二甲双胍是双胍类药物, 为2型糖尿病临床一线用药[1], 对于改善糖代谢和胰岛素抵抗[2]等有重要作用, 且可以保护心血管[3], 是目前唯一被糖尿病指南推荐为有明确心血管保护作用的降糖药物[4]。此外, 二甲双胍临床治疗及预防价值不断被发现, 如用于多囊卵巢综合征[5]、肥胖、非酒精性脂肪性肝病[6], 且有抗癌[7]、抗衰老[8]等疗效。二甲双胍具有显著个体差异性, 大约30%的患者在服用正常剂量的二甲双胍时会出现较为明显的胃肠道反应, 少数患者还会发生更为严重的乳酸性酸中毒[9]。
二甲双胍多采用口服给药, 生物利用率为55% ± 16%, 主要通过小肠吸收。其进入人体后几乎不与血红蛋白结合, 不易被代谢, 主要以原形(79%)通过尿液排泄, 约20%可以在粪便中检测到[10], 极小部分(0.11% ± 0.02%)通过胆汁排泄[11]。
药物转运体在药物药代动力学过程中起到了重要作用, 与药物疗效、不良反应以及毒性等密切相关[12], 其功能的变化直接影响药物的吸收、分布、代谢和排泄[13]。二甲双胍的体内吸收、分布、排泄过程均有药物转运体的参与[14]。本文对基于药物转运体机制的二甲双胍体内过程的研究进展进行综述。
1 有机阳离子转运体有机阳离子转运体(organic cation transporters, OCTs)属于SLC22基因家族, 其底物以亲水性阳离子小分子化合物为主, 在人体肝脏、肾脏和肠等器官均有表达[15]。
OCTs在二甲双胍的吸收、组织分布过程扮演重要角色。① 在小肠部位, 由分布于肠细胞顶膜的OCT3负责将二甲双胍转运进入肠细胞; 而肠细胞基底侧膜的OCT1负责二甲双胍进一步的转运[16, 17]; 另有研究发现OCT1在肠细胞的顶膜侧也有分布[18]。Jensen等[19]通过实验发现OCT1敲除小鼠的小肠浓度显著降低; Shirasaka等[20]的实验结果显示, 正常小鼠的绝对生物利用度为46.8%, OCT3敲除小鼠绝对生物利用度显著下降至32.6%。② 二甲双胍在肝脏的吸收主要通过位于肝细胞窦状隙膜(基底侧膜)的OCT1将其从血液中转运进入肝脏, OCT3也有少量贡献[21]。在OCT1敲除小鼠体内实验中, 正常小鼠的二甲双胍肝浓度是OCT1敲除小鼠的30倍左右, 验证了OCT1在肝脏摄取二甲双胍过程的重要性[22]。细胞实验也显示口服降糖药瑞格列奈和罗格列酮可以通过抑制OCT1来显著减少二甲双胍的细胞摄取[23]。③ 肾脏中介导二甲双胍的阳离子转运体主要为OCT2, 多位于肾小管细胞基底外侧并将二甲双胍转运进入近曲肾小管内细胞[24]。若抑制OCT2将减少二甲双胍的体内清除, 可能会增加二甲双胍的不良反应, 尤其是乳酸酸中毒[25]。Ding等[26]的健康受试者实验表明, OCT抑制剂兰索拉唑可以增加二甲双胍血浆浓度-时间曲线下面积17%。综上所述, 临床上要尽可能避免瑞格列奈、罗格列酮和兰索拉唑以及其他可以影响OCTs功能的药物与二甲双胍合用, 以免影响二甲双胍的治疗效果。
OCT1基因多态性是造成二甲双胍药效个体差异的重要因素, 如rs622342基因型影响了二甲双胍对于南印度地区二型糖尿病患者的治疗药效[27]。低活性基因型OCT1降低了二甲双胍在肝脏中的分布[25], 其基因变体包括: Arg61Cys (181C > T, 单核苷酸多态性(SNP) rs12208357), Gly401Ser (1201G > A, SNP rs34130495), Met420del (1256delATG, SNP rs72552763)和Gly465Arg (1393G > A, SNP rs34059508)。与此相反, 622342A > C突变可以使二甲双胍在肝脏中的浓度升高[28]。此外, OCT1基因多态性R61C(C > T)、G401S(G > A)、G465R(G > A)和M420del也影响了患多囊卵巢综合征的糖尿病女性患者的二甲双胍个体治疗效果[29]; 对于使用二甲双胍治疗去势抵抗性前列腺癌的患者, OCT1的C等位基因与二甲双胍毒性降低有关[30]。OCT2基因位点突变与服用二甲双胍的2型糖尿病患者的肾脏清除相关[31], 进而影响血浆乳酸水平和高乳酸血症的发生。有研究证实中国人群中存在OCT2基因多态性, 并且表明808G > T基因多态性与二甲双胍肾清除降低有关[32]。同时在群体药代动力学模型中, OCT2-808G > T和OCTN1-917C > T变体显著影响二甲双胍的清除率[33]。一项中国2型糖尿病患者的临床研究表明, OCT2-808G > T可以通过推迟二甲双胍的消除从而增强其降血糖作用[34]。此外, Song等[35]的卵母细胞结果表明, OCT2的3种基因突变型OCT2-T199I、-T201M和-A270S可使OCT2对二甲双胍的摄取能力显著下降。
OCT3的基因多态性对二甲双胍的转运也有影响。Chen等[36]的研究发现, OCT3 T400I (c.1199C > T)和V423F (c.1267G > T)变体使得二甲双胍在细胞的吸收量显著降低, 但T44M(c.131C > T)变体可以使二甲双胍在细胞的吸收量明显升高50%以上。临床上二甲双胍的组织分布和治疗效果有个体差异, 可用阳离子转运体的基因多态性解释[37]。
2 多药及毒性化合物外排转运蛋白多药及毒性化合物外排转运蛋白(multidrug and toxin extrusion proteins, MATEs)是SLC47基因家族的一员, 底物主要为有机阳离子, 分布在肝脏和肾脏等器官, 对于二甲双胍的最终排泄过程有很大影响[38]。
研究显示, 肝脏胆小管的管腔侧有MATE1分布, 负责将极少部分的二甲双胍排泄进入胆汁[19]。在肾脏中, MATE1[39]、MATE2-K[40]主要表达于肾近端小管的刷状缘膜, 将二甲双胍从肾小管细胞中转运进入尿液[41]。在MATE1基因敲除小鼠体内, 二甲双胍血浆、肾脏组织浓度均显著升高, 尿排泄量可减少14%[42]。Ma等[43]通过大鼠的实验认为, 联合给予β受体阻断剂阿替洛尔后二甲双胍的尿排泄降低与MATE1的下调有关。临床健康志愿者实验表明, MATE1抑制剂甲氧苄啶可导致二甲双胍肾清除率显著减少(从31~21 h–1), 半衰期延长(从2.7~3.6 h), 导致Cmax和AUC分别增加了38%和37%[44]; 在健康志愿者体内, MATE抑制剂乙胺嘧啶使二甲双胍肾清除率降低了35%, Cmax和AUC均显著增加[45], 故临床上要避免甲氧苄啶、乙胺嘧啶等MATE抑制剂与二甲双胍的合用。
MATEs基因变异会引起二甲双胍的临床治疗个体差异。MATE1等位基因rs2252281T > C和rs2289669G > A患者体内二甲双胍降糖治疗效果增强; 但MATE2等位基因rs12943590G > A二甲双胍的降血糖活性降低[46]。需要提出的是, 携带OCT1 (A > C, SNP rs622342)和MATE1 (G > A, SNP rs2289669)基因变体的患者二甲双胍抗高血糖作用得到增强, OCT2的SNP c.808 (G > T) (rs316019)和MATE1的SNP g.-66T > C (rs2252281)对二甲双胍肾消除的影响会相互抵消[47]。临床上对患者的MATEs基因多态性应予以重视。
3 质膜单胺蛋白转运体质膜单胺转运体(plasma membrane monoamine transporter, PMAT)是一种新的多特异性有机阳离子转运蛋白, 属于SLC29基因家族, 运输各种生物胺和外源性阳离子, 在人肠道有所表达, 主要分布在刷状缘侧[48]。
有研究证实PMAT是二甲双胍在胃肠道吸收中的主要转运体之一。Han等[49]通过单层Caco-2细胞模型实验, 表明PMAT在二甲双胍的肠道吸收贡献率为20%。在去极化和酸性pH值条件下, PMAT介导二甲双胍转运初始速率会大大提高[50]。动力学分析表明, 二甲双胍PMAT介导吸收动力学具有S形, 猜测PMAT蛋白可能包含一个大的底物结合口袋, 它允许不同底物在不同的位点相互作用或多个分子同时识别同一基板[50]。但PMAT的基因多态性对二甲双胍药代动力学的影响未见报道。
4 五羟色胺转运体五羟色胺转运体(serotonin reuptake transporter, SERT)是一种约为630个氨基酸残基的蛋白质, 属于SLC6基因家族[51], 是体内重要神经递质五羟色胺的转运体, 广泛分布于大脑边缘系统、胃肠道细胞膜、肥大细胞和五羟色胺能神经突触前膜上[52], 五羟色胺转运体的活性与体内部分抗抑郁症的药物药效相关[53]。
Han等[49]的Caco-2细胞模型实验表明SERT在二甲双胍的肠道吸收贡献率为20%。此外, Yee等[54]研究发现, 二甲双胍是SERT的底物同时可以抑制SERT, 临床上二甲双胍胃肠道不良反应的发生可能与其抑制五羟色胺的摄取有关[55]。所以在临床上要警惕由其抑制SERT介导的药物-药物相互作用的发生, 避免与SERT的底物如安非他酮、利他林和丙咪嗪等药物联合使用。
5 硫胺素转运体2硫胺素转运体即维生素B1转运体(thiamine transporter, THTR), 属于SLC19基因家族, 底物主要为维生素B1, 在肝、肾、小肠、胎盘和肌肉等组织广泛分布[56]。
THTR的吸收机制是pH值和电化学梯度敏感[57]。Liang等[58]利用人胚胎肾293细胞模型, 证实二甲双胍是THTR-2 (SLC19A3)的一种底物和抑制剂, 同时二甲双胍可以抑制硫胺素的吸收(IC50 = 680 μmol·L-1), 而硫胺素(维生素B1) 缺乏可能与乳酸酸中毒的发生有关[59]。在临床服用二甲双胍时要警惕由THTR-2介导的二甲双胍-药物或二甲双胍-维生素相互作用的发生。
6 肉碱/有机阳离子体1肉碱/有机阳离子体1 (carnitine/organic cation 1, OCTN1) 属于SLC22基因家族, 主要在小肠刷状缘膜表达, 在气管、骨髓、肝脏、肾脏等部位有少量表达[60]。
有研究表明OCTN1参与二甲双胍口服后的小肠吸收过程, 口服二甲双胍(175 mg·kg-1)后最大血浆浓度在OCTN1基因敲除小鼠(OCTN1-/-)明显低于野生型小鼠[61]。Futatsugi等[62]利用转染HEK293细胞实验表明OCTN1基因变种L503F转运二甲双胍的效率提高; 而在亚洲人和高加索人中发现的OCTN1基因变种I306T转运二甲双胍的效率则降低。关于OCTN1基因多态性对二甲双胍药效的影响尚未见报道。
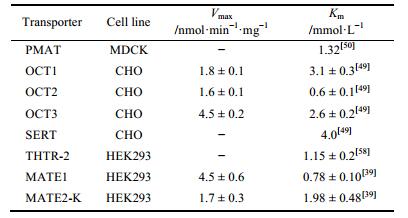
7 结语近年的研究发现, 由转运体介导的药物相互作用是导致药物不良反应的一个重要机制, 所以对于药物相互作用具体机制的研究是减少不良反应发生的重要方法之一。二甲双胍作为2型糖尿病临床的一线用药, 临床治疗及预防价值越来越引起重视, 其在体内的吸收、分布、排泄有多种转运体参与, 见图 1。肠道细胞OCT1、PMAT、SERT等多种转运体介导二甲双胍的肠吸收, 贡献比例分别为25%、20%、20%[49]; 肝脏摄取主要由OCT1摄取[22], 极少量药物通过MATE1排到胆汁[19]; 而肾脏中则由OCT2摄取[24], MATE1、MATE2-K负责外排[41], 部分主要转运体的转运参数见表 1。临床上患者在服用二甲双胍联合使用其他药物时, 可能会增加药物-药物相互作用发生的可能性, 甚至发生不良反应及毒性。因此, 阐明基于药物转运体机制的二甲双胍体内过程, 将有助于促进二甲双胍临床合理用药, 提高疗效和降低不良反应发生的风险。

|
Figure 1 Pharmacokinetics process of metformin mediated by transporter in vivo[19, 54, 58, 61]. MATE: Multidrug and toxin extrusion proteins; OCT: Organic cation transporters; OCTN: Carnitine/organic cation; PMAT: Plasma membrane monoamine transporter; SERT: Serotonin reuptake transporter; THTR: Thiamine transporter |
| Table 1 Apparent affinities of transporters toward metformin |
| [1] | Mu YM, Ji LN, Ni G, et al. Expert consensus on clinical application of metformin[J]. Chin J Diabetes (中国糖尿病杂志), 2014, 22: 673–681. |
| [2] | Zhong HW, LANG JM, Ye JH, et al. Effects of metformin on insulin resistance and leptin levels in patients with metabolic syndrome[J]. Chin Med Her (中国医药导报), 2011, 8: 80–81. |
| [3] | UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complica tions in overweight patients with type 2 diabetes (UKPDS 34)[J]. Lancet, 1998, 352: 854–865. DOI:10.1016/S0140-6736(98)07037-8 |
| [4] | Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement-executive summary[J]. Endocr Pract, 2013, 19: 536–557. DOI:10.4158/EP13176.CS |
| [5] | Yu Q. Expert consensus on diagnosis and treatment of polycystic ovary syndrome[J]. Chin J Pract Gynecol Obstetr (中国实用妇科与产科杂志), 2007, 23: 474. |
| [6] | Barbero-Becerra VJ, Santiago-Hernandez JJ, Villegas-Lopez FA, et al. Mechanisms involved in the protective effects of metformin against nonalcoholic fatty liver disease[J]. Curr Med Chem, 2012, 19: 2918–2923. DOI:10.2174/092986712800672094 |
| [7] | XUE Chao-jun, L K-x. Advances of the anti-tumor research of metformin[J]. Acta Pharm Sin (药学学报), 2015, 50: 1210–1216. |
| [8] | Qi H, Liu TT, Li GR. Effect and mechanism of metformin on prolonging life span[J]. Chin J Clin Pharm Ther (中国临床药理学与治疗学), 2012, 17: 1295–1301. |
| [9] | Audia P, Feinfeld DA, Dubrow A, et al. Metformin-induced lactic acidosis and acute pancreatitis precipitated by diuretic, celecoxib, and candesartan-associated acute kidney dysfunction[J]. Clin Toxicol (Phila), 2008, 46: 164–166. DOI:10.1080/15563650701355314 |
| [10] | Tucker GT, Casey C, Phillips PJ, et al. Metformin kinetics in healthy subjects and in patients with diabetes mellitus[J]. Br J Clin Pharmacol, 1981, 12: 235–246. DOI:10.1111/j.1365-2125.1981.tb01206.x |
| [11] | Zamek-Gliszczynski MJ, Bao JQ, Day JS, et al. Metformin sinusoidal efflux from the liver is consistent with negligible biliary excretion and absence of enterohepatic cycling[J]. Drug Metab Dispos, 2013, 41: 1967–1971. DOI:10.1124/dmd.113.053025 |
| [12] | Nies AT, Herrmann E, Brom M, et al. Vectorial transport of the plant alkaloid berberine by double-transfected cells expressing the human organic cation transporter 1 (OCT1, SLC22A1) and the efflux pump MDR1 P-glycoprotein (ABCB1)[J]. Naunyn Schmiedebergs Arch Pharmacol, 2008, 376: 449–461. DOI:10.1007/s00210-007-0219-x |
| [13] | Graham DGG, Punt J, Arora M, et al. Clinical pharmacoki netics of metformin[J]. Clin Pharmacokinet, 2011, 2: 81–98. |
| [14] | Markovic TP, Jenkins AB, Campbell LV, et al. The determi nants of glycemic responses to diet restriction and weight loss in obesity and NIDDM[J]. Diabetes Care, 1998, 21: 687–694. DOI:10.2337/diacare.21.5.687 |
| [15] | Ito N, Ito K, Ikebuchi Y, et al. Organic cation transporter/solute carrier family 22a is involved in drug transfer into milk in mice[J]. J Pharm Sci, 2014, 103: 3342–3348. DOI:10.1002/jps.24138 |
| [16] | Choi MK, Song IS. Organic cation transporters and their pharmacokinetic and pharmacodynamic consequences[J]. Drug Metab Pharmacokinet, 2008, 23: 243–253. DOI:10.2133/dmpk.23.243 |
| [17] | Muller J, Lips KS, Metzner L, et al. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT)[J]. Biochem Pharmacol, 2005, 70: 1851–1860. DOI:10.1016/j.bcp.2005.09.011 |
| [18] | Han TK, Everett RS, Proctor WR, et al. Organic cation transporter 1 (OCT1/mOct1) is localized in the apical mem brane of Caco-2 cell monolayers and enterocytes[J]. Mol Pharmacol, 2013, 84: 182–189. DOI:10.1124/mol.112.084517 |
| [19] | Jensen JB, Sundelin EI, Jakobsen S, et al. [11C]-Labeled metformin distribution in the liver and small intestine using dynamic positron emission tomography in mice demonstrates tissue-specific transporter dependency[J]. Diabetes, 2016, 65: 1724–1730. DOI:10.2337/db16-0032 |
| [20] | Shirasaka Y, Lee N, Zha W, et al. Involvement of organic cation transporter 3 (Oct3/Slc22a3) in the bioavailability and pharmacokinetics of antidiabetic metformin in mice[J]. Drug Metab Pharmacokinet, 2016, 31: 385–388. DOI:10.1016/j.dmpk.2016.04.005 |
| [21] | Sogame Y, Kitamura A, Yabuki M, et al. A comparison of uptake of metformin and phenformin mediated by hOCT1 in human hepatocytes[J]. Biopharm Drug Dispos, 2009, 30: 476–484. DOI:10.1002/bdd.v30:8 |
| [22] | Wang DS, Jonker JW, Kato Y, et al. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin[J]. J Pharmacol Exp Ther, 2002, 302: 510–515. DOI:10.1124/jpet.102.034140 |
| [23] | Bachmakov I, Glaeser H, Fromm MF, et al. Interaction of oral antidiabetic drugs with hepatic uptake transporters: focus on organic anion transporting polypeptides and organic cation transporter 1[J]. Diabetes, 2008, 57: 1463–1469. DOI:10.2337/db07-1515 |
| [24] | Kimura N, Okuda M, Inui K. Metformin transport by renal basolateral organic cation transporter hOCT2[J]. Pharm Res, 2005, 22: 255–259. DOI:10.1007/s11095-004-1193-3 |
| [25] | Tzvetkov MV, Vormfelde SV, Balen D, et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin[J]. Clin Pharmacol Ther, 2009, 86: 299–306. DOI:10.1038/clpt.2009.92 |
| [26] | Ding Y, Jia Y, Song Y, et al. The effect of lansoprazole, an OCT inhibitor, on metformin pharmacokinetics in healthy subjects[J]. Eur J Clin Pharmacol, 2014, 70: 141–146. DOI:10.1007/s00228-013-1604-7 |
| [27] | Umamaheswaran G, Praveen RG, Damodaran SE, et al. Influence of SLC22A1 rs622342 genetic polymorphism on metformin response in South Indian type 2 diabetes mellitus patients[J]. Clin Exp Med, 2015, 15: 511–517. DOI:10.1007/s10238-014-0322-5 |
| [28] | Tarasova L, Kalnina I, Geldnere K, et al. Association of genetic variation in the organic cation transporters OCT1, OCT2 and multidrug and toxin extrusion 1 transporter protein genes with the gastrointestinal side effects and lower BMI in metformin-treated type 2 diabetes patients[J]. Pharmacogenet Genomics, 2012, 22: 659–666. DOI:10.1097/FPC.0b013e3283561666 |
| [29] | Gambineri A, Tomassoni F, Gasparini DI, et al. Organic cation transporter 1 polymorphisms predict the metabolic response to metformin in women with the polycystic ovary syndrome[J]. J Clin Endocrinol Metab, 2010, 95: E204–208. DOI:10.1210/jc.2010-0145 |
| [30] | Joerger M, van Schai RH, Becker ML, et al. Multidrug and toxin extrusion 1 and human organic cation transporter 1 polymorphisms in patients with castration-resistant prostate cancer receiving metformin (SAKK 08/09)[J]. Prostate Cancer Prostatic Dis, 2015, 18: 167–172. DOI:10.1038/pcan.2015.8 |
| [31] | Duan R, Pak C, Jin P. Single nucleotide polymorphism associated with mature miR-125a alters the processing of pri-miRNA[J]. Hum Mol Genet, 2007, 16: 1124–1131. DOI:10.1093/hmg/ddm062 |
| [32] | Wang ZJ, Yin OQ, Tomlinson B, et al. OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine[J]. Pharmacogenet Genomics, 2008, 18: 637–645. DOI:10.1097/FPC.0b013e328302cd41 |
| [33] | Yoon H, Cho HY, Yoo HD, et al. Influences of organic cation transporter polymorphisms on the population pharmacokinetics of metformin in healthy subjects[J]. AAPS J, 2013, 15: 571–580. DOI:10.1208/s12248-013-9460-z |
| [34] | Hou W, Zhang D, Lu W, et al. Polymorphism of organic cation transporter 2 improves glucose-lowering effect of metformin via influencing its pharmacokinetics in Chinese type 2 diabetic patients[J]. Mol Diagn Ther, 2015, 19: 25–33. DOI:10.1007/s40291-014-0126-z |
| [35] | Song IS, Shin HJ, Shin JG. Genetic variants of organic cation transporter 2 (OCT2) significantly reduce metformin uptake in oocytes[J]. Xenobiotica, 2008, 38: 1252–1262. DOI:10.1080/00498250802130039 |
| [36] | Chen L, Pawlikowski B, Schlessinger A, et al. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin[J]. Pharmacogenet Genomics, 2010, 20: 687–699. DOI:10.1097/FPC.0b013e32833fe789 |
| [37] | Pan X. Review of antidiabetic mechanism of metformin[J]. Chin J Diabetes (中国糖尿病杂志), 2016, 24: 665–668. |
| [38] | YANG Yuan-yuan, M Y-m. Progress in the study of multidrug and toxin extrusion proteins[J]. Acta Pharm Sin (药学学报), 2014, 49: 1105–1110. |
| [39] | Tanihara Y, Masuda S, Sato T, et al. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters[J]. Biochem Pharmacol, 2007, 74: 359–371. DOI:10.1016/j.bcp.2007.04.010 |
| [40] | Otsuka M, Matsumoto T, Morimoto R, et al. A human transporter protein that mediates the final excretion step for toxic organic cations[J]. Proc Natl Acad Sci U S A, 2005, 102: 17923–17928. DOI:10.1073/pnas.0506483102 |
| [41] | Ohta KY, Inoue K, Yasujima T, et al. Functional characteristics of two human MATE transporters: kinetics of cimetidine transport and profiles of inhibition by various compounds[J]. J Pharm Pharm Sci, 2009, 12: 388–396. |
| [42] | Tsuda M, Terada T, Mizuno T, et al. Targeted disruption of the multidrug and toxin extrusion 1 (mate1) gene in mice reduces renal secretion of metformin[J]. Mol Pharmacol, 2009, 75: 1280–1286. DOI:10.1124/mol.109.056242 |
| [43] | Ma YR, Huang J, Shao YY, et al. Inhibitory effect of atenolol on urinary excretion of metformin via down-regulating multidrug and toxin extrusion protein 1 (rMate1) expression in the kidney of rats[J]. Eur J Pharm Sci, 2015, 68: 18–26. DOI:10.1016/j.ejps.2014.12.002 |
| [44] | Grun B, Kiessling MK, Burhenne J, et al. Trimethoprim-metformin interaction and its genetic modulation by OCT2 and MATE1 transporters[J]. Br J Clin Pharmacol, 2013, 76: 787–796. DOI:10.1111/bcp.12079 |
| [45] | Kusuhara H, Ito S, Kumagai Y, et al. Effects of a MATE protein inhibitor, pyrimethamine, on the renal elimination of metformin at oral microdose and at therapeutic dose in healthy subjects[J]. Clin Pharmacol Ther, 2011, 89: 837–844. DOI:10.1038/clpt.2011.36 |
| [46] | Stocker SL, Morrissey KM, Yee SW, et al. The effect of novel promoter variants in MATE1 and MATE2 on the phar macokinetics and pharmacodynamics of metformin[J]. Clin Pharmacol Ther, 2013, 93: 186–194. DOI:10.1038/clpt.2012.210 |
| [47] | Christensen MM, Pedersen RS, Stage TB, et al. A gene-gene interaction between polymorphisms in the OCT2 and MATE1 genes influences the renal clearance of metformin[J]. Phar macogenet Genomics, 2013, 23: 526–534. DOI:10.1097/FPC.0b013e328364a57d |
| [48] | Wang J. The plasma membrane monoamine transporter (PMAT): Structure, function, and role in organic cation dispo sition[J]. Clin Pharmacol Ther, 2016, 100: 489–499. DOI:10.1002/cpt.442 |
| [49] | Han TK, Proctor WR, Costales CL, et al. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in Caco-2 cell monolayers[J]. J Pharmacol Exp Ther, 2015, 352: 519–528. DOI:10.1124/jpet.114.220350 |
| [50] | Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intes tine[J]. Drug Metab Dispos, 2007, 35: 1956–1962. DOI:10.1124/dmd.107.015495 |
| [51] | Barker EL, Kimmel HL, Blakely RD. Chimeric human and rat serotonin transporters reveal domains involved in recognition of transporter ligands[J]. Mol Pharmacol, 1994, 46: 799–807. |
| [52] | Chen JX, Pan H, Rothman TP, et al. Guinea pig 5-HT trans porter: cloning, expression, distribution, and function in intes tinal sensory reception[J]. Am J Physiol, 1998, 275: G433–448. |
| [53] | Seeman P, Madras BK. Anti-hyperactivity medication: meth ylphenidate and amphetamine[J]. Mol Psychiatry, 1998, 3: 386–396. DOI:10.1038/sj.mp.4000421 |
| [54] | Yee SW, Lin L, Merski M, et al. Prediction and validation of enzyme and transporter off-targets for metformin[J]. J Pharmacokinet Pharmacodyn, 2015, 42: 463–475. DOI:10.1007/s10928-015-9436-y |
| [55] | Dujic T, Zhou K, Tavendale R, et al. Effect of serotonin transporter 5-HTTLPR polymorphism on gastrointestinal intolerance to metformin: a GoDARTS study[J]. Diabetes Care, 2016, 39: 1896–1901. DOI:10.2337/dc16-0706 |
| [56] | Ganapathy V, Smith SB, Prasad PD. SLC19: the folate/thiamine transporter family[J]. Pflugers Arch, 2004, 447: 641–646. DOI:10.1007/s00424-003-1068-1 |
| [57] | Nabokina SM, Subramanian VS, Valle JE, et al. Adaptive regulation of human intestinal thiamine uptake by extracellular substrate level: a role for THTR-2 transcriptional regulation[J]. Am J Physiol Gastrointest Liver Physiol, 2013, 305: G593–599. DOI:10.1152/ajpgi.00237.2013 |
| [58] | Liang X, Chien HC, Yee SW, et al. Metformin is a substrate and inhibitor of the human thiamine transporter, THTR-2 (SLC19A3)[J]. Mol Pharm, 2015, 12: 4301–4310. DOI:10.1021/acs.molpharmaceut.5b00501 |
| [59] | Amrein K, Ribitsch W, Otto R, et al. Severe lactic acidosis reversed by thiamine within 24 hours[J]. Crit Care, 2011, 15: 457. DOI:10.1186/cc10495 |
| [60] | Tamai I, Yabuuchi H, Nezu J, et al. Cloning and characteri zation of a novel human pH-dependent organic cation trans porter, OCTN1[J]. FEBS Lett, 1997, 419: 107–111. DOI:10.1016/S0014-5793(97)01441-5 |
| [61] | Nakamichi N, Shima H, Asano S, et al. Involvement of carnitine/organic cation transporter OCTN1/SLC22A4 in gas trointestinal absorption of metformin[J]. J Pharm Sci, 2013, 102: 3407–3417. DOI:10.1002/jps.23595 |
| [62] | Futatsugi A, Masuo Y, Kawabata S, et al. L503F variant of carnitine/organic cation transporter 1 efficiently transports metformin and other biguanides[J]. J Pharm Pharmacol, 2016, 68: 1160–1169. DOI:10.1111/jphp.2016.68.issue-9 |
 2017, Vol. 52
2017, Vol. 52