2. 北京市理化分析测试中心, 北京100089
2. Beijing Centre for Physical and Chemical Analysis, Beijing 100089, China
牛蒡子 (Arctii Fructus) 为菊科 (Asteraceae) 牛蒡属 (Arctium) 植物牛蒡Arctium lappa L.的干燥成熟果实, 我国有牛蒡属植物2种。牛蒡为两年生草本植物, 在全国大部分地区均有分布, 喜温暖湿润气候, 耐寒、耐热性较强, 多生于山坡、山谷、林缘、林中, 海拔750~3 500 m处。目前从牛蒡子中分离得到的化合物主要为木脂素类、脂肪酸以及其他类成分等[1-6]。现代研究表明, 牛蒡子中所含主要成分木脂素类化合物具有抗肿瘤、抗炎、抗病毒、改善肾脏代谢功能、降血糖等多种生理活性[7-11]。
为了进一步揭示牛蒡子的药效物质基础, 本实验利用现代色谱分离技术及光谱鉴定技术, 依据牛蒡子的传统水煎剂用法重点对牛蒡子水溶性成分进行了研究, 共从牛蒡子中分离鉴定了10个化合物, 其中包括1个新的8-O-4'型木脂素和9个首次从牛蒡子中分离得到的单体化合物。这些化合物大多为糖苷类化合物, 包括5个木脂素的糖苷类化合物、3个酚苷类化合物以及1个苯乙醇苷类化合物 (图 1)。
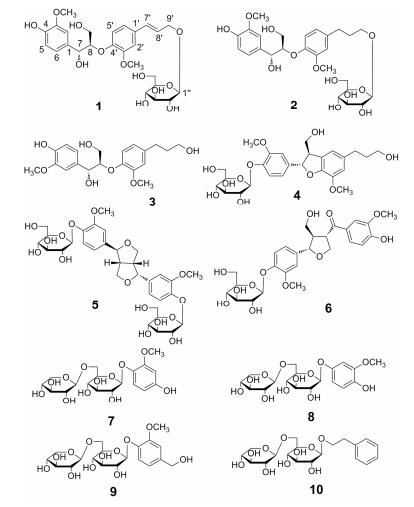
|
Figure 1 Structures of compounds 1-10 |
化合物1, 白色无定形粉末, UV (MeOH) λmax (logε): 230 (3.99)、280 (3.68) nm; [α]D25-29.71 (c 0.1, MeOH-H2O, 1:1); CD (MeOH) λmax (Δε): 257 (-1.23)、302 (-0.57) nm; 高分辨质谱HR-ESI-MS m/z 561.192 1 [M+Na]+ (calcd. for C26H34O12Na, 561.194 8) 提示化合物1分子式为C26H34O12。IR显示该化合物结构中含有羟基 (3 384 cm–1) 和苯环 (1 602、1 511、1 453 cm–1)。
化合物1的1H NMR (500 MHz, DMSO-d6) 谱中 (表 1), 显示有6个芳香质子信号δH 7.05 (1H, brs, H-2)、6.67 (1H, d, J = 8.0 Hz, H-5)、6.75 (1H, d, J = 8.0 Hz, H-6)、6.96 (1H, brs, H-2')、6.96 (1H, d, J = 8.0 Hz, H-5') 和6.87 (1H, d, J = 8.0 Hz, H-6'), 表明有两个ABX自旋体系苯环存在。此外, 还观察到两个连氧次甲基质子信号δH 4.70 (1H, J = 4.0 Hz, H-7)、4.26 (1H, m, H-8);两个连氧亚甲基质子信号δH 3.56 (1H, J = 11.0 Hz, H-9)、3.22 (1H, overlap, H-9)、4.40 (1H, dd, J = 11.0, 5.5 Hz, H-9')、4.19 (1H, overlap, H-9'); 以及两个烯烃氢信号δH 6.54 (1H, d, J = 15.5 Hz, H-7')、6.23 (1H, dt, J = 15.5, 5.5, H-8'), 表明了一个丙三醇单元和一个烯丙醇单元的存在。同时还观察到两个甲氧基信号δH 3.71 (3H, s)、3.78 (3H, s), 以及一个β-葡萄糖端基质子信号δH 4.19 (1H, d, J = 7.5 Hz, H-1")。在13C NMR (表 1) 中共显示了26个碳信号, 除了2个甲氧基碳信号、6个葡萄糖碳信号外, 剩下的18个碳信号可归属为一个木脂素的碳骨架[1]。
| Table 1 1H NMR (500 MHz) and 13C NMR (125 MHz) data ofcompound 1 (in DMSO-d6, J in Hz) |
化合物1的HMBC谱中 (图 2), H-7与C-1、C-2、C-6、C-8、C-9的相关, H-7'与C-1'、C-2'、C-6'、C-8'、C-9'的相关, 以及H-8与C-4'的相关充分说明了化合物1是一个8-O-4'型木脂素。葡萄糖端基质子H-1"与C-9'的相关表明葡萄糖连接在化合物1的9'位上, 甲氧基质子信号δH 3.71 (3H, s)、3.78 (3H, s) 分别与C-3、C-3'存在着远程相关点, 因此两个甲氧基分别位于化合物1的C-3、C-3'。
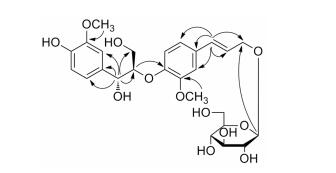
|
Figure 2 Key HMBC of compound 1 |
通过文献调研可知, 对于8-O-4'型木脂素苷类化合物, C-7和C-8的绝对构型可以利用在氘代氯仿中测定苷元H-7的偶合常数并结合CD图谱进行确定[12], 而葡萄糖的绝对构型则可以通过气相色谱进行确定[13]。因此, 采用纤维素酶对化合物1进行水解[13], 并分别获得苷元1a以及单体葡萄糖。苷元1a在氘代氯仿中H-7的偶合常数为7.5 Hz, 说明C-7和C-8的相对构型为苏式。结合化合物1在CD谱中257 nm处的负Cotton效应, 确定了该化合物绝对构型为7R, 8R。单体葡萄糖经三甲基硅烷基咪唑衍生化后进行气相色谱分析, 并与标准D-型葡萄糖对比确定化合物中葡萄糖单元为D型[13]。
综上所述, 化合物1的化学结构被确定为 (7R, 8R)-4, 7, 9, 9'-tetrahydroxy-3, 3'-dimethoxy-8-4'-oxyneolign-7'-ene-9'-O-β-D-glucopyranoside。
实验部分JASCO P-2000旋光仪; Nicolet 5700傅里叶变换红外光谱仪; JASCO J-815圆二色谱仪; Bruker 500 MHz核磁共振仪; Agilent 6520 HPLC-Q-TOF质谱仪; Agilent 1200型高效液相色谱仪, 包括四元高压梯度泵、自动脱气机、二极管阵列检测器、自动进样器、柱温箱。Shimadzu制备型高效液相色谱仪。大孔树脂HP-20为日本三菱化学株式会社生产, 反向硅胶C-18为YMC公司生产, 凝胶Sephadex LH-20为GE公司生产。
牛蒡子于2011年11月采于黑龙江省五常镇, 经中国医学科学院药物研究所马林教授鉴定为牛蒡Arctium lappa L.的干燥成熟果实。标本 (ID-S-2434) 存放于中国医学科学院、北京协和医学院药物研究所植物标本室。
1 提取与分离牛蒡子 (100 kg) 经80%乙醇回流提取 (3×2 h), 得浸膏 (约4.2 kg)。浸膏经水 (10 L) 分散后, 用乙酸乙酯萃取 (3×10 L)。所得水部位 (400 g) 进行HP-20大孔吸附树脂 (4.0 kg) 柱色谱, 用H2O (20 L)、15% EtOH (20 L)、30% EtOH (20 L)、50% EtOH (20 L)、95% EtOH (20 L) 梯度洗脱。
取15% EtOH洗脱部分 (40 g) 进行中压反相柱色谱, 用水-甲醇进行梯度洗脱 (0:100~100:0), 共得到17个部分 (Fr.1~Fr.17), 其中Fr.5再经Sephadex LH-20和制备型高效液色谱相分离得到化合物7 (18 mg)、8 (100 mg)、9 (27 mg)。
取30% EtOH洗脱部分 (86.4 g) 进行中压反相柱色谱, 用水-甲醇进行梯度洗脱 (0:100~100:0), 共得到13个部分 (Fr.1~Fr.13), 其中Fr.8再经Sephadex LH-20和制备型高效液相色谱分离得到化合物10 (23 mg); Fr.9再经Sephadex LH-20和制备型高效液相色谱分离得到化合物1 (16 mg)、2 (31 mg)、3 (14 mg)、5 (15 mg); Fr.10再经Sephadex LH-20和制备型高效液相色谱分离得到化合物4 (15 mg)、6 (19 mg)。
2 结构鉴定化合物1 白色无定形粉末, UV (MeOH) λmax (logε): 230 (3.99)、280 (3.68) nm; [α]D25-29.71 (c 0.1, MeOH-H2O, 1:1); CD (MeOH) λmax (Δε): 257 (-1.23)、302 (-0.57) nm; 高分辨质谱HR-ESI-MS m/z 561.192 1 [M+Na]+ (calcd. for C26H34O12Na, 561.194 8) 提示化合物1分子式为C26H34O12。IR显示该化合物结构中含有羟基 (3 384 cm–1) 和苯环 (1 602、1 511、1 453 cm–1)。核磁数据见表 1。
化合物2 白色无定形粉末。ESI-MS: m/z 541.2 [M+H]+, C26H36O12。1H NMR (500 MHz, DMSO-d6): δH 6.97 (1H, brs, H-2), 6.90 (1H, d, J = 8.5 Hz, H-5'), 6.81 (1H, brs, H-2'), 6.75 (1H, d, J = 8.5 Hz, H-6), 6.68 (1H, d, J = 8.5 Hz, H-5), 6.66 (1H, d, J = 8.5 Hz, H-6'), 4.70 (1H, brs, H-7), 4.16 (1H, dd, J = 9.5, 4.5 Hz, H-8), 4.10 (1H, d, J = 7.5 Hz, H-1"), 3.78 (1H, overlap, H-9'a), 3.74 (3H, s, 3-OCH3), 3.71 (3H, s, 3'-OCH3), 3.63 (1H, dd, J = 11.5, 5.5 Hz, H-6"a), 3.55 (1H, d, J = 11.0 Hz, H-9a), 3.43 (1H, m, H-6"b), 3.41 (1H, overlap, H-9'b), 3.21 (1H, m, H-9b), 3.10 (1H, m, H-5"), 3.04 (1H, m, H-4"), 3.01 (1H, m, H-3"), 2.93 (1H, m, H-2"), 2.56 (2H, t, J = 7.5 Hz, H-7'), 1.77(2H, m, H-8'); 13C NMR (125 MHz, DMSO-d6): δC 133.4 (C-1), 111.5 (C-2), 147.4 (C-3), 145.9 (C-4), 115.1 (C-5), 119.5 (C-6), 71.5 (C-7), 85.3 (C-8), 60.6 (C-9), 135.3 (C-1'), 113.4 (C-2'), 150.0 (C-3'), 146.8 (C-4'), 116.6 (C-5'), 120.7 (C-6'), 31.6 (C-7'), 31.7 (C-8'), 68.4 (C-9'), 103.4 (C-1"), 73.8 (C-2"), 77.1 (C-3"), 70.7 (C-4"), 76.0 (C-5"), 61.6 (C-6"), 56.1 (3-OCH3), 55.9 (3'-OCH3)。与文献[12]报道的化合物 (7R, 8R)-7, 9, 9'-trihydroxy-3, 3'-dimethoxy-8-O-4'-neolignan-9'-O-β-D-glucopyranoside的波谱数据基本一致, 故鉴定化合物2为 (7R, 8R)-7, 9, 9'-trihydroxy-3, 3'-dimethoxy-8-O-4'-neolignan-9'-O-β-D-glucopyranoside。
化合物3 白色无定形粉末。ESI-MS: m/z 379.1 [M+H]+, C20H26O7。1H NMR (500 MHz, DMSO-d6): δH 6.96 (1H, brs, H-2), 6.90 (1H, d, J = 8.0 Hz, H-5), 6.78 (1H, brs, H-2'), 6.75 (1H, d, J = 8.0 Hz, H-5'), 6.66 (1H, d, J = 8.0 Hz, H-6), 6.63 (1H, d, J = 8.0 Hz, H-6'), 4.70 (1H, brs, H-7), 4.15 (1H, dd, J = 9.5, 4.5 Hz, H-8), 3.74 (3H, s, 3-OCH3), 3.71 (3H, s, 3'-OCH3), 3.55 (1H, m, H-9a), 3.39 (2H, overlap, H-9'), 3.23 (1H, overlap, H-9b), 2.51 (2H, overlap, H-7'), 1.67(2H, m, H-8'); 13C NMR (125 MHz, DMSO-d6): δC 133.4 (C-1), 111.5 (C-2), 147.4 (C-3), 145.9 (C-4), 115.2 (C-5), 119.5 (C-6), 71.5 (C-7), 85.4 (C-8), 60.6 (C-9), 135.7 (C-1'), 113.3 (C-2'), 150.0 (C-3'), 146.8 (C-4'), 116.7 (C-5'), 120.6 (C-6'), 31.7 (C-7'), 34.9 (C-8'), 60.5 (C-9'), 56.1 (3-OCH3), 55.9 (3'-OCH3)。与文献[14]报道的化合物 (7R, 8R)-7, 9, 9'-trihydroxy-3, 3'-dimethoxy-8-O-4'-neolignan的波谱数据基本一致, 故鉴定化合物3为 (7R, 8R)-7, 9, 9'-trihydroxy-3, 3'-dimethoxy-8-O-4'-neolignan。
化合物4 白色无定形粉末。ESI-MS:m/z 523.2 [M+H]+, C26H34O11。1H NMR (500 MHz, DMSO-d6): δH 7.05 (1H, d, J = 8.5 Hz, H-5), 6.95 (1H, d, J = 2.0 Hz, H-2), 6.83 (1H, dd, J = 8.5, 2.0 Hz, H-6), 6.68 (2H, brs, H-2', H-6'), 4.96 (1H, d, J = 5.5 Hz, H-7), 4.87 (1H, d, J = 7.5 Hz, H-1"), 3.77 (3H, s, 3-OCH3), 3.74 (3H, s, 3'-OCH3), 3.69 (1H, overlap, H-6"a), 3.64 (1H, overlap, H-8), 3.59 (1H, overlap, H-9'a), 3.41 (3H, overlap, H-9'b, H-9), 3.41 (1H, m, H-6"b), 3.27 (2H, m, H-3", H-5"), 3.23 (1H, m, H-2"), 3.15 (1H, m, H-4"), 2.52 (1H, overlap, H-7'), 1.68 (1H, m, H-8'); 13C NMR (125 MHz, DMSO-d6): δC 136.0 (C-1), 110.9 (C-2), 146.6 (C-3), 146.0 (C-4), 115.8 (C-5), 118.3 (C-6), 87.0 (C-7), 54.0 (C-8), 63.6 (C-9), 135.6 (C-1'), 113.0 (C-2'), 143.8 (C-3'), 149.4 (C-4'), 129.3 (C-5'), 116.9 (C-6'), 32.0 (C-7'), 35.2 (C-8'), 60.7 (C-9'), 100.5 (C-1"), 73.7 (C-2"), 77.5 (C-3"), 70.1 (C-4"), 77.3 (C-5"), 61.1 (C-6"), 56.2 (3-OCH3), 56.2 (3'-OCH3)。与文献[15]报道的化合物 (7S, 8R)-dihydrodehydrodiconiferylalcohol-4-O-β-D-glucopyranoside的波谱数据基本一致, 故鉴定化合物4为 (7S, 8R)-dihydrodehydrodiconiferylalcohol-4-O-β-D-glucopyranoside。
化合物5 白色无定形粉末。ESI-MS: m/z 683.2 [M+H]+, C32H42O16。1H NMR (500 MHz, DMSO-d6): δH 7.04 (1H, d, J = 8.5 Hz, H-5), 7.03 (1H, d, J = 8.5 Hz, H-5'), 6.95 (2H, brs, H-2, H-2'), 6.83 (1H, d, J = 8.5 Hz, H-6), 6.82 (1H, d, J = 8.5 Hz, H-6'), 4.88 (1H, d, J = 7.5 Hz, H-1"), 4.86 (1H, d, J = 7.5 Hz, H-1"'), 4.79 (1H, d, J = 6.0 Hz, H-7), 4.37 (1H, d, J = 6.5 Hz, H-7'), 4.09 (1H, d, J = 9.0 Hz, H-9'a), 3.76 (6H, s, 3-OCH3, 3'-OCH3), 3.69 (2H, overlap, H-6"a, H-6"'a), 3.41 (5H, overlap, H-9, H-9'b, H-6"b, H-6"'b), 3.27 (4H, m, H-3", H-5", H-3"', H-5"'), 3.23 (2H, m, H-2", H-2"'), 3.15 (2H, m, H-4", H-4"'), 3.09 (1H, t, J = 3.5 Hz, H-8), 2.83 (1H, dd, J = 14.5, 6.0 Hz, H-8'); 13C NMR (125 MHz, DMSO-d6): δC 135.7 (C-1), 110.8 (C-2), 149.4 (C-3), 146.4 (C-4), 115.6 (C-5), 119.1 (C-6), 87.1 (C-7), 54.5 (C-8), 70.8 (C-9), 132.8 (C-1'), 110.5 (C-2'), 149.0 (C-3'), 145.9 (C-4'), 115.3 (C-5'), 118.1 (C-6'), 81.6 (C-7'), 49.7 (C-8'), 69.3 (C-9'), 100.6 (C-1"), 73.7 (C-2"), 77.5 (C-3"), 70.1 (C-4"), 77.3 (C-5"), 61.1 (C-6"), 100.6 (C-1"), 73.7 (C-2"), 77.5 (C-3"), 70.1 (C-4"), 77.3 (C-5"), 61.1 (C-6"), 56.1 (3-OCH3), 56.1 (3'-OCH3)。与文献[16]报道的化合物 (7S, 8R, 7'R, 8'R)-pinoresinol-4, 4'-di-O-β-D-glucopyrano side的波谱数据基本一致, 故鉴定化合物5为 (7S, 8R, 7'R, 8'R)-pinoresinol-4, 4'-di-O-β-D-glucopyranoside。
化合物6 白色无定形粉末。ESI-MS: m/z 537.1 [M+H]+, C26H32O12。1H NMR (500 MHz, DMSO-d6): δH 7.55 (1H, d, J = 8.0 Hz, H-6), 7.50 (1H, brs, H-2), 7.05 (1H, d, J = 8.0 Hz, H-6'), 6.98 (1H, brs, H-2'), 6.88 (1H, d, J = 8.0 Hz, H-5), 6.86 (1H, d, J = 8.0 Hz, H-5'), 4.88 (1H, d, J = 7.5 Hz, H-1"), 4.58 (1H, d, J = 8.0 Hz, H-7'), 4.12 (1H, overlap, H-8'), 4.11 (1H, overlap, H-9a), 3.99 (1H, m, H-9b), 3.82 (3H, s, 3-OCH3), 3.76 (3H, s, 3'-OCH3), 3.65 (1H, d, J = 11.5 Hz, H-6"a), 3.49 (2H, m, H-9'), 3.44 (1H, overlap, H-6"b), 3.25 (2H, m, H-3", H-5"), 3.23 (1H, m, H-2"), 3.15 (1H, m, H-4"), 2.54 (1H, m, H-8); 13C NMR (125 MHz, DMSO-d6): δC 128.5 (C-1), 112.1 (C-2), 148.1 (C-3), 152.5 (C-4), 123.8 (C-5), 119.2 (C-6), 197.9 (C-7), 53.6 (C-8), 70.6 (C-9), 136.0 (C-1'), 111.3 (C-2'), 146.4 (C-3'), 149.2 (C-4'), 115.5 (C-5'), 115.5 (C-6'), 83.2 (C-7'), 49.2 (C-8'), 60.3 (C-9'), 100.5 (C-1"), 73.7 (C-2"), 77.5 (C-3"), 70.2 (C-4"), 77.4 (C-5"), 61.1 (C-6"), 56.1 (3-OCH3), 56.1 (3'-OCH3)。与文献[17]报道的化合物 (8S, 7'S, 8'R)-4, 4', 9'-trihydroxy-3, 3'-dimethoxy-7', 9-epoxylignan-7-oxo-4'-O-β-D-glucopy ranoside的波谱数据基本一致, 故鉴定化合物6为 (8S, 7'S, 8'R)-4, 4', 9'-trihydroxy-3, 3'-dimethoxy-7', 9-epoxylignan-7-oxo-4'-O-β-D-glucopyranoside。
化合物7 白色无定形粉末。ESI-MS: m/z 435.1 [M+H]+, C18H26O12。1H NMR (500 MHz, DMSO-d6): δH 6.92 (1H, d, J = 8.5 Hz, H-6), 6.37 (1H, J = 2.0 Hz, H-3), 6.23 (1H, dd, J = 8.5, 2.0 Hz, H-5), 4.63 (1H, d, J = 7.5 Hz, H-1'), 4.15 (1H, d, J = 7.5 Hz, H-1"), 3.89 (1H, d, J = 11.5 Hz, H-6'a), 3.68 (3H, s, 2-OCH3), 3.65 (1H, dd, J = 11.5, 5.5 Hz, H-5"a), 3.55 (1H, dd, J = 11.5, 7.0 Hz, H-6'b), 3.39 (1H, overlap, H-5'), 3.25 (1H, m, H-4'), 3.20 (1H, m, H-3'), 3.13 (1H, m, H-2"), 3.12 (1H, m, H-4"), 3.06 (1H, t, J = 8.0 Hz, H-3"), 2.95 (1H, overlap, H-2'), 2.95 (1H, t, J = 11.5 Hz, H-5"b); 13C NMR (125 MHz, DMSO-d6): δC139.9 (C-1), 150.3 (C-2), 101.3 (C-3), 153.2 (C-4), 106.7 (C-5), 117.8 (C-6), 101.9 (C-1'), 73.8 (C-2'), 77.2 (C-3'), 70.2 (C-4'), 76.3 (C-5'), 68.8 (C-6'), 104.4 (C-1"), 73.9 (C-2"), 77.0 (C-3"), 70.1 (C-4"), 66.1 (C-5"), 56.0 (2-OCH3)。与文献[18]报道的化合物2-methoxy-4-hydroxyphenol-1-O-β-D-xylopy ranosyl-(1→6)-O-β-D-glucopyranoside的波谱数据基本一致, 故鉴定化合物7为2-methoxy-4-hydroxyphenol-1-O-β-D-xylopyranosyl-(1→6)-O-β-D-glucopyranoside。
化合物8 白色无定形粉末。ESI-MS: m/z 435.1 [M+H]+, C18H26O12。1H NMR (500 MHz, DMSO-d6): δH 6.64 (1H, d, J = 8.5 Hz, H-5), 6.61 (1H, d, J = 2.0 Hz, H-2), 6.48 (1H, dd, J = 8.5, 2.0 Hz, H-6), 4.63 (1H, d, J = 7.5 Hz, H-1'), 4.15 (1H, d, J = 7.5 Hz, H-1"), 3.89 (1H, d, J = 11.5 Hz, H-6'a), 3.72 (3H, s, 3-OCH3), 3.65 (1H, dd, J = 11.5, 5.5 Hz, H-5"a), 3.55 (1H, dd, J = 11.5, 7.0 Hz, H-6'b), 3.45 (1H, overlap, H-5'), 3.25 (1H, m, H-4'), 3.20 (1H, m, H-3'), 3.13 (1H, m, H-2"), 3.12 (1H, m, H-4"), 3.06 (1H, t, J = 8.0 Hz, H-3"), 2.96 (1H, t, J = 11.5 Hz, H-5"b), 2.95 (1H, overlap, H-2'); 13C NMR (125 MHz, DMSO-d6): δC 151.2 (C-1), 104.6 (C-2), 148.3 (C-3), 141.8 (C-4), 115.8 (C-5), 108.4 (C-6), 102.0 (C-1'), 73.7 (C-2'), 77.1 (C-3'), 70.3 (C-4'), 76.2 (C-5'), 69.1 (C-6'), 102.7 (C-1"), 73.9 (C-2"), 77.0 (C-3"), 70.1 (C-4"), 66.2 (C-5"), 56.0 (3-OCH3)。与文献[19]报道的化合物3-methoxy-4-hydroxyphenol-1-O-β-D-xylopy ranosyl-(1→6)-O-β-D-glucopyranoside的波谱数据基本一致, 故鉴定化合物8为3-methoxy-4-hydroxyphenol-1-O-β-D-xylopyranosyl-(1→6)-O-β-D-glucopyranoside。
化合物9 白色无定形粉末。ESI-MS: m/z 449.1 [M+H]+, C19H28O12。1H NMR (500 MHz, DMSO-d6): δH 7.08 (1H, d, J = 8.5 Hz, H-5), 6.94 (1H, d, J = 2.0 Hz, H-2), 6.81 (1H, dd, J = 8.5, 2.0 Hz, H-6), 4.84 (1H, d, J = 7.5 Hz, H-1'), 4.42 (2H, d, J = 5.5 Hz, H-7), 4.15 (1H, d, J = 7.5 Hz, H-1"), 3.97 (1H, d, J = 11.5 Hz, H-6'a), 3.72 (3H, s, 3-OCH3), 3.64 (1H, dd, J = 11.5, 5.5 Hz, H-5"a), 3.56 (1H, dd, J = 11.5, 7.0 Hz, H-6'b), 3.47 (1H, m, H-5'), 3.25 (1H, overlap, H-2'), 3.25 (1H, m, H-3'), 3.25 (1H, overlap, H-4"), 3.16 (1H, m, H-4'), 3.05 (1H, ddd, J = 13.5, 9.0, 5.0 Hz, H-3"), 2.94 (1H, m, H-2"), 2.89 (1H, t, J = 11.5 Hz, H-5"b); 13C NMR (125 MHz, DMSO-d6): δC 136.5 (C-1), 111.5 (C-2), 149.0 (C-3), 145.5 (C-4), 115.9 (C-5), 119.2 (C-6), 63.3 (C-7), 100.7 (C-1'), 73.7 (C-2'), 77.2 (C-3'), 70.0 (C-4'), 76.4 (C-5'), 68.6 (C-6'), 104.4 (C-1"), 73.9 (C-2"), 76.9 (C-3"), 70.0 (C-4"), 66.0 (C-5"), 56.0 (3-OCH3)。与文献[20]报道的化合物4-hydroxy-3-methoxybenzylalcohol-4-O-β-D-xylopyranosyl-(1→6)-O-β-D-glucopyranoside的波谱数据基本一致, 故鉴定化合物9为4-hydroxy-3-methoxybenzylalcohol-4-O-β-D-xylopyranosyl-(1→6)-O-β-D-glucopyranoside。
化合物10 白色无定形粉末。ESI-MS: m/z 417.1 [M+H]+, C19H28O10。1H NMR (500 MHz, DMSO-d6): δH 7.16~7.26 (5H, m, H-2~H-6), 4.19 (1H, d, J = 7.5 Hz, H-1'), 4.18 (1H, d, J = 7.5 Hz, H-1"), 3.92 (1H, d, J = 11.5 Hz, H-6'a), 3.67 (2H, overlap, H-8a, H-5"a), 3.53 (1H, dd, J = 11.5, 7.0 Hz, H-6'b), 3.27 (1H, overlap, H-8b), 3.13 (1H, m, H-4'), 3.13 (1H, overlap, H-5'), 3.10 (1H, m, H-3'), 3.08 (2H, m, H-2", H-4"), 3.01 (1H, t, J = 8.0 Hz, H-3"), 2.97 (1H, t, J = 11.5 Hz, H-5"b), 2.94 (1H, overlap, H-2'), 2.85 (2H, t, J = 7.5 Hz, H-7)。与文献[21]报道的化合物2-phenethyl β-primeveroside的波谱数据基本一致, 故鉴定化合物10为2-phenethyl β-primeveroside。
| [1] | Yang YN, Huang XY, Feng ZM, et al. New butyrolactone type lignans from Arctii Fructus and their anti-inflammatory activities[J]. J Agric Food Chem, 2015, 63: 7958–7966. DOI:10.1021/acs.jafc.5b02838 |
| [2] | Yang YN, Huang XY, Feng ZM, et al. Hepatoprotective activity of twelve novel 7'-hydroxy ligninglucosides from Arctii Fructus[J]. J Agric Food Chem, 2014, 62: 9095–9102. DOI:10.1021/jf501859x |
| [3] | Yang YN, Zhang F, Feng ZM, et al. Two new neolignanglu-cosides from Arctii Fructus[J]. J Asian Nat Prod Res, 2012, 14: 981–985. DOI:10.1080/10286020.2012.729050 |
| [4] | He J, Huang XY, Yang YN, et al. Two new compounds from the fruits of Arctium lappa[J]. J Asian Nat Prod Res, 2016, 18: 423–428. DOI:10.1080/10286020.2016.1145671 |
| [5] | Huang XY, Feng ZM, Yang YN, et al. Four new neolignan-glucosides from the fruits of Arctium lappa[J]. J Asian Nat Prod Res, 2015, 17: 504–511. DOI:10.1080/10286020.2015.1039525 |
| [6] | Liu JH, Cui QX, Cheng S. Studies on the physical-chemical properties and fatty acid composition of Lappa seed oil[J]. China Oils Fats (中国油脂), 2000, 25: 51–53. |
| [7] | Hirose M, Yamaguchi T, Lin C, et al. Effects of arctiin on PhIP-induced mammary, colon and pancreatic carcinogenesis in female Sprague-Dawley rats and MelQx-induced hepatocar-cinogenesis in male F344 rats[J]. Cancer Lett, 2000, 155: 79–88. DOI:10.1016/S0304-3835(00)00411-0 |
| [8] | Awale S, Lu J, Kalauni SK, et al. Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation[J]. Cancer Res, 2006, 66: 175l–1757. DOI:10.1158/0008-5472.CAN-05-1130 |
| [9] | Chae SH, Kim PS, Cho JY, et al. Isolation and identification of inhibitory compounds on TNF-α production from Magnoliae fargesii[J]. Arch Pharm Res, 1998, 21: 67–69. DOI:10.1007/BF03216755 |
| [10] | Gao Y, Dong X, Kang YG, et al. Activity of in vitro anti-influenza virus of arctigenin[J]. Chin Tradit Herb Drugs (中草药), 2002, 33: 724–726. |
| [11] | Xu Z, Wang X, Zhou M, et al. The antidiabetic activity of total lignan from Fructus Arctii against alloxan-induced diabetes in mice and rats[J]. Phytother Res, 2008, 22: 97–101. DOI:10.1002/(ISSN)1099-1573 |
| [12] | Gan ML, Zhang YL, Lin S, et al. Glycosides from the root of Iodes cirrhosa[J]. J Nat Prod, 2008, 71: 647–654. DOI:10.1021/np7007329 |
| [13] | Xu K, Jiang JS, Feng ZM, et al. Bioactive sesquiterpenoid and polyacetyleneglycosides from Atractylodes lancea[J]. J Nat Prod, 2016, 79: 1567–1575. DOI:10.1021/acs.jnatprod.6b00066 |
| [14] | Miyase T, Ueno A, Takizawa N, et al. Studies on the glycosides of Epimedium grandiflorum Morr R. var. thunbergianum (MIQ.) NAKAI. Ⅱ[J]. Chem Pharm Bull, 1987, 35: 3713–3719. DOI:10.1248/cpb.35.3713 |
| [15] | Jayaprakasha GK, Ohnishi-Kameyama M, Ono H, et al. Phenolic constituents in the fruits of Cinnamomum zeylanicum and their antioxidant activity[J]. J Agric Food Chem, 2006, 54: 1672–1679. DOI:10.1021/jf052736r |
| [16] | Schumacher B, Scholle S, Hölzl J, et al. Lignans isolated from valerian: identification and characterization of a new olivil derivative with partial agonistic activity at A1 adenosine receptors[J]. J Nat Prod, 2002, 65: 1479–1485. DOI:10.1021/np010464q |
| [17] | Chen JJ, Wei HB, Xu YZ, et al. Antioxidant lignans from the roots of Vladimiria muliensis[J]. Planta Med, 2013, 79: 1470–1473. DOI:10.1055/s-00000058 |
| [18] | Luecha P, Umehara K, Miyase T, et al. Antiestrogenic constituents of the Thai medicinal plants Capparis flavicans and Vitex glabrata[J]. J Nat Prod, 2009, 72: 1954–1959. DOI:10.1021/np9006298 |
| [19] | Kitajima J, Kamoshita A, Ishikawa T, et al. Glycosides of Atractylodes japonica[J]. Chem Pharm Bull, 2003, 51: 152–157. DOI:10.1248/cpb.51.152 |
| [20] | Disadee W, Mahidol C, Sahakitpichan P, et al. Unprecedented furan-2-carbonyl C-glycosides and phenolic diglycosides from Scleropyrum pentandrum[J]. Phytochemistry, 2012, 74: 115–122. DOI:10.1016/j.phytochem.2011.11.001 |
| [21] | Saimaru H, Orihara Y. Biosynthesis of acteoside in cultured cells of Olea europaea[J]. J Nat Med, 2010, 64: 139–145. DOI:10.1007/s11418-009-0383-z |
 2017, Vol. 52
2017, Vol. 52


