2. 南开大学 药学院, 天津 300353;
3. 南开大学 药物化学生物学国家重点实验室, 天津 300353;
4. 天津中医药大学中药学院, 天津 300193
2. College of Pharmacy, Nankai University, Tianjin 300353, China;
3. State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin 300353, China;
4. School of Chinese Materia Medica Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China
“四气五味”是中医药性理论组成的核心内容之一。“四气”为药物的寒、热、温、凉四种药性, “五味”指酸、甘、苦、辛、咸五种味道。二者关系密切, “有味必有其气, 有气斯有性”, 共为中医临床用药的重要依据。早期中药的性味是指口尝的滋味, 随着中医临床理论的发展逐渐演变为以“功效属性”为主的抽象的概念及内涵。“消渴”最早出自《内经》, 又被称为“病消”“消瘅”等[1]。中医理论认为: “上焦多饮、中焦多食、下焦多尿”, 与西医糖尿病症状有相似之处, 故现代中医将糖尿病归于消渴的范畴[2]。但传统药性理论因缺少化学以及生物学的表征, 难以和现代科学有机的融合。
利用网络药理学技术对治疗糖尿病药物作用机制研究已取得较大进展[3], 如Yang等[4]研究了消渴安的作用机制, 发现小檗碱、芒果苷、辛醇和人参皂苷等化合物, 通过抑制氧化应激反应、抑制炎症因子表达等机制发挥抗糖尿病的综合作用。Li等[5]对葛根芩连汤的作用机制进行了研究, 通过将已知抗糖尿病的相关靶点与该复方预测的化合物靶点进行聚类和功能分析, 发现一批具有潜在治疗作用的药效成分。将预测的化合物与相应的靶点通过LigandFit进行对接验证, 并建立预测模型, 通过计算其拓扑学特性实现了对万余种中药成分的抗糖尿病作用的虚拟筛选[6, 7]。尽管网络药理学、生物信息学及分子对接等虚拟评价策略为复杂体系和疾病的研究提供了便捷的方法, 但现阶段的研究与经典的中医理论、药效成分的体内暴露情况以及量效权重关系等还缺少较为深入的结合与探讨。
本研究以系统论为指导, 结合还原论的研究手段[8], 首先建立了消渴病治疗中药的数据库, 在对中医的用药规律系统分析的基础上, 选用代表性的药物和关键药效成分, 结合网络药理学和蛋白互作分析等手段, 尝试对其用药规律以及药性关联内涵的解析。希望通过梳理“药材-化合物-靶点-通路-功能”的相互关联关系, 来诠释中医治疗消渴病的潜在分子机制及协同配伍的内在机制, 为探索中医药的奥秘, 揭示中药药性理论及配伍规律提供新的研究方法和策略。
材料与方法软件与数据库软件为中医传承辅助平台V2.5; ChemDraw12.0; Cytoscapev2.6.0。数据库有:《中华医典》第五版、《中华人民共和国卫生部药品标准-中药成方制剂》、traditional Chinese medicine integrated database (TCMID) (http://www.megabionet.org/tcmid/)、PharmMapper (http://lilab.ecust.edu.cn/pharmmapper/)、CTD (http://ctdbase.org/)、ChEMBL (https://www.ebi.ac.uk/chembl/)、Uniprot (http://www.uniprot.org/)、molecule annotation system (MAS, http://bioinfo.capitalbio.com/mas3/)、KEGG (http://www.kegg.jp/)、String9.1 (http://string91.embl.de/)。
方剂收集 方剂来源分为两部分。第一部分来自于《中华医典》第五版中方书类、伤寒金匮类、温病类、综合医书类四部分医书中收录的古方, 包含有万余首方药; 第二部分来自于《中华人民共和国卫生部药品标准-中药成方制剂》收录的4 049个现代处方。以消渴、消瘅和糖尿病等作为关键字, 对具有糖尿病治疗作用的处方进行搜索收集和整理。
方剂分析
配伍规律分析将筛选出的处方去除重复后, 输入到中医传承辅助平台V2.5软件中。运用该软件中的频次统计和组方规律的分析功能, 通过改变参数支持度及置信度, 进行配伍规律分析。
单体成分筛选 目标活性单体的筛选, 通过PubMed (https://www.ncbi.nlm.nih.gov/pubmed)和中国知网(http://www.cnki.net/)数据库筛选具有糖尿病相关治疗作用且在药材中含量丰富, 并具有代表性的化合物。单体成分的信息通过TCMID数据库查询整理。
靶点通路预测
靶点的预测 将所获得的活性化合物结构在ChemDraw软件中进行整理和保存后, 再投入PharmMapper数据库, 得到化合物对应靶点。将其对应的英文名称输入CTD和ChEMBL数据库, 导出目标结构的化合物对应靶点的基因数据。利用Uniprot网站, 规范和统一上述3个数据库中涉及的靶点名称。
通路预测 运用MAS 3.0数据库预测出靶点对应的通路, 并利用KEGG数据库筛选出糖尿病相关通路, 采用Cytoscape v2.6.0软件绘制出“化合物-靶点-通路”关系图。
结果 1 治疗糖尿病方剂中单味药的用药频次统计从伤寒金匮类、温病类、综合类等经典医书及现代中药成方制剂中, 共计获得了624个治疗消渴和糖尿病的处方。将其分别录入中医传承辅助平台V2.5的数据库中统计用药频次。结果显示, 624个处方中共涉及药材435味, 频次≥10%的中药有27味(表 1), 以苦甘、寒凉和温平的药物为主, 其中麦冬、甘草、人参、黄连、地黄和知母等常出现在治疗消渴的常用方剂中。
| Table 1 Frequency of herbs in the Chinese traditional patent medicine for treating Xiaoke |
设置不同的支持度, 对上述处方中的核心配伍组合进行深度分析。结果如图 1所示, 支持度为7.5%, 主要出现甘草、黄柏、葛根、知母、黄芩、芍药和麦冬等17味中药以及41种配伍组合模式。

|
Figure 1 Network on core combinations in depth of Xiaoke formulae (support degree: 7.5%, confidence≥0.9) |
现代研究认为药物的性味是可以拆分的, 通过对药味的物质基础的辨析发现, 酸味成分多以有机酸和鞣质等为主; 苦味成分多含黄酮、生物碱和甙类; 甘味成分多为糖类和甙类; 辛味的主要成分为挥发油、萜类及生物碱; 而咸味成分包含了无机盐和蛋白质等[9]。从支持度7.5%条件下筛选出的17味中药出发, 查询整理其中具有糖尿病治疗作用的主要药效成分, 共计收集21种代表性化合物。如表 2[7, 10-28]所示, 消渴常用药材的药效物质多为甘苦类成分, 其中主要涉及了皂苷、黄酮、多糖、生物碱、木质素、酚及萜类等, 并以多糖、皂苷和黄酮类为主。
| Table 2 Active compounds and structure types of high frequency herbs for treating Xiaoke |
选取表 2中多糖和无机盐以外的16个药效成分, 通过ChemDraw、PharmMapper、CTD和ChEMBL软件和数据库进行靶点通路分析, 共得到1 153个潜在的靶点, 将其投入MAS 3.0数据库共得到所对应的112条通路。以KEGG提供的相关疾病通路为依据, 筛选得到糖尿病相关20条通路, 共涉及182个靶点, 结果如图 2所示。其中与糖尿病直接相关的通路5条(1型及2型糖尿病通路、胰岛素信号通路、胰岛素及胰腺分泌通路); 间接相关通路4条(内质网蛋白质加工、肾素-血管紧张素-醛固酮系统、醛固酮调节钠重吸收、低氧诱导因子信号通路); 脂代谢相关的PPAP和脂肪细胞因子通路2条, 炎症相关通路9条[雷帕霉素靶蛋白(mTOR)、Wnt、MAPK、PI3K-Akt、肿瘤坏死因子(TNF)、FoxO、转化生长因子-β (TGF-β)、p53和Jak-STAT]。
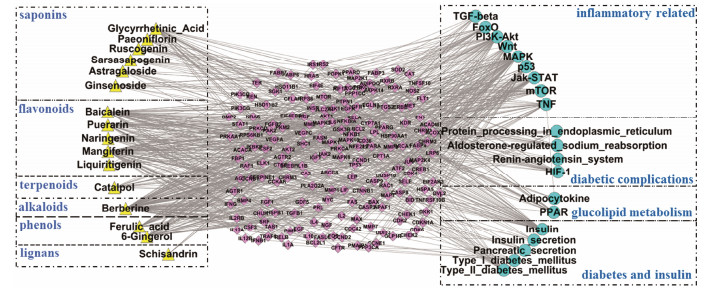
|
Figure 2 The "compound-target-pathway" network of medicinal composition related to Xiaoke. Yellow triangle represents compound, pink rhombus represents target, blue circle represents pathways. Compounds were classified by structure type, and pathway were classified by function |
现代研究认为2型糖尿病主要成因是β细胞功能低下、胰岛素抵抗和遗传因素。高血糖症、游离脂肪酸、脂肪因子、炎症因子、氧化应激和线粒体功能紊乱等因素均可导致胰岛素抵抗, 这些诱因主要发生在肝、骨骼肌和脂肪细胞中[29]。已有研究证实, 饮食脂肪含量过高刺激的慢性炎症可促进胰岛素抵抗, 也是导致心脏、肾脏方面并发症的原因[30]。β细胞功能障碍是由糖毒、脂毒和淀粉样蛋白形成所导致[31], 而这些过程是多靶点多通路共同作用的结果(图 3)。胰腺β细胞产生的胰岛素能促使肝中糖原合成、增加骨骼肌糖摄取、抑制脂肪酸从脂肪组织中释放, 从而达到降低血糖的目的[32]。在胰岛素抵抗的细胞机制中, 胰岛素受体底物(IRS)磷酸化后, 会导致下游PI3K、Akt等一系列信号的变化。游离脂肪酸(FFA)和TNF-α可增加蛋白激酶C (PKC)的表达, 激活c-Jun氨基末端激酶(JNK)和核因子-κB激酶抑制剂(IKK)等, 它们与蛋白酪氨酸磷酸酶1B均可抑制胰岛素受体酪氨酸激酶活性, 从而抑制IRS的磷酸化[33, 34]。而脂肪酸和脂肪因子的增加, 会导致脂肪特异性蛋白脂联素的降低。脂联素是一种胰岛素增敏激素, 可以降低mTOR对IRS1的不良反应, 还可激活腺苷5'-磷酸腺苷活化蛋白激酶(AMPK)和p38-MAPK通路, 增加脂肪酸代谢及葡萄糖转运体4型(GLUT4)的膜转移[35]。激活脂肪细胞中PPAR-γ, 可增加脂肪细胞的数量, 降低TNF-α的表达, 增加GLUT1/4的合成与转运。此外, 在这些过程中还涉及了PI3K、JAK-STAT、MAPK、mTOR和FOX等多条信号通路[29]。
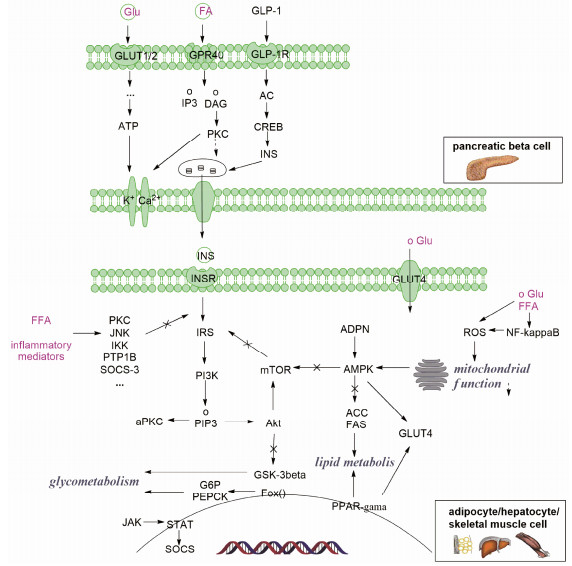
|
Figure 3 Mechanisms of β-cell dysfunction and insulin resistance in type 2 diabetes |
胰岛β细胞的损伤通常由高血糖、FFA和细胞毒素以及细胞因子沉积所导致[36]。高糖能够诱导产生活性氧簇(ROS)和氧化应激, ROS能够增强核因子(NF)-κB活性并导致线粒体氧化损伤, 直接损伤β细胞[37]。高FFA在线粒体氧化过程中积累的长链酰基辅酶A、PKC, 会影响钾通道的开放, 减少胰岛素的分泌[38]。因此, 调节糖脂代谢, 控制血糖和FFA, 促进胰岛素分泌, 改善胰岛素抵抗, 抗炎抗氧化保护胰岛细胞功能是预防和治疗糖尿病的有效手段[31]。
6 消渴治疗代表药对的网络机制分析依据中医传承辅助平台软件可得到高频出现的经典药对, 从中选取知母-黄柏和黄芪-葛根, 从配伍机制入手进一步分析了其主要药效成分在调节糖脂代谢方面的协同机制。并根据String靶点蛋白功能分析与KEGG通路解析, 将其作用机制初步聚焦在糖尿病、糖尿病并发症、糖脂代谢、炎症相关的4类通路上, 结合药性理论分析了其不同用药组合各自的特色。
6.1 知母-黄柏知母黄柏药对出自《兰室秘藏》, 又名疗本滋肾丸。知母味苦寒, 质柔性润, 有清热泻火, 滋阴润燥之功, 主治肺热燥咳、内热消渴。黄柏苦寒沉降, 能清热燥湿, 长于泻肾之火, 清下焦湿热。知母-黄柏是典型的治疗消渴的药对, 主治阴虚火旺, 骨蒸盗汗。
黄柏的主要活性成分被认为是小檗碱, 知母主要代表成分有芒果苷和菝葜皂苷元, 其具体的调控糖脂代谢的作用机制见图 4所示。在糖生成、代谢和转运方面, 小檗碱可以激活AMPK, 从而增加GLUT4的表达和转运, 促进葡萄糖转运[39]; 小檗碱可抑制6-磷酸葡萄糖(G6P)和磷酸烯醇丙酮酸羧激酶(PEPCK)靶蛋白, 从而抑制糖异生[40]。芒果苷可以抑制葡萄糖释放, 同时激活丙酮酸脱氢酶增加葡萄糖的利用[41]。在脂代谢方面, 对接和文献均显示小檗碱和芒果苷可激活PPAR, 调节脂代谢[42, 43]; 并抑制固醇调节元件结合蛋白(SREBP), 从而抑制胆固醇合成[44, 45]。此外, 对接结果还显示小檗碱、芒果苷和菝葜皂苷元可以作用于白介素、MAPK、NF-κB家族等系列靶点, 抑制JNK、IKK、IL和TNF等炎症介质的产生, 从而改善炎症诱导的胰岛素抵抗[18]。知母主要在抑制糖原分解, 促进糖利用, 调节脂代谢, 改善胰岛素抵抗方面起作用。黄柏与知母配伍, 可以在调节脂代谢, 改善胰岛素抵抗方面起到协同增效作用, 同时还可以激活AMPK, 促进葡萄糖转运。
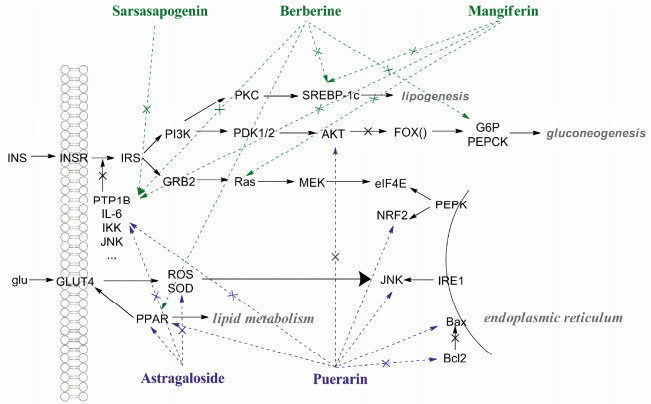
|
Figure 4 Synergy molecular mechanism of berberine, mangiferin and sarsasapogen in Zhimu-Huangbai and astragaloside and puerarin in Huangqi-Gegen in treatment of Xiaoke |
黄芪葛根汤出自于《政治汇补》, 临床上用于治疗气阴两虚的高血压和糖尿病[46]。方中黄芪味甘性温、补气固表和脱毒排脓, 可用于糖尿病、慢性肾炎蛋白尿; 葛根味甘辛性凉, 具有解肌热、生津止渴、升阳止泻功效, 二者合用益气效果显著。有研究显示, 黄芪中的黄芪甲苷与葛根中的葛根素两个化合物配伍使用可降低2型糖尿病大鼠的血脂和血糖, 改善胰岛细胞损伤[47]。
黄芪甲苷作用于超氧化物歧化酶(SOD), 抑制氧化应激反应, 减少细胞损伤, 同时降低TNF-α水平, 也可以抑制一系列NF-κB相关基因的表达[48]。通常内质网应激通路会在糖尿病的发生过程中被激活, 造成肾脏损伤[49], 而葛根素可以作用于该通路中的B细胞淋巴瘤-2 (Bcl2)、Bcl-2相关X蛋(Bax)、NF-E2相关因子2 (NRF2)、真核起始因子4E (EIF4E)等多个靶点, 发挥治疗糖尿病肾病的作用[50, 51]。此外, 葛根素还可以作用于Akt、细胞外调节蛋白激酶(ERK)、JNK等一系列炎症靶点, 通过抑制炎症因子的表达, 减少胰岛素抵抗[52]。另外, 黄芪甲苷和葛根素均能提高PPAR的表达, 促进葡萄糖转运以及调节脂肪代谢[53, 54] (图 4)。由此可见, 黄芪与葛根配伍, 不仅能够改善胰岛素抵抗, 减少细胞损伤, 还能够减轻糖尿病过程中对肾的损伤, 起到补肾益气的作用。
讨论高血糖在中医中被形容为“甘浊积聚”, 消渴的基本病机认为是“阴亏燥热、五脏虚损”, 主要累及肺、脾、肾三脏。研究发现, 中医的“肺”除了组织学上的肺以外, “肺气”也与肾上腺素受体及植物神经的调控关系密切[55]; 现代研究推测中医的“脾”可能相当于现代医学的胰腺[56], 而调节脾胃主要与营养精微物质的消化与利用有关; 而中医“肾”的功能涉及了现代医学的泌尿、生殖、免疫、内分泌以及中枢神经等多个系统[57]。总之, 不论中医与西医均通过药物来调节和干预脏器的糖脂代谢及神经内分泌等, 实现其对糖尿病及其并发症的治疗。
综上所述, 通过对中药治疗消渴的药性理论的网络药理学的整合分析, 本文研究发现甘味中的皂苷类成分主要作用于胰岛素、胰岛素及胰腺分泌等通路, 刺激胰岛素分泌, 改善胰岛素抵抗, 促进葡萄糖的利用, 并可以增加葡萄糖激酶的活性, 加速葡萄糖氧化分解, 体现了补气固本、生津止渴的“补和”之效; 苦味中的黄酮和生物碱等成分主要作用于MAPK、PI3K-Akt、PPAR等通路, 参与了调控炎症因子, 促进糖异生, 改善内分泌, 调节糖脂代谢等生理过程, 反映了清热燥湿, 固肾健脾的“燥坚”之功。针对消渴病“上消、中消、下消”的症状, 中医药主要通过抗炎、调节免疫, 调整糖脂代谢, 提高胰岛素利用, 改善糖尿病并发症等来“清热去燥、补肾固涩”, 是多方面的综合干预结果。但具体的作用机制只是预测和文献对应的结果, 还需要分子生物学手段进行验证。希望本研究策略和方法能为糖尿病的综合治疗提供参考和借鉴。
| [1] | Zhuang QZ. Research of the Academic History of Diabetes in Ancient Times (古代消渴病学术史研究)[D]. Beijing:China Academy of Chinese Medical Sciences, 2006. |
| [2] | Lun ZE. Traditional Chinese Medical Literature Study About Common Chronic Diabetic Complications[糖尿病(消渴病)临床常见慢性并发症的中医文献研究] [D]. Beijing:China Academy of Chinese Medical Sciences, 2010. |
| [3] | Bai Y, Fan XM, Sun H, et al. The mechanism of rosiglitazone compound based on network pharmacology[J]. Acta Pharm Sin (药学学报), 2015, 50: 284–290. |
| [4] | Yang ZZ, Liu W, Zhang F, et al. Deciphering the therapeutic mechanisms of Xiao-Ke-An in treatment of type 2 diabetes in mice by a Fangjiomics approach[J]. Acta Pharmacol Sin, 2015, 36: 699–707. DOI:10.1038/aps.2014.138 |
| [5] | Li HY, Zhao LH, Zhang B, et al. A network pharmacology approach to determine active compounds and action mechanisms of ge-gen-qin-lian decoction for treatment of type 2 diabetes[J]. J Evid Based Complement Alternat Med, 2014, 2014: 495840. |
| [6] | Gu JY, Chen LR, Yuan G, et al. A drug-target network-based approach to evaluate the efficacy of medicinal plants for type Ⅱ diabetes mellitus[J]. J Evid Based Complement Alternat Med, 2013, 2013: 203614. |
| [7] | Vitali F, Mulas F, Marini P, et al. Network-based target ranking for polypharmacological therapies[J]. J Biomed Inform, 2013, 46: 876–881. DOI:10.1016/j.jbi.2013.06.015 |
| [8] | Bai G, Hou YY, Jiang M, et al. Integrated systems biology and chemical biology approach to exploring mechanisms of traditional Chinese medicines[J]. Chin Herb Med, 2016, 8: 99–106. DOI:10.1016/S1674-6384(16)60017-5 |
| [9] | Zhang JY, Cao H, Gong SX, et al. Expression of sweet-taste of Chinese materia medica and its application in clinical compatibility[J]. Chin Tradit Herb Drugs (中草药), 2016, 47: 533–539. |
| [10] | Xiao ZQ. Studies on Quality Control and Anti-diabetic Activities of Polysaccharides From Liriope spicata var. Prolifera (湖北麦冬多糖质量控制与抗糖尿病活性研究)[D]. Wuhan:Huazhong University of Science & Technology, 2014. |
| [11] | Yuan CL, Sun L, Yuan ST, et al. Pharmacological activities and possible mechanism of effective components in Ophiopogonis radix[J]. Chin J New Drugs (中国新药杂志), 2013, 22: 2496–2502. |
| [12] | Kalaiarasi P, Kaviarasan K, Pugalendi KV. Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats[J]. Eur J Pharmacol, 2009, 612: 93–97. DOI:10.1016/j.ejphar.2009.04.003 |
| [13] | Kang HE, Sohn SI, Baek SR, et al. Liquiritigenin pharmacokinetics in a rat model of diabetes mellitus induced by streptozotocin:greater formation of glucuronides in the liver, especially M2, due to increased hepatic uridine 5'-diphosphoglucuronic acid level[J]. Metab Clin Exp, 2010, 59: 1472–1480. DOI:10.1016/j.metabol.2010.01.012 |
| [14] | Li XF. The Study on the Hypoglycemic Active Components of Trichosanthis Radix (天花粉降糖活性的成分研究)[D]. Chongqing:Southwest University, 2011. |
| [15] | Zhao GL, Yu LM, Gao WL, et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress[J]. Acta Pharmacol Sin, 2016, 37: 354–367. DOI:10.1038/aps.2015.136 |
| [16] | Feng YK, Liu ZZ, Peng Y, et al. Validated LC-MS method for simultaneous quantitation of catalpol and harpagide in rat plasma:application to a comparative pharmacokinetic study in normal and diabetic rats after oral administration of Zeng-YeDecoction[J]. Biomed Chromatogr, 2013, 27: 1503–1510. DOI:10.1002/bmc.v27.11 |
| [17] | Sellamuthu PS, Arulselvan P, Muniappan BP, et al. Mangiferin from salacia chinensis prevents oxidative stress andprotects pancreatic β-cells in streptozotocin-induced diabetic rats[J]. J Med Food, 2013, 16: 719–727. DOI:10.1089/jmf.2012.2480 |
| [18] | Yuan YL, Lin BQ, Zhang CF, et al. Timosaponin B-Ⅱ ameliorates palmitate-induced insulin resistance and inflammation via IRS-1/PI3K/Akt and IKK/NF-κB pathways[J]. Am J Chin Med, 2016, 44: 755–769. DOI:10.1142/S0192415X16500415 |
| [19] | Yin YH, Qi FH, Song ZH, et al. Ferulic acid combined with astragaloside IV protects against vascular endothelial dysfunction in diabetic rats[J]. Biosci Trends, 2014, 8: 217–226. DOI:10.5582/bst.2014.01081 |
| [20] | Jeong SI, Kim SJ, Kwon TH, et al. Schizandrin prevents damage of murine mesangial cells via blocking NADPH oxidase-induced ROS signaling in high glucose[J]. Food Chem Toxicol, 2012, 50: 1045–1053. DOI:10.1016/j.fct.2011.11.028 |
| [21] | Huang CL, Zhang JL, Li FL, et al. Effect of pachymaran to the antioxidant capacity and Bax, Bcl-2 protein expression of renal tissue in mice with type Ⅱ diabetes[J]. J Food Sci Biotechnol (食品与生物技术学报), 2016, 35: 82–88. |
| [22] | Shao Y, Yu Y, Li C, et al. Synergistic effect of quercetin and 6-gingerol treatment in streptozotocin induced type 2 diabetic rats and poloxamer P-407 induced hyperlipidemia[J]. RSC Adv, 2016, 6: 12235–12242. DOI:10.1039/C5RA16493A |
| [23] | Xu Z, Lefevre GM, Gavrilova O, et al. Mapping of longrange INS promoter interactions reveals a role for calciumactivated chloride channel ANO1 in insulin secretion[J]. Proc Natl Acad Sci U S A, 2014, 111: 16760–16765. DOI:10.1073/pnas.1419240111 |
| [24] | He MY, Deng YX, Shi QZ, et al. Comparative pharmacokinetic investigation on baicalin and wogonoside in type 2 diabetic and normal rats after oral administration of traditional Chinese medicine Huanglian Jiedu decoction[J]. J Ethnopharmacol, 2014, 155: 334–342. DOI:10.1016/j.jep.2014.05.033 |
| [25] | Zhang W, Liu CQ, Wang PW, et al. Puerarin improves insulin resistance and modulates adipokine expression in rats fed a high-fat diet[J]. Eur J Pharmacol, 2010, 649: 398–402. DOI:10.1016/j.ejphar.2010.09.054 |
| [26] | Xing BH, Yang FZ, Wu XH. Naringenin enhances the efficacy of human embryonic stem cell-derived pancreatic endoderm in treating gestational diabetes mellitus mice[J]. J Pharmacol Sci, 2016, 131: 93–100. DOI:10.1016/j.jphs.2016.04.014 |
| [27] | Han F, Zhou DC, Yin X, et al. Paeoniflorin protects diabetic mice against myocardial ischemic injury via the transient receptor potential vanilloid 1/calcitonin gene-related peptide pathway[J]. Cell Biosci, 2016, 6: 37. DOI:10.1186/s13578-016-0085-7 |
| [28] | Li CJ, Chen P. Lowering sugar effect and mechanism of angelica polysaccharide to wister diabetic rats induced by STZ[J]. J Qiqihar Med Coll (齐齐哈尔医学院学报), 2007, 28: 1158–1161. |
| [29] | Samuel VT, Shulman GI. Mechanisms for insulin resistance:common threads and missing links[J]. Cell, 2012, 148: 852–871. DOI:10.1016/j.cell.2012.02.017 |
| [30] | Wei XC, Song HW, Yin L, et al. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes[J]. Nature, 2016, 539: 294–298. DOI:10.1038/nature20117 |
| [31] | Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes:principles of pathogenesis and therapy[J]. Lancet, 2005, 365: 1333–1346. DOI:10.1016/S0140-6736(05)61032-X |
| [32] | Dinneen S, Gerich J, Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus[J]. N Engl J Med, 1992, 327: 707–713. DOI:10.1056/NEJM199209033271007 |
| [33] | Moller DE. Potential role of TNF-α in the pathogenesis of insulin resistance and type 2 diabetes[J]. Trends Endocrinol Metab, 2000, 11: 212–217. DOI:10.1016/S1043-2760(00)00272-1 |
| [34] | Gao ZG, Zhang XY, Zuberi A, et al. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes[J]. Mol Endocrinol, 2004, 18: 2024–2034. DOI:10.1210/me.2003-0383 |
| [35] | Wang J, Yang X, Zhang JJ. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells[J]. Cell Signal, 2016, 28: 1099–1104. DOI:10.1016/j.cellsig.2016.05.007 |
| [36] | Patti ME, Biles JA, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes:potential role of PGC1 and NRF1[J]. Proc Natl Acad Sci U S A, 2003, 100: 8466–8471. DOI:10.1073/pnas.1032913100 |
| [37] | Dong KL, Ni H, Wu ML, et al. ROS-mediated glucose metabolic reprogram induces insulin resistance in type 2 diabetes[J]. Biochem Biophys Res Commun, 2016, 476: 204–211. DOI:10.1016/j.bbrc.2016.05.087 |
| [38] | Ma HJ, Song GY. Mitochondrial disfunction and insulin resistance[J]. Int J Endoerinol Metab (国际内分泌代谢杂志), 2011, 31: 321–323. |
| [39] | Qian Q, Liu XF, He W, et al. TG accumulation inhibitory effects of Jinqi formula by AMPK signaling pathway[J]. J Ethnopharmacol, 2012, 143: 41–48. DOI:10.1016/j.jep.2012.05.052 |
| [40] | Zhang M, Lv XW, Li J, et al. Sodium caprate augments the hypoglycemic effect of berberine via AMPK in inhibiting hepatic gluconeogenesis[J]. Mol Cell Endocrinol, 2012, 363: 122–130. DOI:10.1016/j.mce.2012.08.006 |
| [41] | Apontes P, Liu ZB, Su K, et al. Mangiferin stimulates carbohydrate oxidation and protects against metabolic disorders induced by high-fat diets[J]. Diabetes, 2014, 63: 3626–3636. DOI:10.2337/db14-0006 |
| [42] | Mahmoud-Awny M, Attia AS, Abd-Ellah MF, et al. Mangiferin mitigates gastric ulcer in ischemia/reperfused rats:involvement of PPAR-γ, NF-κB and Nrf2/HO-1 signaling pathways[J]. PLoS One, 2015, 10: e0132497. DOI:10.1371/journal.pone.0132497 |
| [43] | Zhao WH, Liu LJ, Wang YL, et al. Effects of a combination of puerarin, baicalin and berberine on the expression of proliferator-activated receptor-γ and insulin receptor in a rat model of nonalcoholic fatty liver disease[J]. Exp Ther Med, 2016, 11: 183–190. |
| [44] | Wei SN, Zhang M, Yu Y, et al. Berberine attenuates development of the hepatic gluconeogenesis and lipid metabolism disorder in type 2 diabetic mice and in palmitateincubated HepG2 cells through suppression of the HNF-4α miR122 pathway[J]. PLoS One, 2016, 11: e0152097. DOI:10.1371/journal.pone.0152097 |
| [45] | Xing XM, Li DY, Chen DL, et al. Mangiferin treatment inhibits hepatic expression of acyl-coenzyme A:diacylglycerol acyltransferase-2 in fructose-fed spontaneously hypertensive rats:a link to amelioration of fatty liver[J]. Toxicol Appl Pharmacol, 2014, 280: 207–215. DOI:10.1016/j.taap.2014.08.001 |
| [46] | Wang CY, Chen YF, Li WM, et al. Effect of Huangqi Gegen decoction on experimental diabetes mellitus and insulin resistance[J]. Chin J Exp Tradit Med Formula (中国实验方剂学杂志), 2011, 17: 144–149. |
| [47] | Tian ZY, Wang HM, Tian W, et al. Regulation of puerarin and astragaloside A on rats modeled with type 2 diabetes mellitus[J]. J Hebei United Univ (Health Sci) (河北联合大学学报(医学版)), 2013, 15: 453–455. |
| [48] | Chen T, Wang RN, Jiang WJ, et al. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing Rho signaling[J]. Inflammation, 2016, 39: 483–492. DOI:10.1007/s10753-015-0272-4 |
| [49] | Xie H, Huang L, Li YY, et al. Endoplasmic reticulum stress and renal lesion in mice with combination of high-fat diet and streptozotocin-induced diabetes[J]. Acta Cir Bras, 2016, 31: 150–155. DOI:10.1590/S0102-865020160030000001 |
| [50] | Zhu X, Wang K, Zhang K, et al. Puerarin protects human neuroblastoma SH-SY5Y cells against glutamate-induced oxidative stress and mitochondrial dysfunction[J]. J Biochem Mol Toxicol, 2016, 30: 22–28. DOI:10.1002/jbt.2016.30.issue-1 |
| [51] | Zhang J, Guo WS, Tian BX, et al. Puerarin attenuates cognitive dysfunction and oxidative stress in vascular dementia rats induced by chronic ischemia[J]. Int J Clin Exp Pathol, 2015, 8: 4695–4704. |
| [52] | Yu DD, Mu S, Zhao DY, et al. Puerarin attenuates glucocorticoid-induced apoptosis of hFOB1.19 cells through the JNK-and Akt-mediated mitochondrial apoptotic pathways[J]. Int J Mol Med, 2015, 36: 345–354. |
| [53] | Lee OH, Seo DH, Park CS, et al. Puerarin enhances adipocyte differentiation, adiponectin expression and antioxidant response in 3T3-L1 cells[J]. Biofactors, 2010, 36: 459–467. DOI:10.1002/biof.v36.6 |
| [54] | Wu H, Gao Y, Shi HL, et al. A stragaloside IV improves lipid metabolism in obese mice by alleviation of leptin resistance and regulation of thermogenic network[J]. Sci Rep, 2016, 6: 30190. DOI:10.1038/srep30190 |
| [55] | Bai G, Hou YY, Jiang M, et al. Correlation analysis between visceral manifestation theories on Xuanfa and effect of adrenergic receptors[J]. Chin Herb Med, 2014, 6: 85–92. DOI:10.1016/S1674-6384(14)60013-7 |
| [56] | Wu L. A Preliminary Study on Glycopathological Mechanisms of Pancreatic Damage and Effects of Chinese Herbal Compounds in Diabetic Rats (糖尿病大鼠胰腺损害及补中益气丸、金匮肾气丸作用的糖病理学机制初步研究)[D]. Guangzhou:Guangzhou University of Chinese Medicine, 2009. |
| [57] | Wu RX. Kidney Vital Essence Yin and Yang Theory and Clinical Application Study (肾精气阴阳理论及临床应用研究)[D]. Jinan:Shandong University of Traditional Chinese Medicine, 2010. |
 2017, Vol. 52
2017, Vol. 52


