2. 解放军302医院全军中医药研究所, 北京 100039;
3. 解放军302医院中西医结合肝病诊疗与研究中心, 北京 100039;
4. 中国医学科学院肿瘤医院, 北京 100021
2. China Military Institute of Chinese Medicine, 302 Military Hospital, Beijing 100039, China;
3. Integrative Medicine Center, 302 Military Hospital, Beijing 100039, China;
4. Cancer Hospital, Chinese Academy of Medical Sciences, Beijing 100021, China
何首乌为蓼科植物何首乌Polygonum multiflorum Thunb.的干燥块根, 临床应用有生首乌和制首乌之别, 生品能够解毒、消痈、截疟和润肠通便, 炮制品可以补肝肾、益精血和乌须发等[1], 传统认为无毒。然而, 近年来有关何首乌及其制剂导致肝损伤不良反应的报道大量增加[2], 引起国内外广泛关注。本课题组[3]前期研究提示何首乌引起的肝损伤可能是一种特异质肝损伤, 并建立了内毒素[脂多糖(lipopolysa ccharide, LPS)]诱导的何首乌特异质肝损伤评价模型。在此基础上, 课题组在整体动物水平, 采用中药活性物质敲出/敲入策略[4]和单体化合物验证, 研究发现顺式二苯乙烯苷(顺式-2, 3, 5, 4'-四羟基二苯乙烯-2-O-β-D-葡萄糖苷, cis-SG)是何首乌重要的易感物质[5, 6]。易感物质是药物含有的对某种疾病易感的成分, 通过控制易感物质含量的高低可以有效降低发生的疾病风险。何首乌中天然存在的主要是反式二苯乙烯苷(反式-2, 3, 5, 4'-四羟基二苯乙烯-2-O-β-D-葡萄糖苷, trans-SG), cis-SG是在光照条件下由trans-SG转化而来, 提示在何首乌晾晒、提取、干燥和贮存等生产炮制环节及平常所泡药酒阶段, trans-SG均有可能发生光转化生成cis-SG。针对这一现象和问题, 本文拟考察cis-SG转化量与制首乌特异质肝损伤的相关性, 并探讨其可能的安全限度, 为从质量角度建立何首乌肝损伤风险控制手段提供参考。
材料与方法实验动物 SPF级雄性SD大鼠, 体重180~200 g, 购于军事医学科学院实验动物中心, 合格证号SCXK-(军) 2012-0004, 于解放军302医院实验动物中心分笼饲养, 自由饮水和进食。实验动物中心室温(25 ± 2) ℃, 湿度50%~70%, 室内保持12 h照明与黑暗交替, 并定期进行消毒。
药品与试剂 制何首乌(radix Polygoni multiflori Preparata, RPMP, 批号15050401) 购于北京绿野药业有限公司, 其炮制标准执行《中国药典》和《北京市中药饮片炮制规范》[7, 8]; cis-SG (批号16091802, 供含量测定用, 以98%计)、trans-SG (批号16081506, 供含量测定用, 以98%计)均购于成都普菲德生物技术有限公司; LPS (批号046M4045V)、戊巴比妥钠(批号57-33-0)、乙腈(色谱级, 批号WXBB6406V)购于美国Sigma公司; 10%中性甲醛(固定液) (批号20160620) 购于北京中科万邦生物科技有限公司; AST测定试剂盒(MDH法)、ALT测定试剂盒(乳酸脱氢酶法)均购于美国Beckman Coulter公司; 大鼠IL-6酶联免疫吸附测定试剂盒(SEA079Ra 96T)、大鼠TNF-α酶联免疫吸附测定试剂盒(SEA133Ra 96T)和大鼠PPAR-γ酶联免疫吸附测定试剂盒(SEA886Ra 96T)均购于美国Cloud-Clone公司; 水为超纯水, 其余试剂均为分析纯。
仪器 Agilent1200高效液相色谱仪(HP-12000 DAD检测器) (美国Agilent科技有限公司); ZF-1型三用紫外分析仪(北京启航博达科技有限公司); XS205DU电子天平(瑞士Mettler Toledo公司); 旋转蒸发仪R205B (SENCO) (上海申生科技有限公司); KQ-500DE型数控超声波清洗器(昆山市超声仪器有限公司); AU5400全自动生化分析仪(日本OLYMPUS光学株式会社); ELx808吸收光酶标仪(美国BioTek公司); KD-P摊片机(浙江省金华市科迪仪器设备有限公司); Leica RM2235石蜡切片机[徕卡显微系统(上海)有限公司]; JB-L7石蜡包埋机(武汉俊杰电子有限公司); Nikon Ni-U三人共览显微镜(日本Nikon公司)等。
样品制备 基于本课题组前期实验结果, 何首乌50%乙醇提取物对肝细胞毒性影响最大, 故本实验采用50%乙醇提取制首乌[9]。
未光照样品制备 取制首乌粗粉适量, 加8倍量50%乙醇超声提取, 共提取2次, 每次30 min, 合并提取液, 减压浓缩回收乙醇得浓缩液, 临用前用去离子水按生药量配制成相应浓度。
光照样品制备 各取3份制首乌粗粉适量, 分别加8倍量50%乙醇超声提取, 共提取2次, 每次30 min, 合并提取液, 置于平皿内, 在365 nm紫外灯下照射一定时间至溶液中trans-SG转化为cis-SG且cis-SG含量分别为0.10%、0.35%和0.70%左右, 减压浓缩回收乙醇得浓缩液, 临用前用去离子水按生药量分别配制成相应浓度。
样品中cis-SG含量测定
色谱条件 ZORBAX Eclipse Plus C18色谱柱(250 mm × 4.6 mm, 5 μm); 流动相为水(A)-乙腈(B); 检测波长280 nm; 柱温30 ℃; 流速1 mL·min-1; 线性梯度洗脱, 0~8 min, 5%~35% A, 8~10 min, 35%~45% A, 10~15 min, 45%~80% A, 15~18 min, 80%~5% A.; 进样量10 μL。
混合对照品溶液的制备 取cis-SG对照品和trans-SG对照品适量, 精密称定, 加甲醇制成每1 mL各含0.2 mg的混合对照品溶液。
供试品溶液的制备 分别取上述未经光照和光照后的制首乌浓缩液适量, 用甲醇稀释一定倍数, 过0.22 μm微孔滤膜, 取续滤液, 得供试品溶液。
样品测定 各供试品溶液在上述色谱条件项下进行测定, 采用外标法分别计算各样品中cis-SG含量, 色谱图如图 1所示。
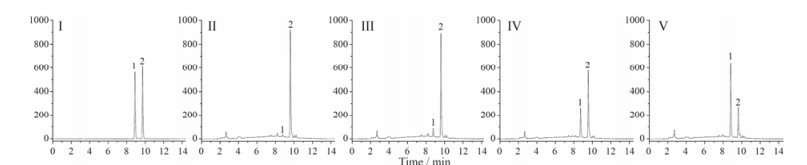
|
Figure 1 HPLC of radix Polygoni multiflori Preparata (RPMP). Ⅰ: Reference substance; Ⅱ: Sample without light treatment; Ⅲ: Sample with cis-SG content of 0.10% after light treatment; Ⅳ: Sample with cis-SG content of 0.35% after light treatment; Ⅴ: Sample with cis-SG content of 0.70% after light treatment; 1: cis-SG, 2: trans-SG. cis-SG: cis-2, 3, 5, 4'-Tetrahydroxystilbene-2-O-β-D-glucoside; trans-SG: trans-2, 3, 5, 4'-Tetrahydroxystilbene-2-O-β-D-glucoside |
基于内毒素模型的特异质肝损伤评价
动物分组 将80只SD大鼠随机分成10组(n = 8):正常对照组(N)、LPS模型组(L)、制首乌未光照样品单独给药组(A)、制首乌光照后cis-SG含量0.10%样品单独给药组(B)、制首乌光照后cis-SG含量0.35%样品单独给药组(C)、制首乌光照后cis-SG含量0.70%样品单独给药组(D)、LPS联合制首乌未光照样品给药组(LA)、LPS联合制首乌光照后cis-SG含量0.10%样品给药组(LB)、LPS联合制首乌光照后cis-SG含量0.35%样品给药组(LC)、LPS联合制首乌光照后cis-SG含量0.70%样品给药组(LD)。制首乌给药剂量基于前期实验研究所得, 为7.56 g·kg-1[10]。
给药与标本采集 将大鼠禁食不禁水12 h后, 称重, 按剂量给药。其中制首乌样品通过灌胃给予, 3 h后尾静脉注射LPS (2.8 mg·kg-1), LPS尾静脉注射7 h后, 腹腔注射戊巴比妥钠(50 mg·kg-1)麻醉大鼠, 下腔静脉取血, 并采集肝脏样品[3, 11]。
检测方法 收集大鼠离体血液, 离心(3 000 r·min-1, 10 min)取血浆, 采用全自动生化分析仪检测ALT和AST水平, 采用ELISA法按试剂盒说明书检测IL-6、TNF-α和PPAR-γ含量; 采集大鼠肝组织, 用10%中性福尔马林固定, 常规病理切片, 采用HE染色法观察肝组织病理学变化, 免疫组化法测定大鼠肝脏中NF-κB p65表达, TUNEL法检测肝细胞的凋亡[以细胞核呈棕褐色染色为阳性标准, 细胞凋亡率% = (凋亡细胞数/细胞总数) ×100%]。
临床肝损伤患者余留药物和产地收集饮片含量分析 通过收集来源于不同产地(湖南、广东、湖北、四川和云南)的制首乌饮片和经解放军302医院确诊为制首乌所致肝损伤患者所服用余留的药物(饮片、药酒及药粉), 并测定cis-SG含量, 测定方法参考“样品中cis-SG含量测定”项。
统计学分析 采用SPSS 19.0软件进行统计分析, 实验数据以x± s表示, 计量资料采用单因素方差分析(ANOVA), 显著性概率水平P < 0.05。
结果 1 血浆ALT和AST测定结果比较与对照组及模型组相比, 单独给药未光照组、单独给药光照后3个不同cis-SG含量组、单用LPS组、LPS联合未光照组及LPS联合光照后cis-SG含量为0.10%组对大鼠血浆AST和ALT活力均无显著性差异(P > 0.05), 而相同剂量光照后cis-SG含量为0.35%和0.70%样品联合LPS给药后, 大鼠血浆AST和ALT活力均显著升高(P < 0.05), 说明样品中cis-SG含量与何首乌肝损伤呈现一定的量-毒关系。结果见图 2。

|
Figure 2 Influence of co-treatment with lipopolysaccharide (LPS) and RPMP with different cis-SG content on plasma ALT and AST activities. N: Normal control group; A: RPMP without light treatment group; B: 0.10% cis-SG in RPMP after light treatment group; C: 0.35% cis-SG in RPMP after light treatment group; D: 0.70% cis-SG in RPMP after light treatment group; L: LPS model group; LA: LPS+ RPMP without light treatment group; LB: LPS+0.10% cis-SG in RPMP after light treatment group; LC: LPS+0.35% cis-SG in RPMP after light treatment group; LD: LPS+0.70% cis-SG in RPMP after light treatment group. The saline-treated group served as a control group and the LPS-treated group served as a model group. n = 8, x± s. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group; ▲P < 0.05, ▲▲P < 0.01, ▲▲▲P < 0.001 vs model group |
LPS会刺激单核/巨噬细胞分泌大量的致炎细胞因子, 与对照组相比, 模型组的TNF-α含量明显升高(P < 0.05), IL-6呈现升高的趋势; 与模型组TNF-α和IL-6含量相比, LPS联合光照后cis-SG含量为0.35%和0.70%样品显著升高(P < 0.05), 而相同剂量未光照样品和光照后cis-SG含量为0.10%样品联合LPS给药后无明显改变(P > 0.05)。与对照组PPAR-γ表达相比, 模型组明显下降(P < 0.05), 单独给药未光照组、单独给药光照后3个不同cis-SG含量组无显著性差异(P > 0.05);在LPS模型上, 未光照样品和光照后cis-SG含量为0.10%样品PPAR-γ表达无明显改变(P > 0.05), 而光照后cis-SG含量为0.35%和0.70%样品PPAR-γ表达显著降低(P < 0.05)。结果见图 3。

|
Figure 3 Influence of co-treatment with LPS and RPMP with different cis-SG content on plasma IL-6, TNF-α and PPAR-γ activities. The saline-treated group served as a control group and the LPS-treated group served as a model group. n = 8, x± s. *P < 0.05, **P < 0.01 vs control group; ▲P < 0.05 vs model group |
与正常对照组相比, 单独给药未光照组和单独给药光照后3个不同cis-SG含量组无明显变化, 大鼠肝组织病理切片可见细胞排列整齐, 偶有炎细胞浸润; 模型组可见大鼠肝组织切片的汇管区炎症细胞增加, 但无明显病理学改变; 相同剂量未光照样品联合LPS给药和相同剂量光照后cis-SG含量为0.10%样品联合LPS给药, 可见汇管区炎症细胞增加, 但无明显病理学改变; 而相同剂量光照后cis-SG含量分别为0.35%和0.70%的样品联合LPS给药后, 可见中央静脉扩张、内膜脱落, 中央静脉周肝细胞出现肿胀、坏死现象, 汇管区被大量的炎细胞浸润。结果见图 4。
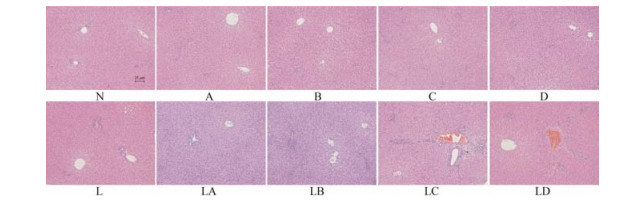
|
Figure 4 Influence of co-treatment with LPS and RPMP with different cis-SG content on pathological morphology (HE staining 100×). The saline-treated group served as a control group and the LPS-treated group served as a model group |
与对照组p65表达相比, 模型组明显升高(P < 0.05), LPS激活了NF-κB通路的活性; 与模型组相比, LPS联合光照后cis-SG含量为0.35%组和LPS联合光照后cis-SG含量为0.70%组, 累积光密度值(IOD)显著升高(P < 0.05);而LPS联合未光照组、LPS联合光照后cis-SG含量为0.10%组的IOD并没有明显改变(P > 0.05)。以细胞核内出现棕黄色颗粒为阳性标准, 根据IOD大小判断阳性表达量的高低。结果见图 5。

|
Figure 5 Influence of co-treatment with LPS and RPMP with different cis-SG content on the expression of p65 (immunohistochemical staining 200×). The saline-treated group served as a control group and the LPS-treated group served as a model group. n = 8, x± s. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group; ▲P < 0.05, ▲▲P < 0.01 vs model group |
与对照组及模型组相比, LPS联合光照后cis-SG含量为0.35%组和LPS联合光照后cis-SG含量为0.70%组, 肝细胞凋亡数和凋亡率显著升高(P < 0.05);而其他组的细胞凋亡率并没有明显改变(P > 0.05)。结果见图 6。
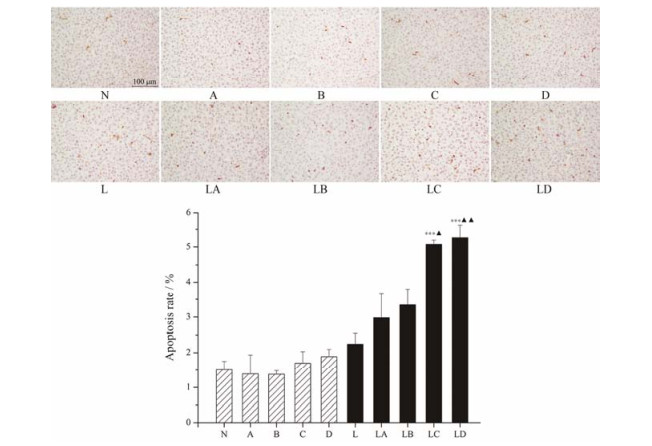
|
Figure 6 Influence of co-treatment with LPS and RPMP with different cis-SG content on apoptosis of liver tissue (TUNEL staining 200×). The saline-treated group served as a control group and the LPS-treated group served as a model group. n = 8, x± s. ***P < 0.001 vs control group; ▲P < 0.05, ▲▲P < 0.01 vs model group |
如图 7所示, 来源于不同产地(湖南、广东、湖北、四川和云南)收集的制首乌饮片中cis-SG含量普遍较低( < 0.10%), 而肝损伤患者服用的药物中cis-SG含量均明显偏高( > 0.40%), 说明制首乌中cis-SG含量的高低与其特异质肝损伤密切相关。

|
Figure 7 Content of cis-SG in RPMP pieces and medicine |
何首乌造成肝损伤的风险因素是多方面的, 研究发现何首乌肝损伤的发生存在易感人群[12, 13]和易感物质[5, 6], 主要与机体的特异质状态及易感性密切相关。本课题组[14]在此基础上提出了何首乌“免疫应激三因致毒”假说, 当机体处于免疫异常活化时(易感人群), 何首乌的免疫促进成分(如trans-SG)可增强机体免疫, 使肝脏对何首乌中易感成分(如cis-SG)增敏, 从而诱发免疫应激性特异质肝损伤。因此, 通过筛查易感人群和控制易感物质的策略, 均可以有效降低何首乌特异质肝损伤发生的风险。
药物特异质肝损伤的评价模型是国内外研究的热点也是难点[15, 16]。完全在实验室模拟临床的特异质肝损伤尚有较大困难, 国内外已报道的模型大多是针对临床某一方面的模拟。本文采用的内毒素复制的免疫应激易感性模型, 是国际上已较广泛采用的成熟模型, 该模型的优势是评价时间较短、模型稳定及易于操作, 与临床免疫相关的特异质肝损伤的主要通路和机制较为相似[17]。该模型已经成功用于揭示了曲伐沙星(有特异质肝损伤)和左氧氟沙星(肝损伤不明显)[18]、雷尼替丁(有特异质肝损伤)和法莫替丁(肝损伤不明显)[19]等同类别药物在临床上肝损伤风险不同的现象。
本研究在内毒素复制的特异质肝损伤评价模型基础上, 针对何首乌中重要易感物质cis-SG, 探讨制首乌中cis-SG转化量与特异质肝损伤的相关性。结果表明, cis-SG转化量与制首乌的特异质肝损伤存在一定的量−毒关系, 特别是当cis-SG转化量低于0.10%时, 在内毒素易感模型上未见肝损伤现象(图 2); 作者在产地收集并控制光照的制首乌样品中cis-SG含量低于0.10%, 而临床肝损伤患者余留药物的cis-SG含量高于0.40% (图 7)。综合这些结果, 提示控制易感物质cis-SG的含量限度, 可作为降低何首乌肝损伤风险的重要手段; 为降低临床用药风险, 可考虑将cis-SG含量0.10%作为何首乌生产炮制过程的一个重要质控限度。诚然, 由于药物特异质肝损伤的多影响因素的特点, 何首乌特异质肝损伤的易感物质也可能还有其他易感成分。本课题组[20]前期研究发现, 铁离子介导的二苯乙烯苷聚合物, 可能也和何首乌肝损伤风险有关, 但该发现还需要后续的实验证实才能制定风险控制的限度。本文对cis-SG含量限度和肝损伤风险的研究结果, 为从质量角度控制何首乌肝损伤风险提供了参考依据。
此外, 较高转化量的cis-SG (0.35%和0.70%)与内毒素联用, 发现与炎症反应有关的PPAR-γ含量显著减少(P > 0.05); NF-κB p65表达量、炎症因子TNF-α和IL-6含量均显著增加(P < 0.05)。提示较高转化量的cis-SG可能协同LPS作用, 通过下调PPAR-γ表达, 激活NF-κB等信号通路活性, 诱导单核/巨噬细胞分泌TNF-α和IL-6等致炎细胞因子, 引起肝损伤。cis-SG具体激活或参与了哪个免疫因子或靶标的信号传导, 进而在免疫应激大鼠模型上导致肝损伤, 值得进一步研究。
| [1] | China Pharmacopoeia Committee. Chinese Pharmacopoeia(中国药典)[M]. Beijing: China Medical Science Press, 2015: 175. |
| [2] | Lei X, Chen J, Ren J, et al. Liver damage associated with Polygonum multiflorum Thunb.: a systematic review of case reports and case series[J]. Evid Based Complement Alternat Med, 2015, 2015: 459749. |
| [3] | Li CY, Li YF, Tu C, et al. The idiosyncratic hepatotoxicity of Polygonum multiflorum based on endotoxin model[J]. Acta Pharm Sin(药学学报), 2015, 50: 28–33. |
| [4] | Kong WJ, Wang JB, Zhang QC, et al. A novel "target constituent knock-out" strategy coupled with TLC, UPLC-ELSD and microcalorimetry for preliminary screening of antibacterial constituents in Calculus bovis[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2011, 879: 3565–3573. DOI:10.1016/j.jchromb.2011.09.045 |
| [5] | Meng YK, Li CY, Li RY, et al. Mechanism of Polygonum multiflorum induced liver injury: cis-stilbene glucoside induces immunological idiosyncratic hepatotoxicity by suppressing PPAR-γ in a lipopolysaccharide model[J]. Acta Pharmacol Sin, 2017. DOI:10.1038/aps.2017.32 |
| [6] | Li CY, Niu M, Bai ZF, et al. Screening for main components associated with the idiosyncratic hepatotoxicity of a tonic herb Polygonum multiflorum[J]. Front Med, 2017. DOI:10.1007/s11684-017-0508-9 |
| [7] | China Pharmacopoeia Committee. Chinese Pharmacopoeia(中国药典)[M]. Beijing: China Medical Science Press, 2010: 122. |
| [8] | Beijing Food and Drug Administration. Beijing Standardized Processing of Chinese Herbal Pieces(北京市中药饮片炮制规范·上册)[M]. Beijing: Chemical Industry Press, 2008: 139. |
| [9] | Lü Y, Wang JB, Ji Y, et al. Influence of extracting solvent on hepatocytes toxicity of Polygonum multiflorum[J]. Chin J Exp Tradit Med Form(中国实验方剂学杂志), 2013, 20: 268–272. |
| [10] | Li XF, Li N, Tu C, et al. Comparison of crude and prepared Polygonum multiflorum-induced idiosyncratic hepatotoxicity based on lipopolysaccharide model[J]. Chin Tradit Herbal Drugs(中草药), 2015, 46: 1481–1486. |
| [11] | Yee SB, Hanumegowda UM, Copple BL, et al. Endothelial cell injury and coagulation system activation during synergistic hepatotoxicity from monocrotaline and bacterial lipopolysaccharide coexposure[J]. Toxicol Sci, 2003, 74: 203–214. DOI:10.1093/toxsci/kfg106 |
| [12] | Zhu Y, Niu M, Chen J, et al. Comparison between Chinese herbal medicine and Western medicine-induced liver injury of 1985 patients[J]. J Gastroenterol Hepatol, 2016, 31: 1476. DOI:10.1111/jgh.2016.31.issue-8 |
| [13] | Zhu Y, Liu SH, Wang JB, et al. Clinical analysis of drug-induced liver injury caused by Polygonum multiflorum and its preparations[J]. Chin J Integr Tradit West Med(中国中西医结合杂志), 2015, 35: 1442–1447. |
| [14] | Wang JB, Cui HR, Bai ZF, et al. Precision medicine-oriented safety assessment strategy for traditional Chinese medicines: disease-syndrome-based toxicology[J]. Acta Pharm Sin(药学学报), 2016, 51: 1681–1688. |
| [15] | Krueger W, Boelsterli UA, Rasmussen TP. Stem cell strategies to evaluate idiosyncratic drug-induced liver injury[J]. J Clin Transl Hepatol, 2014, 2: 143–152. |
| [16] | Wang Q, Mei H, Zhang YL, et al. The associations between idiosyncratic adverse drug reactions and HLA alleles and their underlying mechanism[J]. Acta Pharm Sin(药学学报), 2013, 48: 799–808. |
| [17] | Roth RA, Ganey PE. Animal models of idiosyncratic drug-induced liver injury—current status[J]. Crit Rev Toxicol, 2011, 41: 723–739. DOI:10.3109/10408444.2011.575765 |
| [18] | Waring JF, Liguori MJ, Luyendyk JP, et al. Microarray analysis of lipopolysaccharide potentiation of trovafloxacin-induced liver injury in rats suggests a role for proinflammatory chemokines and neutrophils[J]. J Pharmacol Exp Ther, 2006, 316: 1080–1087. |
| [19] | Luyendyk JP, Lehman-Mckeeman LD, Nelson DM, et al. Coagulation-dependent gene expression and liver injury in rats given lipopolysaccharide with ranitidine but not with famotidine[J]. J Pharmacol Exp Ther, 2006, 317: 635–643. DOI:10.1124/jpet.105.096305 |
| [20] | Li RF, Feng WW, Li XF, et al. Influence of metal ions on stability of 2, 3, 5, 4'-tetrahydroxy stilbene-2-O-β-D-glucoside contained in Polygoni Multiflori radix[J]. Acta Pharm Sin(药学学报), 2016, 51: 116–121. |
 2017, Vol. 52
2017, Vol. 52


