2. 北京输血医学研究所, 北京 100850;
3. 解放军302医院全军中医药研究所, 北京 100039;
4. 中国医学科学院药用植物研究所, 北京 100193;
5. 火箭军总医院礼士路门诊部, 北京 100820
2. Beijing Institute of Transfusion Medicine, Beijing 100850, China;
3. China Military Institute of Chinese Medicine, 302 Military Hospital, Beijing 100039, China;
4. Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100193, China;
5. Outpatient Department of Rocket Army General Hospital, Beijing 100820, China
何首乌是蓼科植物何首乌(Polygonum multiflorum Thunb.)的干燥块根[1]。何首乌的炮制品具有补肝肾、乌须发、益精血和强筋骨等功效, 广泛用于补益类中药制剂和保健食品。目前研究认为何首乌的主要活性成分是反式二苯乙烯苷(2, 3, 5, 4'-tetrahydroxy-trans-stilbene-2-O-β-glucoside, trans-SG), 在提高记忆力、清除自由基抗氧化、降低胆固醇、抗肿瘤及抑制动脉粥样硬化等方面均显现出良好效果[2]。然而, 近年来关于何首乌及其制剂肝损伤的报道逐渐增多[3], 引起国内外高度关注。本课题组前期实验研究发现何首乌引起的肝损伤可能是一种特异质肝损伤, 并在整体动物水平证实了顺式二苯乙烯苷(2, 3, 5, 4'-tetrahydroxy cis-stilbene-2-O-β-glucoside, cis-SG)是其重要的易感物质[4]。
肝脏作为药物代谢的主要场所, 是药物性肝损伤最主要的靶器官之一[5]。目前药物肝毒性评价常用的肝细胞模型有L02细胞和HepG2细胞等。L02细胞是来源于人正常肝细胞的细胞株, 具有成熟肝细胞的主要表型特征[6]; HepG2细胞则是来源于人肝癌组织的细胞株, 是一种高度分化并显示许多正常肝细胞基因型特征的细胞[7], 被广泛应用于肝癌治疗及肝毒性预测等方面的研究[8]。然而, 由于普通细胞培养为平面培养, 难以维持肝细胞立体结构形态和细胞间联系, 故导致肝细胞在培养过程中较少表达肝脏特有合成和代谢功能相关的蛋白质和代谢物, 与体内肝脏真实情况相差较大。如前期在整体动物水平发现, trans-SG和cis-SG的肝毒性差异在普通二维(two-dimension, 2D)细胞培养模型无法评价。因此, 寻找能够准确、快速、灵敏反映药物肝毒性的新评价模型, 已成为当前中药肝毒性研究亟待解决的问题。
近年来一些研究显示, 三维(three-dimension, 3D)培养条件下获得的具有类器官(organoids)特点的细胞球在维持细胞形态与功能方面, 较常规2D培养具有显著优势[9]。类器官3D培养技术的发展为再生医学和药物安全性评价等方面的研究开辟了新的途径[10]。本研究将基于以上两种细胞, 采用液滴重叠法构建类器官3D培养体系, 通过评测细胞生长状态和肝脏功能等相关指标, 选择合适的细胞构建类器官评价模型, 进一步用于何首乌易感物质的肝毒性评价。
材料与方法药物、试剂与仪器 对乙酰氨基酚(acetaminophen, APAP)、阿司匹林(aspirin, ASP)、丙戊酸(valproic acid, VPA)、环孢霉素(cyclosporine, CSP) (Sigma公司); trans-SG、cis-SG (成都普菲德生物技术有限公司); DMEM培养基(Gibco公司); 胎牛血清(Bioligical Industries公司); DAPI (Santa公司); 白蛋白(albumin, ALB)抗体(Abcam公司); 尿素测试盒(BioAssay Systems公司); 引物合成(北京奥科鼎盛生物科技有限公司); RNA提取试剂盒(QIAGEN公司); cDNA反转录试剂盒、RT-PCR试剂盒(TOYOBO公司); Alamar Blue (Invitrogen公司); 96孔板(Costar公司); CO2培养箱(Thermo Scientific公司); Ensight微孔板检测仪(PerkinElmer公司)。
细胞培养 HepG2和L02细胞培养于含10%胎牛血清、100 u·mL-1青霉素、100 μg·mL-1链霉素的DMEM培养基中, 隔天换液, 于37 ℃、5% CO2饱和湿度培养箱中培养, 待其汇合度达到90%左右传代。
类器官3D模型构建 将HepG2和L02细胞分别以100、200、400、800和1 600个/孔接种于低吸附的96孔U型底板中, 静置10 min左右, 于37 ℃、5% CO2饱和湿度培养箱中培养, 使细胞形成3D结构。培养28天, 期间测量其直径大小并记录3D类器官的变化。
RNA提取及RT-PCR分析 为了比较细胞在2D及3D不同培养条件下, 相关基因表达的差异性, 分别收集2D及3D培养7、14、21和28天的细胞提取RNA。将细胞收集到1.5 mL离心管, PBS洗1次, 加入裂解液, 待其在冰上充分裂解, 放于-80 ℃备用。根据RNeasy® Mini Kit说明书提取RNA。逆转录成cDNA的反应条件: 65 ℃、5 min, 冰上静置2 min; 加反转录试剂后37 ℃ 15 min; 50 ℃ 5 min; 98 ℃ 7 min; 4 ℃ ∞。RT-PCR所用引物序列见表 1, 反应程序: 95 ℃ 3 min; (95 ℃ 10 s; 60 ℃ 35 s) 40 cycles; 65 ℃ 5 s; 95 ℃ ∞。以GAPDH为内参, 采用Livak (2-△△Ct)法进行相对基因表达分析, 并以2D细胞基因表达结果为对照进行数据分析。
| Table 1 Primer sequences used for RT-PCR |
白蛋白表达的检测 通过RT-PCR及免疫荧光染色的方法分别比较两种细胞的白蛋白在基因水平及蛋白水平的表达差异。ALB基因引物参照表 1。免疫荧光染色步骤如下:收集生长7天的细胞球于4%多聚甲醛固定30 min, 10% BSA封闭1 h, 一抗4 ℃孵育过夜, 荧光二抗室温避光孵育1 h; DAPI染色10 min, PBS洗3次。采用激光共聚焦显微镜进行拍照分析。
尿素检测 分别取7、14、21和28天类器官3D培养的L02和HepG2细胞模型及2D培养的细胞, 弃去培养基并用PBS洗2次, 加入含10 mmol·L-1氯化铵的无酚红无血清培养基, 24 h后收上清, 采用BioAssay Systems试剂盒检测尿素生成的含量。具体检测操作步骤按说明书进行, 尿素生成量用细胞数与生成时间进行均一化处理分析。
药物毒性实验 取对数生长期的HepG2细胞接种, 2D培养的细胞以1×104个/孔接种于96孔中, 24 h后进行细胞毒性测试。3D培养的细胞以400个/孔接种于低吸附的96孔板培养7天后, 进行细胞毒性测试。二者在给药24 h后, 加入Alamar blue试剂检测细胞活性。对3D培养的HepG2细胞进行重复给药3次, 隔天给药且半换液, 第7天使用Alamar Blue试剂检测。具体检测分析步骤参照试剂使用说明。
统计学方法 采用Office Excel 2010软件进行数据统计, 数据以x± s表示, 使用SPSS 19.0软件进行单因素方差分析, P < 0.05表示具有显著性差异。
结果 1 3D类器官培养的细胞结构与功能将L02和HepG2细胞接种于96孔板并观察细胞状态, 细胞成对数生长。L02与HepG2细胞以不同密度(100、200、400、800和1 600个/孔)接种于低吸附96孔板中的, 在培养3天后开始形成3D微球结构, 7天结构更加紧密, 可持续培养14天以上。培养前14天, L02与HepG2细胞均可以形成形貌较为规整的细胞球体, 继续培养之后, 由于细胞自身的来源不同, L02细胞仍可维持细胞球的形态, HepG2细胞则呈近球体状态, 相对于L02细胞的3D模型呈松散状态。微球大小与初始细胞数及培养天数有关, 稳定培养可达28天。3D培养的L02和HepG2细胞形态及直径增长曲线, 见图 1。为保证后续研究的一致与稳定性, 最终均选用两种细胞接种密度为400个/孔, 形成紧密结构的细胞球。

|
Figure 1 Morphology of L02 and HepG2 cells in two dimen sional (2D) culture (A) and in three dimensional (3D) culture (B); Diameter of 3D L02 and HepG2 spheroids. L02 and HepG2 cells were seeded at different densities (100, 200, 400, 800 and 1600 per well) (C). Spheroid formation was monitored at 1, 3, 5, 7, 14, 21 and 28 day by light microscopy. n = 5, x± s |
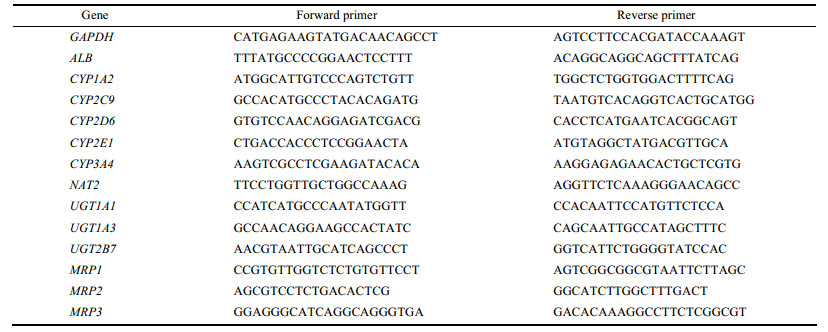
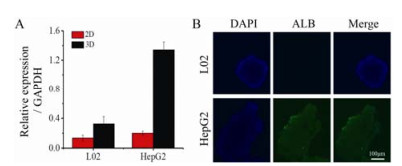
RT-PCR结果显示, 在3D培养状态下, 无论L02细胞还是HepG2细胞, 其ALB的mRNA水平均高于2D培养的细胞, 分别提高2.5和6.7倍; 且3D培养的HepG2细胞较3D培养的L02细胞升高倍数更明显, 可达到约4.5倍。免疫荧光染色检测ALB表达水平, 结果显示, L02几乎不表达, HepG2细胞ALB表达显著高于L02细胞, 见图 2。
|
Figure 2 RT-PCR analysis of albumin (ALB) expression in 2D and 3D of L02 and HepG2 (A); Immunofluorescence detection of ALB in 3D culture of L02 and HepG2 (B). n = 5, x± s |
尿素检测结果显示, 两种细胞在3D培养条件下, 较2D培养尿素生成能力升高, 且在3D培养下, HepG2细胞尿素生成能力显著高于L02细胞, 见图 3。在第21天时, 较2D培养尿素生成, 3D培养的L02细胞球升高8.3倍, HepG2细胞球升高15.5倍。基于以上结果, HepG2在白蛋白表达及尿素生成能力均优于L02, 因此HepG2是更加优选的细胞模型, 后续研究采用HepG2细胞。

|
Figure 3 Urea production of the 2D and 3D L02 and HepG2 cultures. n = 5, x± s |
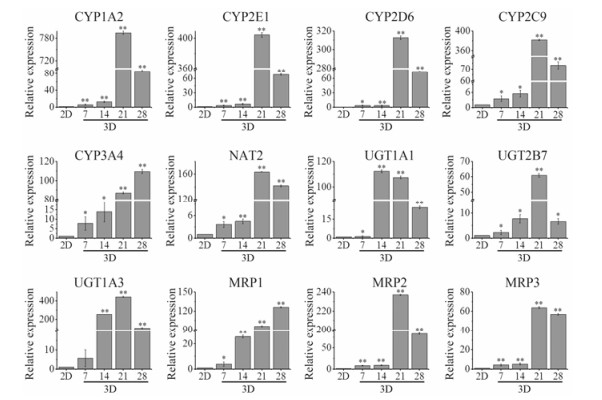
RT-PCR检测结果显示, HepG2细胞在3D培养条件下, 肝功能相关基因表达在第7天开始较2D有不同程度上调, 且大多数药物代谢酶及药物转运体的基因表达在第21天表达最高, 见图 4。在第21天, 相比较2D培养的细胞, 3D培养条件下, Ⅰ相药物代谢CYP1A2上调794.6倍, CYP3A4上调87.0倍, CYP2E1上调404.4倍, CYP2D6上调312.6倍, CYP2C9上调381.9倍, Ⅱ相药物代谢UGT1A3上调445.7倍, 药物转运体MRP2上调236.8倍。本研究观察到涵盖了80%临床药物代谢的CYP450酶(3A4、2D6和2C9) 的功能活性增加。3D培养的模型在肝功能相关基因表达方面显著优于2D培养的细胞。
|
Figure 4 RT-PCR analysis of drug metabolic enzymes and transporters in 2D and 3D culture. n = 5, x± s. *P < 0.05, **P < 0.01 vs 2D culture cells |
通过比较2D与3D HepG2细胞单剂量给药测试细胞毒性的结果, 可发现在3D培养条件下的细胞对药物毒性反应较为敏感, 同时3D培养可以实现重复给药, 反映出更强的细胞毒性。采用已知有肝毒性的APAP为阳性对照, 在2D和3D培养的HepG2细胞加入APAP, 单剂量给药结果显示, 3D培养的类器官较2D培养的细胞对药物毒性更为敏感; 重复给药后3D细胞对药物毒性的敏感性明显增强。通过半数抑制浓度(IC50)值比较可以看出, 在3D培养条件下, APAP重复给药的IC50显著小于单剂量给药, 2D培养的HepG2无法测出IC50。采用ASP为阴性对照, 在2D和3D培养的条件下, 单剂量及重复给药均没有明显的细胞毒性。进一步检测VPA及CSP的肝毒性, 结果显示, 3D培养的HepG2细胞重复给药时, 细胞对药物毒性的敏感性明显增强, 说明3D培养的类器官模型及重复给药能更好地预测药物产生的肝毒性。因此, 可使用该模型探索trans-SG和cis-SG的肝毒性。细胞毒性结果显示, 3D重复给药与2D、3D单剂量给药相比较, 3D重复给药细胞对药物毒性更为敏感, 见图 5。cis-SG在普通2D培养肝细胞模型未检测到IC50, 在类器官3D培养模型上单次给药方式的IC50是肝毒性阳性药CSP的1.9倍, 而trans-SG的IC50比cis-SG高4.1倍, 与前期整体动物水平的毒性强弱基本一致[4]; cis-SG在类器官3D培养模型上多次给药方式的IC50进一步降低, 提示长期用药可能增大何首乌肝损伤风险, 见表 2。

|
Figure 5 Comparison of cytotoxicity of 2D and 3D HepG2 cells in single and repeated dose administration. APAP, Aceta minophen; ASP, Aspirin; VPA, Valproic acid; CSP, Cyclosporine; trans-SG, 2, 3, 5, 4'-Tetrahydroxy trans-stilbene-2-O-β-glucoside; cis-SG, 2, 3, 5, 4'-Tetrahydroxy cis-stilbene-2-O-β-glucoside |
| Table 2 IC50 (mmol·L−1) value of drugs in 2D and 3D culture model |
传统2D培养的细胞呈平面生长, 虽操作简单、费用低, 但是其自身细胞与细胞之间联系缺失, 使细胞极化现象消失, 药物代谢酶和转运体低表达, 导致与正常生理环境存在较大差异[11], 且2D细胞增殖较快, 在一定程度上掩盖了药物毒性所造成的损伤[12], 从而限制了其在药物安全评价的应用, 难以用于特异质肝损伤的评价。3D培养的细胞球(类器官)可模拟体内生长模式, 更接近人体的组织微结构, 模拟肝脏的功能[13], 在评价药物毒性时可重复给药激活多种生物活性, 克服2D细胞模型掩盖药物毒性的缺陷[12], 从而在药物特异质毒性评价方面更具优势。而且, 类器官3D培养模型具有构建周期短、成本低、易于操作和作用机制较易探明等特点[14]。在细胞选择方面, L02来源于正常人肝细胞系, HepG2来源于肿瘤细胞系, 可能是导致在3D模型建立时L02细胞仍可维持细胞球的形态, HepG2细胞则呈近球体状态, 相对于L02细胞的3D模型呈松散状态的原因。但通过L02和HepG2两种细胞白蛋白检测及尿素代谢方面的比较, HepG2优于L02细胞, 因此选用HepG2细胞作为药物肝毒性筛选的模型。
与2D培养细胞相比, 类器官3D培养模型表达的药物代谢酶、转运体及白蛋白显著升高, 与文献[8, 15, 16]报道的3D细胞模型特性一致。此外, 3D细胞培养可进行长期培养, 适用于重复给药的研究。通过IC50值的比较, 结果显示3D类器官在重复给药后对肝损伤药物毒性更为敏感, 说明类器官3D培养模型符合中药肝损伤的特点, 与中药肝毒性的慢性损伤的生理发生过程类似。3D培养的类器官可用于模拟长期低剂量药物对人体肝细胞损害的影响, 比2D细胞模型更符合中药临床使用情况。
前期研究发现, cis-SG是何首乌肝损伤的重要易感物质, 在对cis-SG及trans-SG在3D类器官代谢毒性进行检测后, 3次给药结果显示, cis-SG毒性显著高于trans-SG, 与前期整体动物结果一致。在进一步的工作中, 利用该模型对cis-SG可能引起肝损伤的机制进行探讨, 从药物代谢方面深入研究cis-SG代谢过程对肝细胞引起损伤的机制, 从而找出何首乌引起肝损伤的相关原因, 为中药的肝毒性预测与解决提供思路。
| [1] | China Pharmacopoeia Committee. Chinese Pharmacopoeia(中国药典)[M]. Beijing: China Medical Science Press, 2010: 164. |
| [2] | Yang HL, Ge ZZ, Sun ZX. Advances in pharmacological studies of Polygonum multiflorum Thumb[J]. J Chin Med Mater(中药材), 2013, 36: 1713–1717. |
| [3] | Sun Z, Zhang L. Liver damage related to Polygonum multi florum and its preparations: domestic literature review and analysis[J]. Adv Drug React J(药物不良反应杂志), 2010, 12: 26–30. |
| [4] | Li CY, Niu M, Bai ZF, et al. Screening for main components associated with the idiosyncratic hepatotoxicity of a tonic herb Polygonum multiflorum[J]. Front Med, 2017. DOI:10.1007/s11684-017-0508-9 |
| [5] | Huang YY, Sun R. Research development on the application of systems biology in the discovery and assessment of hepato toxicity TCM[J]. Chin J Pharmacovig(中国药物警戒), 2013, 10: 219–222. |
| [6] | Hu X, Yang T, Li C, et al. Human fetal hepatocyte line, L-02, exhibits good liver function in vitro and in an acute liver failure model[J]. Transplant Proc, 2013, 10: 695–700. |
| [7] | Gerets HH, Hanon E, Cornet M, et al. Selection of cytotoxicity markers for the screening of new chemical entities in a phar maceutical context: a preliminary study using a multiplexing approach[J]. Toxicol In Vitro, 2009, 23: 319–332. DOI:10.1016/j.tiv.2008.11.012 |
| [8] | Luckert C, Schulz C, Lehmann N, et al. Comparative analysis of 3D culture methods on human HepG2 cells[J]. Arch Toxicol, 2017, 91: 393–406. DOI:10.1007/s00204-016-1677-z |
| [9] | Lauschke VM, Hendriks DF, Bell CC, et al. Novel 3D culture systems for studies of human liver function and assessments of the hepatotoxicity of drugs and drug candidates[J]. Chem Res Toxicol, 2016, 29: 1936–1955. DOI:10.1021/acs.chemrestox.6b00150 |
| [10] | Clevers H. Modeling development and disease with organoids[J]. Cell, 2016, 165: 1586–1597. DOI:10.1016/j.cell.2016.05.082 |
| [11] | Tseng H, Balaoing LR, Grigoryan B, et al. A three-dimensional co-culture model of the aortic valve using magnetic levitation[J]. Acta Biomater, 2014, 10: 173–182. DOI:10.1016/j.actbio.2013.09.003 |
| [12] | Ramaiahgari SC, Den Braver MW, Herpers B, et al. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies[J]. Arch Toxicol, 2014, 88: 1083–1095. |
| [13] | Lau TT, Lee LQ, Leong W, et al. Formation of model hepatocellular aggregates in a hydrogel scaffold using degradable genipin crosslinked gelatin microspheres as cell carriers[J]. Biomed Mater, 2012, 7: 065003. DOI:10.1088/1748-6041/7/6/065003 |
| [14] | Qin YY, Liu Y, Liu CB, et al. Research progress of organ culture[J]. Guangdong Med J(广东医学), 2016, 37: 2827–2828. |
| [15] | Gunness P, Mueller D, Shevchenko V, et al. 3D organotypic cultures of human HepaRG cells: a tool for in vitro toxicity studies[J]. Toxicol Sci, 2013, 133: 67–78. DOI:10.1093/toxsci/kft021 |
| [16] | Van Zijl F, Mikulits W. Hepatospheres: three dimensional cell cultures resemble physiological conditions of the liver[J]. World J Hepatol, 2010, 2: 1–7. |
 2017, Vol. 52
2017, Vol. 52