect the translocation of Bax (green channel). Cells with typical characteristics were marked with arrows and enlarged in images a1, a2. (b) GFP-Bax punctate cells and total green fluorescent cells were counted, then the content of punctate cells was calculated. (c) The abnormal nuclei and total fluorescent nuclei (with condensate chromatin, crenation and fractionation) were counted, then the content of abnormal nuclei was calculated. (d) Expressions of Bax separately in mitochondria and cytoplasm were determined by Western blotting. Representative blot (up) and quantified protein levels (down) are shown. Results are presented as x± s from at least 3 independent experiments. #P < 0.05, ###P < 0.001 vs control; *P < 0.05, **P < 0.01, ***P < 0.001 vs model |
为了验证Bax是否转位至了线粒体, 用同法处理BEAS-2B细胞, 收集细胞并且分离胞浆和线粒体组分。在模型组, BEAS-2B细胞胞浆Bax和线粒体Bax蛋白表达相较空白组均显著升高, 且线粒体Bax表达升高的比例更为显著(P<0.001), 表明大量的Bax生成且从胞浆转位至了线粒体。在TSG预处理组, 胞浆Bax和线粒体Bax蛋白表达相较模型组均显著下降(图 4d)。
此外, 分析Bax/Bcl-2比率来观察Bax和Bcl-2的表达情况。H/R处理10 h后, 总Bax蛋白表达相较空白组显著升高(图 5)。在TSG预处理之后, 总Bax蛋白表达显著下降。然而, 在空白组与H/R处理组、H/R处理组与TSG预处理组间没有观察到Bcl-2蛋白表达的显著性差异。
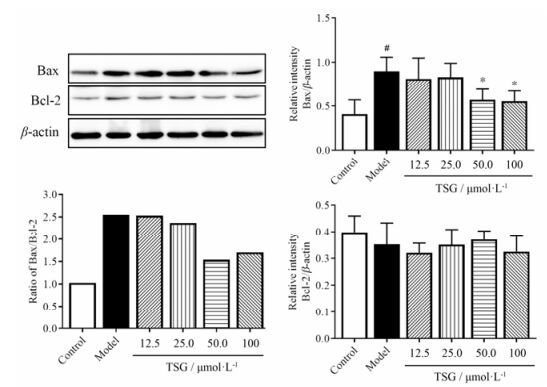
|
Figure 5 TSG inhibited the expression of total Bax in BEAS-2B cells and decreased the Bax/Bcl-2 ratio. Relative expressions of Bax and Bcl-2 were determined by Western blotting. Representative blot (upper left), quantified protein levels (right) and calculated Bax/Bcl-2 ratio (lower left) are shown. Protein levels results are presented as x± s from at least 3 independent experiments. The ratio was calculated as (mean Bax)/(mean Bcl-2). #P < 0.05 vs control; *P < 0.05 vs model |
同时检测了线粒体去极化水平, 观察TSG对H/R损伤诱导的BEAS-2B细胞凋亡的保护作用。正常BEAS-2B细胞在加载JC-1染料后会激发出橙红色荧光(图 6a)。在H/R处理10 h后, 聚集的JC-1从线粒体内释放至胞浆以绿色荧光的单体形式存在, 从而可指示线粒体膜电位的下降。与空白组相比, 解耦联试剂CCCP可彻底抑制线粒体氧化磷酸化, 从而能大幅降低线粒体膜电位, 产生明显的绿色荧光并且显著增加绿/红荧光比值(图 6b), 因此其在本实验中作为阴性对照。TSG可使H/R处理后的BEAS-2B细胞绿色荧光翻转至红色, 且显著降低绿/红荧光比值, 说明TSG可减轻H/R诱导的线粒体去极化。
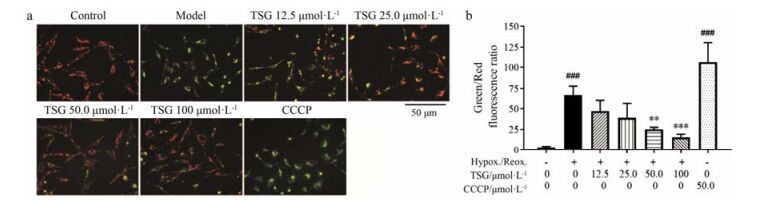
|
Figure 6 TSG inhibited the decline of BEAS-2B cells membrane potential (ΔφM). (a) The cells were imaged through fluorescent microscopy. Representative merged images of green (monomeric JC-1) and red (aggregated JC-1) channels were shown. (b) The fluorescent intensity of BEAS-2B cells were quantificationally assayed through microplate reader and the green/red fluorescence ratio was calculated. Results are presented as x± s from at least 3 independent experiments. ###P < 0.001 vs control; **P < 0.01, ***P < 0.001 vs model |
在免疫荧光实验中, 混合了MitoTracker和anti-Cyt-C通道的荧光。激光共聚焦显微镜观察发现, 生长良好的BEAS-2B细胞胞浆部分呈现橙红色荧光, 指示细胞色素C和线粒体位于相同位置(图 7a)。在H/R处理10 h后, 胞浆部分呈现出明显的绿色荧光拖尾(箭头所示), 表明细胞色素C从线粒体大量释放至胞浆。然而, TSG的预处理可使绿色荧光拖尾缩短, 并且使胞浆荧光颜色从亮绿色翻转至黄色或橙色, 表明其可减轻H/R诱导的细胞色素C的释放。Western blotting结果也证实, TSG可显著抑制H/R诱导的细胞色素C从线粒体向胞浆的释放(图 7b)。
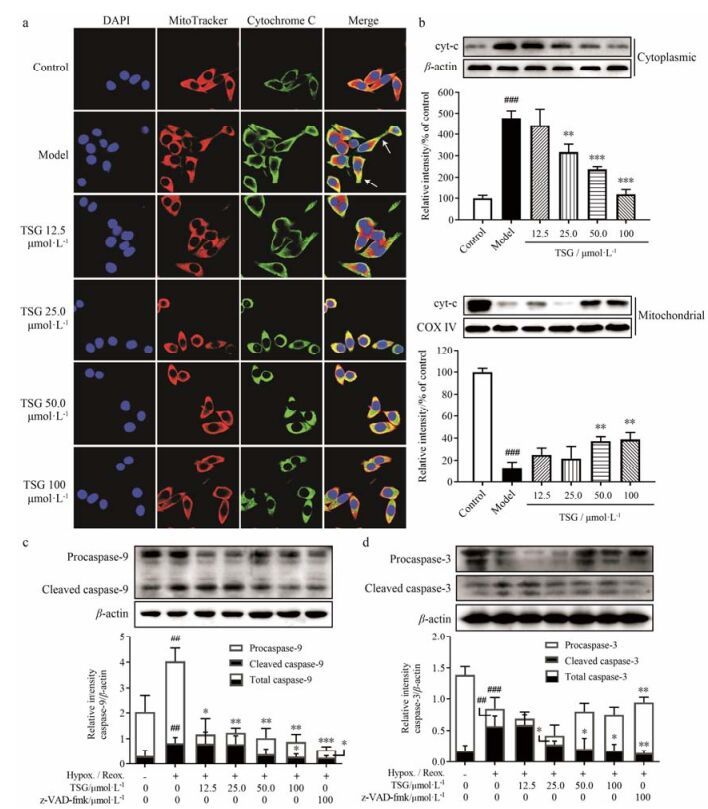
|
Figure 7 TSG inhibited release of cytochrome C and subsequent activation of cleaved caspase-9 and caspase-3. (a) Representative confocal microscopic images showing nuclei (blue channel), mitochondria (red channel), cytochrome C (green channel) and overlaid images are shown. Released cytochrome C was marked with arrows. (b) Expressions of cytochrome C separately in mitochondria and cytoplasm were determined by Western blotting. Representative blot (up) and quantified protein levels (down) are shown. Mean intensity of control group was normalized as 100%. (c, d) Relative expressions of caspase-9 and caspase-3 were determined by Western blotting. Representative blot (up) and quantified protein levels (down) are shown. Results are presented as x± s from at least 3 independent experiments. ##P < 0.01, ###P < 0.001 vs control; *P < 0.05, **P < 0.01, ***P < 0.001 vs model |
作为细胞色素C释放的结果, 后续caspase-9和caspase-3的激活在细胞凋亡中扮演重要的角色。通过实验观察前体的procaspase-9、procaspase-3及它们的激活型caspase-9、caspase-3的表达情况。与空白对照相比, H/R处理10 h可使procaspase-9及激活型caspase-9的表达同时升高(图 7c)。TSG可同时抑制caspase-9的合成和激活, 并呈现较好的量效关系。作为起始caspase, caspase-9可剪切激活下游的效应caspase (即caspase-3等)。与空白对照相比, H/R处理10 h可使激活型caspase-3表达显著升高(图 7d), 同时前体procaspase-3表达相应降低。而TSG可抑制caspase-3的剪切激活, 并呈现较好的量效关系。
4 TSG的抗凋亡效应受MAPK、HIF-1α及p53的调控为了证实TSG是否可调节BEAS-2B细胞中H/R诱导损伤的MAPK信号通路, 通过Western blotting观察了p-p38 MAPK、p-SAPK/JNK1/2和p-ERK1/2在BEAS-2B细胞中的表达(图 8)。在H/R处理后的BEAS-2B细胞中, 磷酸化p38和磷酸化JNK1/2表达明显增加。而TSG的预处理显著降低了p38和JNK1/2在H/R处理的BEAS-2B细胞中的磷酸化蛋白质/总蛋白比, 并存在一定的剂量依赖性。与对照组细胞相比, BEAS-2B细胞经H/R处理后ERK1/2磷酸化水平显著升高。但是, 在H/R模型组和TSG预处理组之间并没有观察到(p-ERK1/2)/(ERK1/2) 比值的显著性差异。这些结果提示, TSG可能通过抑制MAPK通路的激活, 减少p-p38 MAPK、p-SAPK/JNK1/2及下游促凋亡蛋白的表达从而减轻H/R造成的BEAS-2B细胞的损伤。
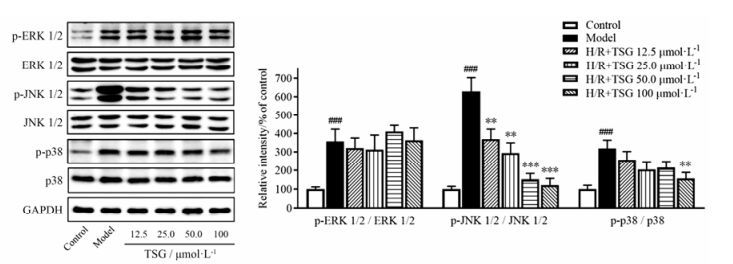
|
Figure 8 TSG inhibited MAPK signaling pathways. Total levels and phosphorylated forms of ERK, JNK and p38 in BEAS-2B cells by H/R injury were detected by Western blotting analysis. Representative blot (left) and quantified protein levels (right) are shown. Results are presented as x± s from at least 3 independent experiments. ###P < 0.001 vs control; **P < 0.01, ***P < 0.001 vs model |
此外还检测了HIF-1α和p53蛋白水平, 以观察TSG对H/R诱导损伤的BEAS-2B细胞的保护作用是否与HIF-1α和p53这对调节细胞缺氧适应的重要蛋白有关。BEAS-2B细胞经H/R处理10 h后, HIF-1α表达明显增高; 而在TSG预处理后, HIF-1α表达呈剂量依赖性下降(图 9)。同时, H/R处理也升高BEAS-2B细胞p53蛋白磷酸化水平, 通过TSG的预处理, p53磷酸化水平下降(图 9)。这些结果表明, TSG可以通过降低HIF-1α的表达和p53的核级联反应, 抑制H/R诱导的细胞凋亡。
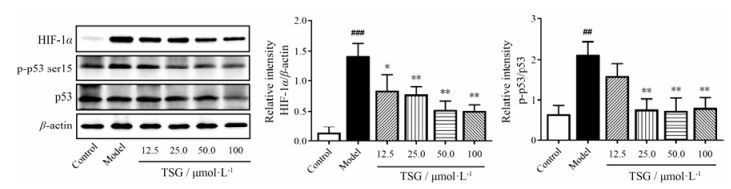
|
Figure 9 TSG inhibited expression of HIF-1α and activation of p53 protein. Total levels of HIF-1α, p53 and phosphorylated (Ser 15) forms of p53 in BEAS-2B cells by H/R injury were detected by Western blotting analysis. Representative blot (left) and quantified protein levels (right) are shown. Results are presented as x± s from at least 3 independent experiments. ##P < 0.01, ###P < 0.001 vs control; *P < 0.05, **P < 0.01 vs model |
H/R及氧化应激损伤频繁发生于人体各组织, 尤其是在呼吸系统, 因为肺和支气管直接与外环境相通并且随时进行气体交换。BEAS-2B细胞广泛应用于肺部相关的研究中。低糖和低血清培养基的氧剥夺模型可诱导细胞缺氧状态。复氧处理干扰细胞的缺氧适应, 从而对细胞造成额外的损伤。基于MAPK通路是转导胞外信号至胞内效应的重要的级联通路, 本研究主要通过MAPK信号通路探讨了何首乌的主要生物活性组分TSG在H/R诱导的BEAS-2B细胞损伤中的保护作用, 即胞外氧化应激刺激和胞内下游的细胞凋亡反应。H/R处理后, 氧化应激状态被认为是启动细胞损伤的主要因素, 尤其是在肺上皮和支气管上皮细胞中, 因为此类细胞与外环境直接相通且无时不在与外界进行气体交换。
无论是缺氧或高氧状态都能诱导细胞损伤, 并且调节BEAS-2B细胞的凋亡[21, 22]。ROS的产生是氧张力的直接指标。SOD反映了缺氧组织损伤的水平, 其作为一个强大的抗氧化剂参与体内氧化还原反应, 将超氧化自由基催化反应为过氧化氢和氧气, 在肺部对氧化应激的响应中扮演了一个有益的角色[23], 避免了组织的进一步氧化损伤[24]。MDA是脂质过氧化的稳定终产物, 与ROS介导的氧化应激相关, 指示了组织损伤的严重程度[25]。在本研究中, 通过观察H/R处理的BEAS-2B细胞产生的ROS、SOD活性和MDA浓度来检测细胞氧化损伤程度。实验结果显示了TSG具有抗H/R诱导的BEAS-2B细胞氧化应激损伤的活性。H/R处理后BEAS-2B细胞ROS和MDA的生成量显著升高, 而TSG能显著逆转这种ROS和MDA的生成, 同时本研究并没有观察到空白对照组、模型组和TSG预处理组之间SOD活性的显著差异。这些结果提示TSG能部分纠正H/R损伤下的BEAS-2B细胞氧张力失衡, 改善氧化应激状态, 且这种效应可能与SOD的参与并没有直接关系。
MAPK信号通路调控细胞的存亡, 其被认为是氧化应激损伤的调控因子[26]。在H/R发生之时, 氧化应激激活MAPK介导细胞存活或凋亡的级联反应[27] (图 10)。p38 MAPK和SAPK/JNK1/2由炎性细胞因子和环境应激因素激活, ERK1/2则由生长因子及细胞因子激活[28]。
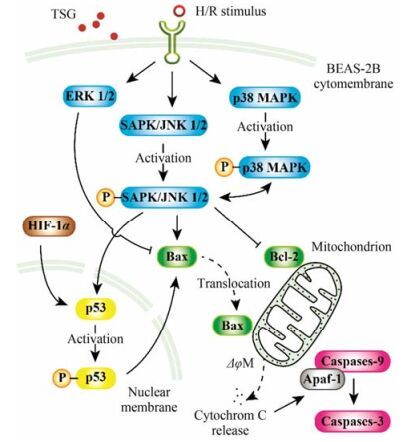
|
Figure 10 TSG is involved in the regulation of MAPK, HIF-1α and p53 signaling pathway in H/R-induced cell apoptosis. Solid arrow: Stimulatory modification or phosphorylating activation; Dashed arrow: Translocation; Bar-headed arrow: Inhibitory modification |
JNK1/2在死亡受体介导的外源性凋亡通路和线粒体介导的内源性凋亡通路中均发挥关键作用。JNK1/2参与促凋亡蛋白Bax的转位和下游的线粒体去极化、细胞色素C的释放及caspase的激活(图 10)。本研究观察了H/R处理后, Bax从BEAS-2B细胞胞浆至线粒体的转位。聚集的Bax在线粒体外膜上形成孔道, 诱使细胞色素C从线粒体内释放至胞浆。细胞色素C在胞浆外与Apaf-1结合、寡聚, 募集始动caspase (如procaspase-9)。自活化的procaspase-9又激活下游效应caspase (如caspase-3和caspase-7)[29], 最终引起DNA断裂和细胞凋亡。TSG抑制Bax的转位、降低Bax/Bcl-2比值, 从而抑制H/R诱导损伤的BEAS-2B细胞下游一系列的功能改变和凋亡行为。值得注意的是, 本研究没有观察到空白对照组、模型组和TSG预处理组之间Bcl-2表达的显著差异。这个结果提示, Bcl-2作为对MAPK通路激活有负反馈调节效应的抑凋亡蛋白, 可能在BEAS-2B细胞上, 对TSG的预处理并不敏感。
此外, JNK还激活p53凋亡通路[30] (图 10)。JNK激活的凋亡通路与p53介导的Bax表达上调有关。在大多数情况下, p38-MAPK与JNK1/2同时被激活[31]。BEAS-2B经4 h的缺氧及6 h的复氧处理后, p38、ERK1/2及JNK1/2的磷酸化水平迅速升高。TSG的预处理可抑制JNK1/2和p38 MAPK的激活但对p-ERK1/2的表达没有显著影响。这些结果提示, TSG抑制H/R诱导的BEAS-2B细胞损伤的作用可能与JNK1/2及p38 MAPK信号通路有关。
另一方面, HIF也是与氧化应激敏感性相关的重要转录因子。缺氧之后的复氧过程可导致p53蛋白的积累和Bax的寡聚化[32], 使细胞经历速发的p53依赖性细胞凋亡[33]。有研究证实, p53蛋白与HIF-1α之间在物质与功能上有着密切的相互作用[34, 35] (图 10)。在本研究中, H/R诱导损伤的BEAS-2B细胞中磷酸化p53和HIF-1α表达均显著升高; 而在TSG预处理组中, 两者表达均显著下降, 且呈良好的量效关系。这提示TSG可通过调控p53和HIF-1α发挥增强BEAS-2B细胞存活和降低H/R损伤的作用。
总之, 此研究表明了TSG能降低BEAS-2B细胞的H/R损伤。这种保护效应能够抑制细胞死亡、提高气管内皮功能、恢复氧化应激平衡及增强细胞抗凋亡能力。TSG通过抑制MAPK的激活, 从而降低Bax/Bcl-2比值、细胞色素C的释放, 抑制caspase-9、caspase-3、磷酸化p53和HIF-1α的表达发挥保护作用。TSG抗H/R损伤的保护作用可能对将来预防和治疗慢性阻塞性肺病、肺水肿、肺气肿和其他肺部缺血再灌注损伤药物研发带来积极意义。
| [1] | Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury[J]. Am J Physiol Cell Physiol, 2002, 282: C227–C241. DOI:10.1152/ajpcell.00112.2001 |
| [2] | Ferrari RS, Andrade CF. Oxidative stress and lung ischemia-reperfusion injury[J]. Oxid Med Cell Longev, 2015, 2015: 590987. |
| [3] | Jiang T, Sun Q, Chen S, et al. Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's disease and Alzheimer's disease[J]. Prog Neurobiol, 2016, 147: 1–19. DOI:10.1016/j.pneurobio.2016.07.005 |
| [4] | Maekawa H, Inagi R. Stress signal network between hypoxia and ER stress in chronic kidney disease[J]. Front Physiol, 2017, 8: 74. |
| [5] | McGuinness AJ, Sapey E. Oxidative stress in COPD: sources, markers, and potential mechanisms[J]. J Clin Med, 2017, 6: E21. DOI:10.3390/jcm6020021 |
| [6] | To T, Zhu J, Larsen K, et al. Progression from asthma to chronic obstructive pulmonary disease(COPD): is air pollution a risk factor?[J]. Am J Respir Crit Care Med, 2016, 194: 429–438. DOI:10.1164/rccm.201510-1932OC |
| [7] | Lü LS. Recent advances on stilbene glucoside from Polygonum multiflorum Thunb[J]. Food Sci(食品科学), 2006, 27: 608–612. |
| [8] | Nonaka G, Miwa N, Nishioka I, et al. Stilbene glycoside gallates and proanthocyanidins from Polygonum multiflorum[J]. Phytochemistry, 1982, 21: 429–432. DOI:10.1016/S0031-9422(00)95282-8 |
| [9] | Wang T, Yang YJ, Wu PF, et al. Tetrahydroxystilbene glucoside, a plant-derived cognitive enhancer, promotes hippocampal synaptic plasticity[J]. Eur J Pharmacol, 2011, 650: 206–214. DOI:10.1016/j.ejphar.2010.10.002 |
| [10] | Hou Y, Yang Q, Zhou L, et al. Tetrahydroxystilbene glucoside improves learning and(or)memory ability of aged rats and may be connected to the APP pathway[J]. Can J Physiol Pharmacol, 2011, 89: 801–809. |
| [11] | Zeng C, Xiao JH, Chang MJ, et al. Beneficial effects of THSG on acetic acid-induced experimental colitis: involve ment of upregulation of PPAR-γ and inhibition of the NF-κb inflammatory pathway[J]. Molecules, 2011, 16: 8552–8568. DOI:10.3390/molecules16108552 |
| [12] | Chao H, Wang Y, Jia W, et al. TSG(2, 3, 4', 5-tetrahydroxys tilbene 2-O-β-D-glucoside)suppresses induction of pro-inflam matory factors by attenuating the binding activity of nuclear factor-κB in microglia[J]. J Neuroinflammation, 2013, 10: 129. |
| [13] | Zhang Y, Shen J, Xu J, et al. Inhibitory effects of 2, 3, 5, 4'-tetrahydroxystilbene-2-O-β-D-glucoside on experimental inflam mation and cyclooxygenase 2 activity[J]. J Asian Nat Prod Res, 2007, 9: 355–363. DOI:10.1080/10286020600727772 |
| [14] | Yao W, Fan W, Huang C, et al. Proteomic analysis for anti-atherosclerotic effect of tetrahydroxystilbene glucoside in rats[J]. Biomed Pharmacother, 2013, 67: 140–145. DOI:10.1016/j.biopha.2012.10.007 |
| [15] | Zhang W, Xu X, Wang Y, et al. Effects of 2, 3, 4', 5-tetrahy droxystilbene 2-O-β-D-glucoside on vascular endothelial dys function in atherogenic-diet rats[J]. Planta Med, 2009, 75: 1209–1214. DOI:10.1055/s-0029-1185540 |
| [16] | Li H, Wang X, Liu Y, et al. Hepatoprotection and hepato toxicity of Heshouwu, a Chinese medicinal herb: context of the paradoxical effect[J]. Food Chem Toxicol, 2016. DOI:10.1016/j.fct.2016.07.035 |
| [17] | Zhou X, Yang Q, Xie Y, et al. Protective effect of tetrahy droxystilbene glucoside against D-galactose induced aging process in mice[J]. Phytochem Lett, 2013, 6: 372–378. DOI:10.1016/j.phytol.2013.05.002 |
| [18] | Xuan L, Shi J, Yao C, et al. Vam3, a resveratrol dimer, inhibits cigarette smoke-induced cell apoptosis in lungs by improving mitochondrial function[J]. Acta Pharmacol Sin, 2014, 35: 779–791. DOI:10.1038/aps.2014.17 |
| [19] | Jiang RT, Yao CS, Bai JY, et al. Effects of Vam3 on sodium nitroprusside-induced apoptosis and SIRT1 and p53 expression in rat articular chondrocytes[J]. Acta Pharm Sin(药学学报), 2014, 49: 608–614. |
| [20] | Liu MJ, Fei SJ, Qiao WL, et al. The protective effect of 17β-estradiol postconditioning against hypoxia/reoxygenation injury in human gastric epithelial cells[J]. Eur J Pharmacol, 2010, 645: 151–157. DOI:10.1016/j.ejphar.2010.06.060 |
| [21] | Pietarinenruntti P, Raivio KO, Saksela M, et al. Antioxidant enzyme regulation and resistance to oxidants of human bronchial epithelial cells cultured under hyperoxic conditions[J]. Am J Respir Cell Mol Biol, 2012, 19: 286–292. |
| [22] | Park E, Yi J, Chung K, et al. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells[J]. Toxicol Lett, 2008, 180: 222–229. DOI:10.1016/j.toxlet.2008.06.869 |
| [23] | Shen CY, Lee J, Su CL, et al. Hypoxia and reoxygenation of the lung tissues induced mRNA expressions of superoxide dismutase and catalase and interventions from different anti oxidants[J]. Transplant Proc, 2008, 40: 2182–2184. DOI:10.1016/j.transproceed.2008.07.080 |
| [24] | Ciancarelli I, Di Massimo C, De Amicis D, et al. Uric acid and Cu/Zn superoxide dismutase: potential strategies and biomarkers in functional recovery of post-acute ischemic stroke patients after intensive neurorehabilitation[J]. Curr Neurovasc Res, 2015, 12: 120–127. DOI:10.2174/1567202612666150311104900 |
| [25] | Yuhai GU, Zhen Z. Significance of the changes occurring in the levels of interleukins, SOD and MDA in rat pulmonary tissue following exposure to different altitudes and exposure times[J]. Exp Ther Med, 2015, 10: 915–920. |
| [26] | Kovalska M, Kovalska L, Pavlikova M, et al. Intracellular signaling MAPK pathway after cerebral ischemia-reperfusion injury[J]. Neurochem Res, 2012, 37: 1568–1577. DOI:10.1007/s11064-012-0752-y |
| [27] | Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia[J]. Mol Neurobiol, 2001, 23: 1–19. DOI:10.1385/MN:23:1 |
| [28] | Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases[J]. Science, 2002, 298: 1911–1912. DOI:10.1126/science.1072682 |
| [29] | Kurokawa M, Kornbluth S. Caspases and kinases in a death grip[J]. Cell, 2009, 138: 838–854. DOI:10.1016/j.cell.2009.08.021 |
| [30] | Oleinik NV, Krupenko NI, Krupenko SA. Cooperation between JNK1 and JNK2 in activation of p53 apoptotic pathway[J]. Oncogene, 2007, 26: 7222–7230. DOI:10.1038/sj.onc.1210526 |
| [31] | Werlen G, Hausmann B, Naeher D, et al. Signaling life and death in the thymus: timing is everything[J]. Science, 2003, 299: 1859–1863. DOI:10.1126/science.1067833 |
| [32] | Zhang X, Li J, Sejas DP, et al. Hypoxia-reoxygenation induces premature senescence in FA bone marrow hematopoietic cells[J]. Blood, 2005, 106: 75–85. DOI:10.1182/blood-2004-08-3033 |
| [33] | Pires IM, Bencokova Z, Milani M, et al. Effects of acute versus chronic hypoxia on DNA damage responses and genomic instability[J]. Cancer Res, 2010, 70: 925–935. DOI:10.1158/0008-5472.CAN-09-2715 |
| [34] | Poon E, Harris AL, Ashcroft M. Targeting the hypoxia-inducible factor(HIF)pathway in cancer[J]. Exp Rev Mol Med, 2009, 11: e26. DOI:10.1017/S1462399409001173 |
| [35] | Fels DR, Koumenis C. HIF-1alpha and p53: the ODD couple?[J]. Trends Biochem Sci, 2005, 30: 426–429. DOI:10.1016/j.tibs.2005.06.009 |
 2017, Vol. 52
2017, Vol. 52


