2. 湖南中医药大学药学院, 湖南 长沙 410208;
3. 江西中医药大学药学院, 江西 南昌 330004;
4. 解放军302医院中西医结合肝病诊疗与研究中心, 北京 100039
2. School of Pharmacy, Hunan University of Traditional Chinese Medicine, Changsha 410208, China;
3. School of Pharmacy, Jiangxi University of Traditional Chinese Medicine, Nanchang 330004, China;
4. Integrative Medical Center for Liver Diseases, 302 Military Hospital, Beijing 100039, China
何首乌为蓼科植物何首乌(Polygonum multiflorum Thunb.)的块根, 是临床常用的传统无毒补益类中药, 但近年来肝损伤事件频发, 使其成为中药安全问题研究热点和难点[1-3]。
尽管何首乌肝损伤病例报道较多, 但考虑到服用何首乌人群巨大, 其发生率相对较低, 结合在正常动物安全性评价表现出的肝损伤不强的特点, 考虑何首乌可能为特异质肝损伤[4, 5]。已有大量文献报道证实, 部分药物与无毒剂量的脂多糖(lipopolysaccharide, LPS)共同作用, 可以产生类似临床特异质肝损伤反应, 而单独给予同等剂量药物则不产生肝损伤, 如曲伐沙星[6]、雷尼替丁[7]、双氯芬酸[8]等, 因此目前认为低剂量LPS介导的药物特异质肝损伤评价模型是研究特异质肝损伤的有效方法之一。
本课题组前期采用广泛认可的低剂量LPS诱导的药物特异质肝损伤评价模型分析了何首乌诱导的肝损伤, 研究发现单独给予低剂量的LPS (2.8 mg·kg-1)和单独给予何首乌(生药2.16 g·kg-1)均不会引起肝脏损伤, 而同等剂量LPS联合何首乌后则产生明显肝脏损伤, 这与其他药物导致的特异质肝损伤评价结果相一致, 也进一步阐明了何首乌肝损伤为免疫性特异质肝损伤[9-11]; 在LPS模型评价基础上, 课题组采用目标成分“敲入敲除”的活性物质筛选模式, 对何首乌肝损伤的物质基础进行了初步研究, 结果表明二苯乙烯类组分或为何首乌肝损伤的潜在物质, 同时应用基因芯片筛查发现PPAR-γ通路与顺式二苯乙烯苷诱导肝损伤存在显著相关性[12]。
PPAR-γ是核受体转录因子, 与多种配体结合可抑制单核巨噬细胞等免疫细胞活性, 进而抑制免疫炎症反应[13-15]。吡格列酮属噻唑烷二酮(thiazolidinediones, TZDs)类化合物, 为高选择性PPAR-γ激动剂, 众多研究发现吡格列酮亦可通过激活PPAR-γ降低多种因素诱导的免疫炎症反应[16-18]。尽管PPAR-γ与顺式二苯乙烯苷诱导的肝损伤存在显著相关性, 但PPAR-γ对何首乌免疫特异质肝损伤的影响及其作用机制不清, 为此本研究采用LPS诱导的免疫炎症特异质肝损伤模型, 探讨PPAR-γ依赖的何首乌免疫性特异质损伤机制。
材料与方法药品与试剂 何首乌, 批号13101701, 购于北京绿野药业有限公司, 经解放军第302医院肖小河研究员鉴定为蓼科植物何首乌(Polygonum multiflorum Thunb.)的干燥块根。LPS、戊巴比妥钠(Sigma公司); 盐酸吡格列酮口腔崩解片(康芝药业); IFN-γ、IL-6、IL-1β、TNF-α ELISA试剂盒(USCN), 丙氨酸转氨酶(ALT)、天冬氨酸转氨酶(AST)试剂盒(南京建成生物工程研究所); P65 (批号: 8242S)、二抗(批号: 7074S) (Cell Signaling Technology)。其他所用试剂均为分析纯。
仪器 SynergyH2全功能微孔板检测仪(美国BioTek); Leica 2016石蜡切片机(上海莱卡仪器有限公司); 爱华KPJ-1A生物组织摊片烤片机(天津天利航空有限公司); Nikon E200光学显微镜。
实验动物 健康雄性SD大鼠, SPF级, 6~8周龄, 180± 20 g, 购于中国人民解放军军事医学科学院实验动物中心, [合格证号SCXK-(军) 2012-0004]。分笼饲养于解放军第302医院动物实验中心, 自由进食及饮水, 室温(25 ± 2) ℃, 湿度60%~80%, 人工光照约12 h/12 h明暗周期, 并定期消毒。
样品制备 基于本课题组前期实验, 50%乙醇冷浸提取何首乌的肝细胞毒性最强, 故本实验采用50%乙醇冷浸制备样品[19]。称取适量生何首乌饮片, 加8倍量的50%乙醇冷浸提取, 共提取2次, 每次48 h, 合并提取液, 减压浓缩回收乙醇, 真空干燥得粗提物, 临用前用去离子水配制成相应浓度(按生药量配制)。
动物分组及给药 将70只SD大鼠随机均分为对照组、LPS组(2.8 mg·kg-1)、何首乌组(生药2.16 g·kg-1)、吡格列酮组(0.5 mg·kg-1)、吡格列酮+LPS组(0.5 mg·kg-1、2.8 mg·kg-1)、何首乌+LPS组(生药2.16 g·kg-1、2.8 mg·kg-1)及何首乌+LPS+吡格列酮组(生药2.16 g·kg-1、2.8 mg·kg-1、0.5 mg·kg-1)。吡格列酮组、吡格列酮+LPS组、何首乌+LPS+吡格列酮组灌胃给予吡格列酮, 每日1次, 连续给药2天[20], 第3天何首乌组、何首乌+LPS组、何首乌+LPS+吡格列酮组灌胃何首乌, 3 h后LPS组、吡格列酮+LPS组、何首乌+LPS组、何首乌+LPS+吡格列酮组给予尾静脉注射LPS[10]。
标本采集 大鼠尾静脉注射LPS 7 h后, 采用1%戊巴比妥钠腹腔注射麻醉, 下腔静脉取血, 3 500 r·min-1离心10 min, 取血浆, 并采集肝脏标本。
肝脏病理学检查 取大鼠肝组织1.5 cm × 1 cm × 0.5 cm, 10%甲醛固定, 常规病理切片, HE染色, 光镜下观察。
指标检测 采集所有大鼠血浆, 采用试剂盒(酶标仪法)检测肝功能指标ALT、AST; 酶联免疫吸附法测定炎症因子TNF-α、IL-1β、IL-6、IFN-γ的水平。
TUNEL标记法检测肝细胞凋亡 采用TUNEL检测分析肝细胞凋亡情况, 具体操作参照试剂盒说明书进行, 以细胞核固缩呈不规则形、被染成棕褐色或分解碎裂成数块为阳性细胞。随机观察3个视野(×200) 中阳性细胞数和总细胞数, 阳性细胞数占总细胞数的比例即为肝细胞凋亡率。
肝组织中PPAR-γ和NF-κB p65蛋白表达检测 石蜡切片常规脱蜡至水, 用高温进行抗原修复, 3%过氧化氢灭活内源性酶后, 5% BSA封闭20 min, 一抗4 ℃过夜孵育, 次日滴加偶联二抗, 室温30 min后DAB显色, 苏木精复染, 透明, 封片, 显微镜观察。阳性显棕黄(褐)色颗粒。各组均在显微镜200倍视野下随机拍摄3张图片, 用Image Pro Plus 6.0图像分析软件, 得到积分光密度值(integrated optical density, IOD)。
统计学分析 数据均以x± s表示, 釆用SPSS 17.0软件进行统计分析, 计量资料两组间比较采用t检验, 组间的比较均采用单因素方差分析(ANOVA), P < 0.05为差异有统计学意义。
结果 1 动物一般情况观察大鼠给予何首乌醇提物后, 1~3 h内出现轻微腹泻现象, 尿液颜色加深; 尾静脉注射LPS后, 出现排便增多现象, 且何首乌联合LPS组(PM+LPS)重于其他各组; 何首乌联合LPS组(PM+LPS)大鼠出现大便稀溏、臭味重等症状; 吡格列酮组(PIO)与对照组无异, 吡格列酮联合LPS组(PIO+LPS)、吡格列酮预防组(PM+LPS+PIO)大鼠排便量略有增加, 大便质地改变不明显, 尿液颜色趋于正常。
2 血浆转氨酶水平检测实验结果显示, 与对照组比较, LPS组、何首乌组(PM)、吡格列酮组(PIO)和吡格列酮联合LPS组(PIO+LPS)大鼠血浆ALT和AST活性均无显著变化(P > 0.05);而何首乌联合LPS组(PM+LPS)的ALT和AST水平显著升高(P < 0.05), 结果表明, 内毒素特异质肝损伤模型制备成功。与何首乌联合LPS组(PM+ LPS)相比, 吡格列酮预防组(PM+LPS+PIO) ALT和AST水平明显降低(P < 0.05), 提示吡格列酮对LPS联合何首乌致特异质肝损伤具有保护作用, 见图 1。
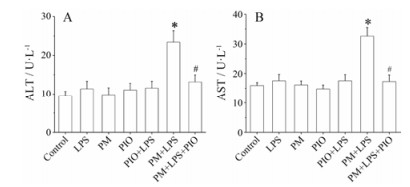
|
Figure 1 Detection of alanine transaminase (ALT, A) and aspartate aminotransferase (AST, B) in serum of rats in groups. LPS: Lipopolysaccharide; PM: Polygonum multiflorum; PIO: Pioglitazone. n = 10, x± s. *P < 0.05 vs LPS group; #P < 0.05 vs PM+LPS group |
HE染色结果显示, 与对照组(A)相比, PM组(C)、PIO组(D)及PIO+LPS组(E)无明显病理学改变; LPS组(B)有轻度的炎症细胞浸润, 未见肝细胞有明显病理学改变; PM+LPS组(F)可见肝细胞索排列紊乱, 中央静脉扩张、内膜脱落并伴有炎症细胞浸润。与PM+LPS组(F)相比, PM+LPS+PIO组(G)能减轻上述肝组织病理学损伤, 肝细胞结构完整、排列整齐。结果提示, 吡格列酮可明显改善LPS联合何首乌引起的肝组织损伤, 见图 2。
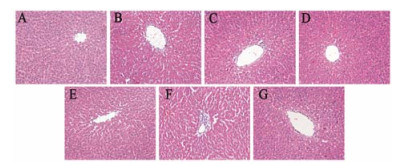
|
Figure 2 Effect of pioglitazone on histologic change of hepatic tissue of rats. A: Control group; B: LPS group; C: PM group; D: PIO group; E: PIO+LPS group; F: PM+LPS group; G: PM+LPS+PIO group. HE staining (×200) |
TUNEL检测结果显示, 对照组(A)、PM组(C)和PIO组(D)无明显肝细胞凋亡; 与LPS组(B)相比, PM+LPS组(F)肝细胞凋亡显著(P < 0.05); PM+ LPS+PIO组(G)凋亡的细胞较PM+LPS组(F)显著减少(P < 0.05), 其中LPS组(B)和PIO+LPS组(E)偶有凋亡细胞的分布, 提示吡格列酮可抑制LPS联合何首乌引起的肝细胞凋亡, 见图 3。
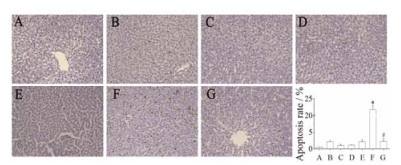
|
Figure 3 Effect of pioglitazone on hepatocytes apoptosis in liver tissue of PM+LPS induced liver injury rats detected with TUNEL staining (×200). A: Control group; B: LPS group; C: PM group; D: PIO group; E: LPS+PIO group; F: LPS+PM group; G: LPS+PM+PIO group. n = 3, x± s. *P < 0.05 vs LPS group; #P < 0.05 vs PM+LPS group |
ELISA法测定大鼠血浆中TNF-α、IL-1β、IL-6和IFN-γ水平, LPS组均高于对照组(均P < 0.05);与LPS组相比, PM+LPS组均显著升高(均P < 0.05);与PM+LPS组相比, 吡格列酮预防(PM+LPS+PIO)组均显著降低(均P < 0.05);而PM组、PIO组和PIO+ LPS组间均无明显差异(均P > 0.05)。结果提示, 吡格列酮可抑制LPS联合何首乌引起的免疫炎症反应, 见图 4。
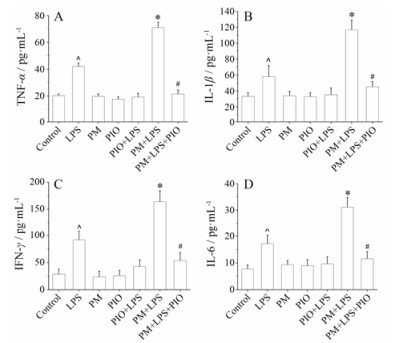
|
Figure 4 Effect of inflammatory cytokine on PM+LPS induced liver injury. TNF-α (A), IL-1β (B), IFN-γ (C) and IL-6 (D) secre tion was measured by ELISA. n = 10, x± s. ^P < 0.05 vs control group; *P < 0.05 vs LPS group; #P < 0.05 vs PM+LPS group |
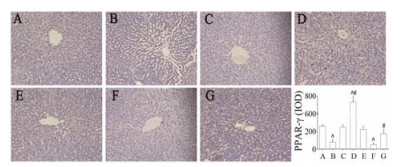
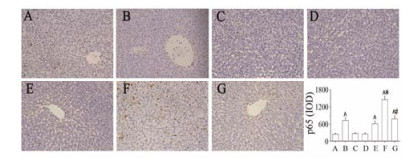
免疫组化检测结果显示, PM+LPS组(F)肝组织中PPAR-γ的表达显著低于对照组(A)及PM+LPS+PIO组(G), PIO组(D)较PM+LPS组(F) PPAR-γ的表达显著增加(P < 0.05);而PM+LPS组(F)肝组织中p65的表达显著高于LPS组(B) (P < 0.01), 吡格列酮预防组(PM+LPS+PIO)较PM+LPS组(F) p65的表达显著降低(P < 0.05), 此结果表明吡格列酮活化PPAR-γ, 并显著抑制p65的表达, 见图 5、图 6。
|
Figure 5 Effect of pioglitazone on the expression of PPAR-γ in liver tissue of rats detected with immunohistochemistry staining (×200). A: Control group; B: LPS group; C: PM group; D: PIO group; E: LPS+PIO group; F: LPS+PM group; G: LPS+PM+PIO group. n = 3, x± s. ^P < 0.05 vs control group; #P < 0.05 vs PM+LPS group |
|
Figure 6 Effect of pioglitazone on the expression of p65 in liver tissue of rats detected with immunohistochemistry staining (×200). A: Control group; B: LPS group; C: PM group; D: PIO group; E: LPS+PIO group; F: LPS+PM group; G: LPS+PM+PIO group. n = 3, x± s. ^P < 0.05 vs control group; *P < 0.05 vs LPS group; #P < 0.05 vs PM+LPS group |
何首乌是传统补益类中药, 但近年来肝损伤事件频发, 引起国内外药品监管部门和研究机构的高度重视。本课题组前期研究证实何首乌肝损伤为免疫性特异质肝损伤, 并阐明何首乌中顺式二苯乙烯苷是其主要的致肝损伤物质, 同时基于前期研究发现PPAR-γ通路与顺式二苯乙烯苷联合LPS诱导的肝损伤存在关联性。
PPAR-γ是一类配体依赖性的转录因子, 属于核受体超家族成员, 大量研究表明PPAR-γ激动剂在细胞增殖分化、凋亡、创伤愈合和炎症调控过程中发挥重要作用[21], PPAR-γ的高选择性激动剂吡格列酮可通过活化PPAR-γ调控NF-κB、信号转导和转录激活因子(signal transducer and activator of transcription, STAT)、活化蛋白-1 (activated protein-1, AP-1) 等信号分子或通路, 抑制免疫炎症相关基因的转录与表达和单核/巨噬细胞活化, 降低炎症细胞浸润和炎症因子释放而发挥作用[22]。
既往研究表明, PPAR-γ激动剂吡格列酮可影响免疫细胞活性, 抑制免疫炎症反应, 进而有效防治肝损伤[20, 22]。Enomoto等[23]研究证实吡格列酮通过抑制枯否细胞中TNF-α的释放而防治LPS诱导的肝损伤; Tomita等[24]研究表明吡格列酮通过上调c-Met的表达而防治非酒精性脂肪肝; Kon等[25]研究也证实吡格列酮可抑制HSC激活, 减少细胞外基质的生成, 延缓四氯化碳诱导大鼠肝纤维化的生成。作者课题组前期研究发现PPAR-γ与何首乌主要致肝损伤物质顺式二苯乙烯苷联合LPS诱导的肝损伤存在负相关, 但关于PPAR-γ对何首乌免疫性特异质肝损伤的作用及机制尚未见报道。因此, 本研究采用低剂量LPS介导的药物特异质肝损伤评价模型, 初步探讨何首乌免疫性特异质肝损伤的机制。
实验证实肝组织PPAR-γ表达量与何首乌免疫性特异质肝损伤呈负相关, 而给予PPAR-γ激动剂吡格列酮可以显著逆转PPAR-γ表达(P < 0.05), 同时显著抑制p65和TNF-α、IL-1β、IL-6等相关免疫炎症因子表达(P < 0.05), 降低肝损伤大鼠血浆中ALT和AST水平(P < 0.05), 减少大鼠肝组织炎症细胞浸润及肝细胞的凋亡, 实验结果初步揭示了PPAR-γ依赖的何首乌免疫性特异质肝损伤机制; 同时, 课题组前期研究证实, 何首乌配伍茯苓可以减毒[26]。因此, 本实验也提示, 可以从PPAR-γ激动剂的角度研究何首乌配伍减毒的可能中药及其减毒机制。
| [1] | Wang T, Wang JY, Jiang ZZ, et al. Study on hepatotoxicity of aqueous extracts of Polygonum multiflorum in rats after 28 play oral administration-analysis on correlation of cholestasis[J]. China J Chin Mater Med(中国中药杂志), 2012, 37: 1445–1450. |
| [2] | Mao HM, Xie LH, Fan X, et al. Effect of ethanol extracts from Polygonum multiflorum Thunb on expressions of signal pathway TLR4/TRIF/IRF-3 in LPS induced rats liver[J]. J Int Pharm Res(国际药学研究杂志), 2016, 43: 496–503. |
| [3] | Lin L, Lin H, Zhang M, et al. A novel method to analyze hepatotoxic components in Polygonum multiflorum using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry[J]. J Hazard Mater, 2015, 299: 249–259. DOI:10.1016/j.jhazmat.2015.06.014 |
| [4] | Chen SJ. Research progress on toxicity and its mechanism of adverse reaction information analysis of Polygonum multiflorum liver injury[J]. Chin J Hosp Pharm(中国医院药学杂志), 2013, 33: 573–577. |
| [5] | Yan LC, Zhao JN, Qiu X. Research progress on security issues of Polygonum multiflorum[J]. Pharmacol Clin Chin Mater Med(中药药理与临床), 2009, 25: 77–80. |
| [6] | Waring JF, Liguori MJ, Luyendyk JP, et al. Microarray analysis of lipopolysaccharide potentiation of trovafloxacin induced liver injury in rats suggests a role for proinflammatory chemokines and neutrophils[J]. J Pharmacol Exp Ther, 2006, 16: 1080–1087. |
| [7] | Luyendyk JP, Lehman-McKeeman LD, Nelson DM, et al. Coagulation-dependent gene expression and liver injury in rats given lipopolysaccharide with ranitidine but not with famotidine[J]. J Pharmacol Exp Ther, 2006, 317: 635–643. DOI:10.1124/jpet.105.096305 |
| [8] | Deng X, Stachlewitz RF, Liguori MJ, et al. Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation[J]. J Pharmacol Exp Ther, 2006, 319: 1191–1199. DOI:10.1124/jpet.106.110247 |
| [9] | Zhu Y, Liu SH, Wang JB, et al. Clinical analysis of drug-induced liver injury caused by Polygonum multiflorum and its preparations[J]. Chin J Integr Tradit West Med(中国中西医结合杂志), 2015, 35: 1442–1447. |
| [10] | Li CY, Li XF, Tu C, et al. The idiosyncratic hepatotoxicity of Polygonum multiflorum based on endotoxin model[J]. Acta Pharm Sin(药学学报), 2015, 50: 28–33. |
| [11] | Li XF, Li N, Tu C, et al. Comparison of crude and prepared Polygonum multiflorum-induced idiosyncratic hepatotoxicity based on lipopolysaccharide model[J]. Chin Tradit Herb Drugs(中草药), 2015, 46: 1481–1486. |
| [12] | Li CY. The Primary Research on Immunological Stress-mediated Idiosyncratic Liver Injury of Polygonum multiflorum(基于免疫应激的何首乌特异质肝损伤的初步研究)[D]. Chengdu: Chengdu University of Traditional Chinese Medicine, 2015. http://d.g.wanfangdata.com.cn/Thesis_D829769.aspx |
| [13] | Ricote M, Li AC, Willson TM, et al. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation[J]. Nature, 1998, 391: 79–82. DOI:10.1038/34178 |
| [14] | De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor κB[J]. Oncogene, 2006, 25: 6868–6886. DOI:10.1038/sj.onc.1209935 |
| [15] | Heikkinen S, Auwerx J, Argmann CA. PPARγ in human and mouse physiology[J]. Biochim Biophys Acta, 2007, 1771: 999–1013. DOI:10.1016/j.bbalip.2007.03.006 |
| [16] | Gupta G, Krishna G, Chellappan DK, et al. Protective effect of pioglitazone, a PPARγ agonist against acetaminophen-induced hepatotoxicity in rats[J]. Mol Cell Biochem, 2014, 393: 223–228. DOI:10.1007/s11010-014-2064-9 |
| [17] | Ohata M, Suzuki H, Sakamoto K, et al. Pioglitazone prevents acute liver injury induced by ethanol and lipopolysaccharide through the suppression of tumor necrosis factor-α[J]. Alcohol: Clin Exp Res, 2004, 28: 139S–144S. |
| [18] | Abd El-Haleim EA, Bahgat AK, Saleh S. Effects of combined PPAR-γ and PPAR-α agonist therapy on fructose induced NASH in rats: modulation of gene expression[J]. Eur J Pharmacol, 2016, 773: 59–70. DOI:10.1016/j.ejphar.2016.01.011 |
| [19] | Lv Y, Wang JB, Ji Y, et al. Influence of extracting solvent on hepatocytes toxicity of Polygonum multiflorum[J]. Chin J Exp Tradit Med Form(中国实验方剂学杂志), 2013, 19: 268–272. |
| [20] | Enomoto N, Takei Y, Hirose M, et al. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-γ, pioglitazone[J]. Pharmacol Exp Ther, 2003, 306: 846–854. DOI:10.1124/jpet.102.047217 |
| [21] | Gelman L, Michalik L, Desvergne B, et al. Kinase signaling cascades that modulate peroxisome proliferator activated receptors[J]. Curr Opin Cell Biol, 2005, 17: 216–222. DOI:10.1016/j.ceb.2005.02.002 |
| [22] | Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPARγ ligands in ischemia-reperfusion injury, inflammation and shock[J]. Cardiovasc Res, 2005, 65: 772–781. DOI:10.1016/j.cardiores.2004.12.008 |
| [23] | Enomoto N, Takei Y, Yamashima S, et al. Protective effect of pioglitazone against endotoxin-induced liver injury through prevention of Kupffer cell sensitization[J]. Alcohol: Clin Exp Res, 2005, 29: 216S–219S. DOI:10.1097/01.alc.0000192394.26573.10 |
| [24] | Tomita K, Azuma T, Kitamura N, et al. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met[J]. Gastroenterology, 2004, 126: 873–885. DOI:10.1053/j.gastro.2003.12.008 |
| [25] | Kon K, Ikejima K, Hirose M, et al. Pioglitazone prevents early-phase hepatic fibrogenesis caused by carbon tetrachloride[J]. Biochem Biophys Res Commun, 2002, 291: 55–61. DOI:10.1006/bbrc.2002.6385 |
| [26] | Gao D, Pang JY, Zhang CE, et al. Poria attenuates idiosyncratic liver injury induced by Polygoni Multiflori Radix Praeparata[J]. Front Pharmacol, 2016, 7: 386. |
 2017, Vol. 52
2017, Vol. 52


