糖尿病(diabetes mellitus)是以慢性高糖血症为特征、由于胰岛β细胞分泌胰岛素绝对或相对不足而产生的代谢性疾病。2011年全世界约3.66亿糖尿病患者, 预计到2030年将达到5.52亿[1]; 我国成年人患病率为9.7%, 糖尿病前期的患病率更高达15.5%[2]。其中, 90%以上患者为2型糖尿病。血糖控制是治疗2型糖尿病、延缓并发症进程的关键。长期慢性高血糖导致并发症发生, 引起大、小血管及周围神经病变, 造成心、脑、肾和眼等器官的损伤[3]。然而, 仍约有一半糖尿病患者的血糖未达到理想水平。开发新型的降糖药物一直是抗糖尿病药物研发的热点。
近年来钠-葡萄糖协同转运蛋白2 (sodium-glucose cotransporter 2, SGLT2) 被认为是降糖药物开发的热门靶点之一, 其SGLT2抑制剂(SGLT2 inhibitor, SGLT2i)通过减少肾脏对葡萄糖的重吸收, 促进尿糖排出从而降低血糖。与其他糖尿病治疗药物相比, SGLT2i独特的非胰岛素依赖的作用方式使其不受患者β细胞功能以及胰岛素敏感性的影响。同时, SGLT2i还具有减轻体重, 降低内脏脂肪量, 降低血压和降低血尿酸等优势[4-6]。值得注意的是, 对于SGLT2i上市后研究发现糖尿病患者服用该类药物后心血管系统获益[7, 8]。2017年美国糖尿病学会诊疗指南里明确提出, 对于血糖控制不佳且患有动脉粥样硬化心血管疾病的患者推荐使用SGLT2i。
选择性对SGLT2i研发是一个重要问题。肾脏每天滤过约180 g葡萄糖, 大部分被SGLTs重吸收[9]。其中, SGLT2是一种低亲和力、高容量的转运体, 主要分布于肾脏近曲小管S1、S2段, 负责大于90%的肾脏葡萄糖重新收[10, 11], 其余由钠-葡萄糖协同转运蛋白1 (sodium-glucose cotransporter 1, SGLT1) 完成。SGLT1是一种高亲和力、低转运能力的转运体, 在肾脏分布于近曲小管较远的S3段。此外, SGLT1分布于小肠刷状缘, 对葡萄糖的吸收具有重要作用, 抑制该转运体会发生严重腹泻等胃肠道不良反应[5, 11]。因此, 需要开发对SGLT2抑制活性较高, 而对SGLT1抑制活性较低的选择性抑制剂。根皮苷(phloridzin)是SGLT1和SGLT2的双重抑制剂, 其选择性差和口服吸收差不能被药用[12]。目前国际上已上市的依帕列净(empagliflozin)是选择性最好的SGLT2i ( > 2 500:1), 其次是达格列净(dapagliflozin, > 1 200:1) 和卡格列净(canagliflozin, > 250:1)[13]。
目前SGLT2i研发通常采用同位素标记的葡萄糖类似物作为底物, 其试剂成本高, 易污染环境; 荧光标记的2-脱氧葡萄糖(2-[N-(7-nitrobenz-2-oxa-1, 3-diazol-4-yl)amino]-2-deoxy-D-glucose, 2-NBDG)转运效率较低, 且不稳定。本研究的目的是建立SGLT2的选择性抑制剂筛选方法。在本实验室前期工作的基础上[14], 构建了人SGLT1、SGLT2稳定过表达细胞系, 采用荧光标记1-脱氧葡萄糖(1-NBDG)作为示踪剂对其功能进行检测。同时, 在整体水平建立SGLT2抑制剂药效学评价方法。
材料与方法药品与试剂 1-NBDG由药明康德新药开发有限公司合成(ET4099-7-P1);根皮苷(phloridzin dihydrate, P3449)、氯化胆碱(choline chloride)购于Sigma公司; 达格列净由芷威(上海)化学科技有限公司(APIchem)合成。其他化学试剂购自国药集团化学试剂北京有限公司。
真核表达载体 pMSCVpuro (Clontech公司), TransT1感受态细胞(Transgen Biotech公司)核酸内切酶EcoRⅠ/XhoⅠ、T4 DNA连接酶(Fermentas公司); 琼脂糖(Sigma公司), DNA Marker (Transgen Biotech公司); 质粒提取试剂盒、DNA转染试剂(Qiagen公司); BCA蛋白检测试剂盒(Thermo公司)。Cocktail蛋白酶抑制剂(Roche公司); anti-SGLT1 (Abcam公司)、anti-SGLT2、anti-GAPDH (Santa Cruz公司); HRP/FITC标记的二抗(中杉金桥公司); 化学发光检测试剂(Tanon公司)。其他试剂为进口或国产分析纯试剂。
仪器 荧光酶标仪(Enspire), 激光共聚焦扫描显微镜(Bio-Rad Radiance 2100 CLSM), 凝胶成像分析系统(Tanon-4100), CO2培养箱(Sanyo MCO-15AC)。
细胞培养 HEK293 (人胚胎肾上皮细胞)购于中国医学科学院基础医学研究所细胞资源中心; 高糖DMEM、低糖DMEM培养基、胎牛血清(FBS)均购自GIBCO公司。细胞培养于高糖DMEM培养液中(含10%胎牛血清、50 u·mL-1青霉素及50 μg·mL-1链霉素), 培养条件为37 ℃、5% CO2、湿度95%。
SGLT1/SGLT2稳定过表达细胞构建 用于过表达的逆转录病毒载体为pMSCVpuro。设计引物通过PCR钓取人全长SGLT1 cDNA (Forward: CCGC TCGAGGCCACCATGGACAGTAGCACCTGGAGC; Reverse: CCGGAATTCTCAGGCAAAATATGCATG GCAAAAG)。人全长SGLT2 cDNA (NCBI Reference Seqμence: NM_003041.3) 由Invitrogen (上海)公司合成。将目的基因连接至pMSCVpuro载体并转化至感受态细胞TransT1。进行EcoRⅠ、XhoⅠ双酶切鉴定, 并测序验证重组克隆中基因序列。进行稳定过表达SGLT1/SGLT2的HEK293细胞株的筛选:将测序正确的质粒转染HEK293细胞, 嘌呤霉素筛选阳性克隆细胞株, 挑选单克隆扩增培养后, 检测目的蛋白SGLT1/SGLT2的表达。
免疫印迹检测 提取细胞总蛋白。用BCA法测定样品蛋白的浓度。制备10%凝胶, 上样量为10 μg, 电泳(80~120 V, 60~90 min), 转膜(320 mA, 60 min)。2% BSA中室温封闭1 h, 加入一抗SGLT1 (1:1 000)、SGLT2 (1:1 000)、GAPDH (1:2 000), 4 ℃孵育过夜, TBST洗膜后加入二抗(1:5 000), 室温封闭2 h, 显影液孵育, 凝胶成像分析系统进行显影。
免疫荧光染色 细胞爬片后, 4 ℃ PBS冲洗2遍, 用4 ℃、2%多聚甲醛固定30 min, 加入PBS冲洗2次。加入甲醇置于-20 ℃脱水15 min, 4 ℃ PBS中复水。用封闭液(2% FBS)进行非特异性位点封闭1 h后, 加入含有一抗(anti-SGLT2 antibody, 1:50) 的封闭液, 4 ℃孵育1 h, PBS冲洗3次。加入含有二抗(FITC-anti-goat antibody, 1:200) 的封闭液, 4 ℃孵育1 h, PBS冲洗3次。10 μg·mL-1碘化吡啶对细胞核进行染色15 min, PBS冲洗3次。封片液(甘油:PBS = 1:9) 封片后, 激光共聚焦扫描显微镜进行观察。
Na+依赖性葡萄糖转运实验 24孔板用多聚赖氨酸预涂, 干燥备用。细胞铺板, 90%融合后, 低糖无血清DMEM培养基处理2 h, Na+-free/Na+ buffer洗一遍, 加入含有1-NBDG (100 μmol·L-1)的摄取液进行葡萄糖摄取。其中, Na+依赖性摄取缓冲液(Na+ buffer)含120 mmol·L-1 NaCl, 4.7 mmol·L-1 KCl, 1.2 mmol·L-1 MgCl2, 2.2 mmol·L-1 CaCl2, 10 mmol·L-1 Hepes, pH 7.4。非特异性摄取缓冲液(Na+-free buffer)含140 mmol·L-1氯化胆碱, 4.7 mmol·L-1 KCl, 1.2 mmol·L-1 MgCl2, 2.2 mmol·L-1 CaCl2, 10 mmol·L-1 Hepes, pH 7.4。37 ℃摄取4 h; 加入预冷的终止液(含有0.5 mmol·L-1根皮苷的摄取缓冲液); 荧光显微镜下观察细胞内荧光强度。0.1 mol·L-1 NaOH裂解细胞, 转移至96孔透明底黑板中, 采用荧光酶标仪检测细胞内1-NBDG的含量(Ex/Em: 485/535 nm)。BCA法测裂解液蛋白浓度, 结果表示为荧光强度/蛋白含量。
动物实验 雄性ICR小鼠(22~24 g)购自北京维通利华实验动物技术有限公司。动物合格证号: SCXK (京) 2012-0001。
正常小鼠实验 将动物随机分为3组, 每组8只:正常血糖组(NG)、dapagliflozin低剂量组(0.25 mg·kg-1)、和高剂量组(4 mg·kg-1)。动物禁食后给药, 进行口服葡萄糖耐量实验(oral glucose tolerance test, OGTT)。取0 min点血作为初始血糖, 灌胃给予2 g·kg-1葡萄糖, 分别监测30、60和120 min时动物血糖的变化, 并计算血糖-时间曲线下面积。
高糖小鼠实验 尾静脉注射四氧嘧啶(69 mg·kg-1); 选择72 h后血糖 > 180 mg·dL-1小鼠作为1型糖尿病(T1DM)模型动物。将模型动物按血糖水平随机分为5组, 每组9只:模型组(HG)、二甲双胍组(200 mg·kg-1)、和dapagliflozin低剂量组(0.25 mg·kg-1)、中剂量组(1 mg·kg-1)、高剂量组(4 mg·kg-1)。单次给药实验, 于灌胃后监测0~24 h血糖变化。多次给药实验, 每天于17:00灌胃给药前监测血糖。
统计学分析 实验数据以x±s表示, 多组间比较采用one-way ANOVA (Prism 5.0), 两组间比较采用Student’s t-test。P < 0.05被认为存在统计学差异。
结果 1 SGLT1稳定过表达细胞构建分别采用RT-PCR、Western blot对稳定过表达HEK293细胞中SGLT1表达进行检测, 结果如图 1所示, 与pMSCVpuro-null转染组相比, pMSCVpuro-SGLT1转染组SGLT1基因及蛋白水平均显著升高, 说明SGLT1稳定过表达HEK293细胞构建成功。
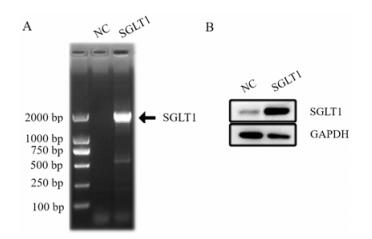
|
Figure 1 Expression of sodium-glucose cotransporter 1 (SGLT1) in stably transfected HEK293 cells. A: RT-PCR analysis of HEK293 cells stably transfected with pMSCVpuro-SGLT1; B: Western blot analysis for human SGLT1. Band represents ~70 kDa |
双酶切鉴定结果如图 2A所示, 第一泳道为pMSCVpuro空载体; 第二泳道质粒的电泳迁移率明显低于pMSCVpuro空载体, 说明其分子质量大于空载体; 第三泳道为pMSCVpuro空载体经Xho I和EcoR I双酶切; 第四泳道为载体经Xho I和EcoR I双酶切后, 在2 000 bp出现明显的条带, 说明目的基因SGLT2已经插入载体。
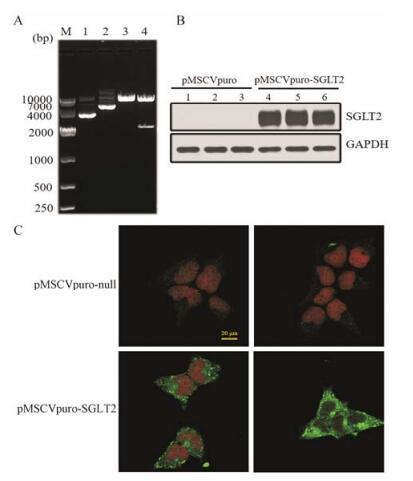
|
Figure 2 Expression of sodium-glucose cotransporter 2 (SGLT2) in stably transfected HEK293 cells. A: SGLT2 expression plasmid digested by restriction enzyme EcoRⅠ and XhoⅠ, and frag ments produced in 0.8% agarose gel electrophoresis. Lane 1: pMSCVpuro; Lane 2: pMSCVpuro-SGLT2; Lane 3: pMSCVpuro digested by EcoRⅠ and XhoⅠ; Lane 4: pMSCVpuro-SGLT2 digested by EcoRⅠ and XhoⅠ. M: DNA marker. B: Western blotting analysis for human SGLT2 in the HEK293 cells stable transfected with pMSCVpuro-SGLT2 compared to pMSCVpuro-null transfected cells. Band represents ~70 kDa. C: Immuno fluorescence staining of HEK293 cells stably transfected with pMSCVpuro-null (upper panels) and pMSCVpuro-SGLT2 (lower panels) using SGLT2 antibody. Cells were grown on poly-L-lysine-coated coverslips for 24 h followed by fixation and im munocytochemistry analysis using CLSM. Green: FITC-tagged hSGLT2; Red: PI counterstain of the nucleus. Magnification, 1 000× |
Western blot检测稳转HEK293细胞中SGLT2蛋白表达情况。与pMSCVpuro-null转染组相比, pMSCVpuro-SGLT2转染组SGLT2蛋白高表达(图 2B); 细胞免疫荧光染色结果显示SGLT2蛋白表达于细胞膜及细胞质(图 2C), 说明SGLT2稳定过表达HEK293细胞构建成功。
3 Na+依赖性葡萄糖转运实验荧光显微镜下观察HEK293细胞对葡萄糖的摄取。如图 3A所示, 与pMSCVpuro-null转染组相比, pMSCVpuro-SGLT1、pMSCVpuro-SGLT2转染组细胞Na+依赖性1-NBDG摄取明显增高。
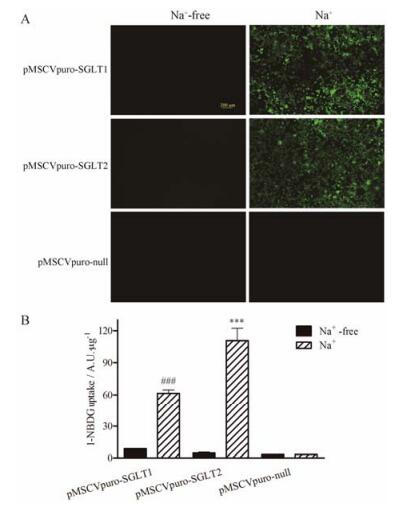
|
Figure 3 Sodium-dependent glucose uptake via SGLT1/SGLT2. A: Fluorescence images of 1-[N-(7-nitrobenz-2-oxa-1, 3-diazol-4-yl)amino]-1-deoxy-D-glucose (1-NBDG) uptake in HEK293 cells (exposure time: 5 s, objective lens: 10×). Na+ dependent 1-NBDG uptake was significantly enhanced in pMSCVpuro-SGLT1/2 transfected cells. B: Results of transport studies. SGLT transport was assessed by 1-NBDG. n = 3, x±s. ###P < 0.001 vs Na+-free group in pMSCVpuro-SGLT1 transfected cells; ***P < 0.001 vs Na+-free group in pMSCVpuro-SGLT2 transfected cells |
采用荧光酶标仪检测细胞裂解液的荧光强度, 如图 3B所示, SGLT1过表达细胞Na+依赖性1-NBDG摄取显著升高, 为非特异性摄取的6.1倍; SGLT2过表达细胞Na+依赖性摄取为非特异性摄取的23.1倍; pMSCVpuro-null转染组Na+依赖性摄取与非特异性摄取相比未有变化。
4 选择性实验阳性药达格列净对SGLT1的半数抑制浓度(IC50)为6.20×10-7 mol·L-1, 远高于对SGLT2的IC50 (2.24×10-10 mol·L-1) (图 4A, B), 说明其对SGLT2的选择性较好。根皮苷对SGLT1和SGLT2抑制的IC50浓度相近, 分别为9.24×10-8和1.76×10-8 mol·L-1 (图 4C, D), 说明其对SGLT2的选择性较差。

|
Figure 4 Selectivity of dapagliflozin (dapa) and phloridzin. A, B: Na+-dependent 1-NBDG uptake was measured in HEK293 cells stably expressing SGLT1 and SGLT2 in presence of various concentrations of dapa. C, D: Na+-dependent 1-NBDG uptake was measured in HEK293 cells stably expressing SGLT1 and SGLT2 in presence of various concentrations of phloridzin. n = 3, x±s |
一次口服给予达格列净能够使正常血糖小鼠(NG) OGTT实验中血糖上升幅度降低(图 5A), 其中, 高剂量组(4 mg·kg-1)能够显著降低其AUC值(图 5B)。对于T1DM小鼠(HG), 达格列净低、中、高剂量组从口服给药后1 h血糖水平即显著降低, 药效维持24 h (图 5C); 能够剂量依赖地降低0~24 h的AUC (图 5D)。每天给药连续20天, 与HG组相比, 达格列净低、中、高剂量组给药24 h后血糖水平均明显降低, 药效优于二甲双胍(图 5E)。
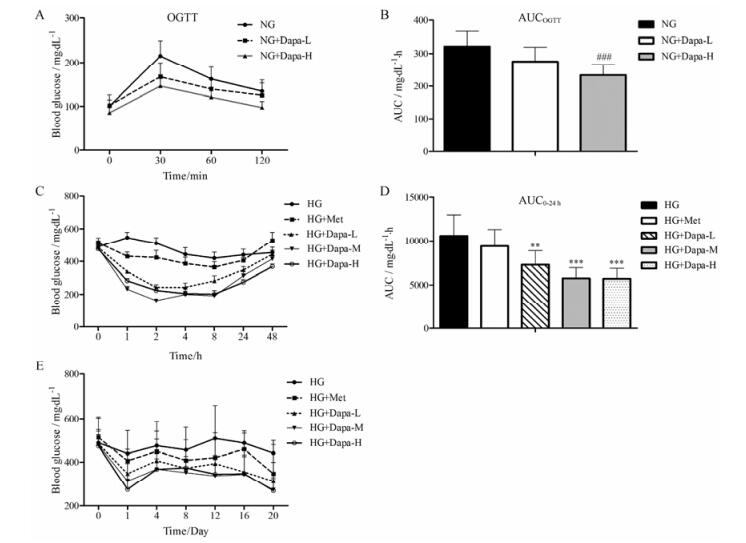
|
Figure 5 Hypoglycemic effects of dapa in mice. A, B: Hypoglycemic effects of dapa in mice with normal glucose levels (NG). Animals were treated with dapa at 2 dosages (0.25, 4 mg·kg-1 for low (L), high (H) dose groups) and then oral glucose tolerance test (OGTT) was performed. A: Changes of blood glucose after the glucose loading; B: Area under the curve in OGTT. C-E: Hypoglycemic effects of dapa in T1DM mice (HG). Animals were daily treated with dapa at 3 dosages (0.25, 1, 4 mg·kg-1 for low (L), middle (M), high (H) dose group) or metformin (Met, 200 mg·kg-1). C: Changes of blood glucose 0-48 h after a single administration; D: Area under the curve for 0-24 h after a single administration; E: Changes of blood glucose during a 20-day administration study. n = 8-9, x±s. ###P < 0.001 vs NG; **P < 0.01, ***P < 0.001 vs HG |
选择性是评价SGLT2i类药物疗效及安全性的重要标准。SGLT2主要表达于肾脏, 而SGLT1除了肾脏, 还表达于肠、心脏、肝和肺中, 对小肠中葡萄糖和半乳糖的重吸收具有重要作用[15]。抑制SGLT1可导致腹泻和严重脱水, 即“葡萄糖-半乳糖吸收不良”[16], 而对其他组织SGLT1的长期抑制风险尚未得到确切的评估。因此, 抑制剂对SGLT2的选择性超过SGLT1显得尤为重要。达格列净是于2012年和2014年分别在欧洲和美国获批, 用于治疗2型糖尿病的SGLT2选择性抑制剂[17]。本研究建立了SGLT2i选择性评价方法, 分别将人SGLT1、SGLT2稳定过表达于HEK293细胞中(图 1, 2), 稳转细胞具有良好的葡萄糖转运功能, 其Na+依赖的葡萄糖摄取远高于非特异性摄取(图 3)。应用该方法进行选择性评价, 发现达格列净对SGLT1抑制的IC50远高于SGLT2 (SGLT1i:SGLT2i > 1:2 500) (图 4A, B), 优于选择性较差的根皮苷(图 4C, D), 与文献报道一致[13]。
目前, 国内外常采用14C或者3H标记的葡萄糖类似物进行葡萄糖转运实验[13, 18, 19]。本方法采用荧光标记避免了环境污染等问题, 优于同位素标记的SGLT2筛选方法。构建的稳转细胞系所表达的转运体具有较高的葡萄糖转运活性, 测得达格列净对SGLT1/SGLT2的IC50 (6.2×10-7 mol·L-1/2×10-10 mol·L-1)均低于文献[13]中以[14C]-AMG为底物测得的IC50 (1.4×10-6 mol·L-1/1.2×10-9 mol·L-1); 测得根皮苷对SGLT1/SGLT2的IC50分别为9.24×10-8 mol·L-1/1.76×10-8 mol·L-1, 低于文献[20]中测得的5.5×10-5 mol·L-1/ 4.0×10-5mol·L-1。鉴于1-NBDG比2-NBDG具有更加稳定的化学性质, 不易被细胞代谢[21], 作为荧光示踪剂1-NBDG优于2-NBDG。与以往采用2-NBDG的方法[14, 22]相比, 本方法采用1-NBDG进行葡萄糖摄取的转运效率更高。最后, 本方法采用了HEK293 (人胚胎肾细胞)构建的稳定转染细胞模型更加接近SGLT2的生理状态。
此外, 本研究建立了整体水平的SGLTi药效评价方法。对阳性药达格列净进行降糖作用评价, 发现其在正常小鼠中能够改善口服葡萄糖耐量; 在高糖小鼠中, 给药后1 h即可降低餐后血糖, 与文献报道一致[23]; 动态监测血糖变化, 发现降糖作用持续24 h。在20天的给药过程中, 该药能够平稳降糖, 其效果优于二甲双胍(图 5)。
本研究构建了SGLT1/SGLT2稳定过表达细胞模型, 采用新的、较稳定的荧光脱氧葡萄糖1-NBDG作为示踪剂, 建立完善的SGLT2选择性抑制剂的体外筛选方法, 具有无同位素污染、高灵敏度、高选择性和良好稳定性等特点; 并在整体水平完善了SGLT2i药效学评价方法。该技术体系将为新的SGLT2高选择性抑制剂研发奠定实验基础。
| [1] | Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030[J]. Diabetes Res Clin Pract, 2011, 94: 311–321. DOI:10.1016/j.diabres.2011.10.029 |
| [2] | Yang WY, Lu JM, Weng JP, et al. Prevalence of diabetes among men and women in China[J]. N Engl J Med, 2010, 362: 1090–1101. DOI:10.1056/NEJMoa0908292 |
| [3] | Aronson D. Hyperglycemia and the pathobiology of diabetic complications[J]. Adv Cardiol, 2008, 45: 1–16. DOI:10.1159/000115118 |
| [4] | Jabbour SA. SGLT2 inhibitors to control glycemia in type 2 diabetes mellitus: a new approach to an old problem[J]. Postgrad Med, 2014, 126: 111–117. |
| [5] | Dardi I, Kouvatsos T, Jabbour SA. SGLT2 inhibitors[J]. Biochem Pharmacol, 2016, 101: 27–39. DOI:10.1016/j.bcp.2015.09.005 |
| [6] | Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium-dependent glucose co-transporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus[J]. J Diabetes Investig, 2017. DOI:10.1111/jdi.12644 |
| [7] | Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes[J]. N Engl J Med, 2015, 373: 2117–2128. DOI:10.1056/NEJMoa1504720 |
| [8] | Zaccardi F, Webb DR, Htike ZZ, et al. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis[J]. Diabetes Obes Metab, 2016, 18: 783–794. DOI:10.1111/dom.2016.18.issue-8 |
| [9] | Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications[J]. Diabet Med, 2010, 27: 136–142. DOI:10.1111/dme.2010.27.issue-2 |
| [10] | Jabbour SA, Goldstein BJ. Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes[J]. Int J Clin Pract, 2008, 62: 1279–1284. DOI:10.1111/j.1742-1241.2008.01829.x |
| [11] | Neumiller JJ, White JR Jr, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus[J]. Drugs, 2010, 70: 377–385. DOI:10.2165/11318680-000000000-00000 |
| [12] | Toggenburger G, Kessler M, Semenza G. Phlorizin as a probe of the small-intestinal Na+, D-glucose cotransporter. A model[J]. Biochim Biophys Acta, 1982, 688: 557–571. DOI:10.1016/0005-2736(82)90367-4 |
| [13] | Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors[J]. Diabetes Obes Metab, 2012, 14: 83–90. DOI:10.1111/dom.2012.14.issue-1 |
| [14] | Zhang XL, Yang XM, Li J, et al. A new fluorescence method for determination of sodium-glucose cotransporter 2 activity[J]. J Peking Univ (Health Sci) (北京大学学报医学版), 2012, 44: 725–731. |
| [15] | Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease[J]. J Intern Med, 2007, 261: 32–43. DOI:10.1111/jim.2007.261.issue-1 |
| [16] | Turk E, Zabel B, Mundlos S, et al. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotrans porter[J]. Nature, 1991, 350: 354–356. DOI:10.1038/350354a0 |
| [17] | Vivian EM. Dapagliflozin: a new sodium-glucose cotrans porter 2 inhibitor for treatment of type 2 diabetes[J]. Am J Health Syst Pharm, 2015, 72: 361–372. DOI:10.2146/ajhp140168 |
| [18] | Dienel GA, Cruz NF, Sokoloff L, et al. Determination of glucose utilization rates in cultured astrocytes and neurons with [14C] deoxyglucose: progress, pitfalls, and discovery of intracellular glucose compartmentation[J]. Neurochem Res, 2017, 42: 50–63. DOI:10.1007/s11064-015-1650-x |
| [19] | Jiang Z, Yu B, Li Y, et al. Effect of three statins on glucose uptake of cardiomyocytes and its mechanism[J]. Med Sci Monit, 2016, 22: 2825–2830. DOI:10.12659/MSM.897047 |
| [20] | Castaneda F, Kinne RK. A 96-well automated method to study inhibitors of human sodium-dependent D-glucose trans port[J]. Mol Cell Biochem, 2005, 280: 91–98. DOI:10.1007/s11010-005-8235-y |
| [21] | Yamada K, Saito M, Matsuoka H, et al. A real-time method of imaging glucose uptake in single, living mammalian cells[J]. Nat Protoc, 2007, 2: 753–762. DOI:10.1038/nprot.2007.76 |
| [22] | Kanwal A, Singh SP, Grover P, et al. Development of a cell-based nonradioactive glucose uptake assay system for SGLT1 and SGLT2[J]. Anal Biochem, 2012, 429: 70–75. DOI:10.1016/j.ab.2012.07.003 |
| [23] | Meng W, Ellsworth BA, Nirschl AA, et al. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes[J]. J Med Chem, 2008, 51: 1145–1149. DOI:10.1021/jm701272q |
 2017, Vol. 52
2017, Vol. 52


