2. 承德医学院, 河北 承德 067000;
3. 秦皇岛市第一医院, 河北 秦皇岛 066000;
4. 解放军302医院中西医结合医学中心, 北京 100039;
5. 株洲千金药业股份有限公司, 湖南 株洲 412000
2. Chengde Medical University, Chengde 067000, China;
3. First Hospital of Qinhuangdao, Qinhuangdao 066000, China;
4. Integrative Medicine Center, 302 Military Hospital, Beijing 100039, China;
5. Qianjin Pharmaceutical Co., LTD, Zhuzhou 412000, China
炎性小体 (inflammasome) 是一类由胞浆分子模式识别受体 (pattern-recognition receptor, PRR) 参与组装的多蛋白复合体, 通过PRR识别病原微生物及内源性危险信号 (病原相关分子模式和危险相关分子模式) , 在细胞内参与先天性免疫防御功能。目前发现能够形成炎性小体的PRR主要有NOD样受体 (NOD-like receptor, NLR) 及PYHIN蛋白家族受体[1]。其中, NLRP3 (NACHT、LRR and PYD domains-containing protein 3) 炎性小体是目前研究最多的炎性小体, 能够识别多种外源性和内源性刺激, 通过招募并激活caspase-1, 促进促炎因子IL-1β和IL-18的剪切成熟与分泌, 引起一系列炎症反应[2], 在2型糖尿病、阿尔茨海默病、痛风等多种疾病中起到一定作用[3-5]。为此, 基于NLRP3炎性小体的抗炎药物发现及作用机制分析对于相关疾病的防治具有重要意义。
五味子甲素 (deoxyschizandrin, schisandrin A) 是五味子 [Schisandra chinensis (Turcz.) Baill] 干燥果实中分离得到的木脂素类成分之一[6], 在抗氧化、抑制细胞凋亡、调节免疫等方面具有广泛的药理作用[7-10]。其中, 在抗炎方面, 已有文献表明五味子甲素在体外能够有效抑制脂多糖 (lipopolysaccharide, LPS) 诱导的炎症反应[11]。然而, 目前对于五味子甲素是否可以抑制NLRP3炎症小体的活化目前未有报道, 为此, 本次实验以小鼠骨髓来源巨噬细胞 (BMDM) 作为研究对象, 首次研究五味子甲素对BMDM中NLRP3炎性小体活性的抑制作用及初步机制, 以期为相关炎症疾病的治疗药物研发提供参考。
材料与方法材料和试剂 小鼠骨髓来源巨噬细胞 (BMDM), 购自中国科学院上海细胞所; 五味子甲素对照品 (批号: 141112) , 购自成都普菲德生物技术有限公司, 纯度 > 98%; 三磷酸腺苷 (ATP)、尼日利亚菌素 (Nig) 均购自Sigma, USA; ultrapure LPS购自Invivogen, USA; NF-κB (p65) 和F(ab') 2羊抗兔IgG荧光抗体均购自Cell Signaling Technology, USA; NLRP3、凋亡相关斑点样蛋白 (apoptosis associated speck-like protein containing CARD domain, ASC) 和胱天蛋白酶-1 (caspase-1) 抗体均购自Adipogen, USA; IL-1β抗体购自R&D, USA; 辣根过氧化物酶标记羊抗小鼠IgG抗体、辣根过氧化物酶标记羊抗兔IgG抗体均购自Santa Cruz, USA; PVDF膜(孔径0.45 μm) 购自Millipore, USA; DA PI核染料购自Life Technologies, USA; opti-MEM无血清培养基及其他所有细胞培养试剂均购自Gibco, USA。
细胞培养 将BMDM细胞用含10%胎牛血清、100 u·mL-1青霉素、100 μg·mL-1链霉素的DMEM高糖培养基在37 ℃、5% CO2培养箱中培养, 3天传代一次, 取对数期细胞进行实验。
CCK-8法检测细胞毒性 取培养至对数生长期的BMDM细胞, 接种于96孔板中, 每孔100 μL, 每孔中细胞数为1×105个。BMDM细胞分为正常组、0.1% DMSO组、不同浓度的五味子甲素组 (6.25、12.5、25、50、100、200、400、800和1 600 μmol·L-1), 每组设5个复孔, 孵育12 h。随后每孔加入10 μL CCK-8溶液, 培养40 min后, 测定在酶标仪上450 nm处的吸光度值。
刺激方式[12] 取培养至对数生长期的BMDM细胞, 接种于12孔板中, 每孔1 mL, 每孔中细胞数为1×106个, 培养过夜后, 培养基替换为opti-MEM无血清培养基, 同时加入100 ng·mL-1 LPS预处理3 h。随后, 将培养基替换为不同浓度的五味子甲素对照品溶液 (25、50、100、200 μmol·L-1, opti-MEM无血清培养基作为溶剂) 作用1 h。随后, 分别加入ATP (1 mmol·L-1) 和Nig (5 μmol·L-1) 刺激45 min。
样品处理 将细胞上清吸去后PBS洗板2次, 置于冰上, 每孔加入1×loading buffer 200 μL, 10 min后刮下细胞, 吸取细胞裂解液, 水浴煮沸, 冷却后作为细胞裂解样品。吸取细胞上清加入1/4体积三氯乙酸 (TCA) 冰上静置15 min, 14 000 r·min-1、4 ℃离心10 min, 弃去上清, 加冰丙酮洗3次, 95 ℃金属浴挥干丙酮, 待丙酮挥发后加1×loading buffer 100 μL振荡混匀, 水浴煮沸, 冷却后作为上清样品。
Western blot测定蛋白的表达 BCA法测定蛋白质的浓度, 取蛋白样品30 μg进行SDS-聚丙烯酰胺凝胶电泳, 将凝胶中分离的蛋白转移至PVDF膜上, 5%脱脂奶室温封闭2 h后, 分别加入caspase-1、IL-1β、ASC、NLRP3、GAPDH一抗溶液4 ℃孵育过夜, 加入辣根过氧化物酶标记的相应二抗溶液, 加入ECL发光液后经过化学发光成像系统 (Tanon 4200SF) 显影分析, 结果用ImageJ2X软件定量分析。
免疫荧光分析NF-κB (p65) 入核情况[13] 将细胞按每毫升1.8×105个接种于96孔板, 培养过夜。加入不同浓度的五味子甲素对照品溶液 (25、50、100、200 μmol·L-1) 作用4 h。随后加入LPS (100 ng·mL-1) 刺激0.5 h。弃上清, PBS洗1次 (每孔200 μL)。用4% 多聚甲醛 (每孔100 μL) 室温固定20 min。弃去固定液, PBS洗2次。加入含0.1% Triton X-100的PBS (每孔100 μL) 冰上打孔15 min, 随后PBS洗1遍。加入封闭液 (含5% BSA的PBS, 每孔100 μL) 室温封闭1 h。加NF-κB (p65) 一抗(1:300) , 每孔50 μL 4 ℃孵育过夜。用含0.1% Tween-20的PBS洗3次 (每孔200 μL), 每次5 min。加入荧光二抗 (1:300, 离心并过膜, 每孔50 μL) 室温避光孵育1 h。用含0.1% Tween-20的PBS洗4次, 每次5 min。加入DAPI染核5 min (1:10 000, 避光) , PBS洗3次 (每孔200 μL)。甘油-PBS (1:1) 混合液保存 (每孔100 μL), 上机检测。p65及DAPI荧光信号采用Thermo Scientific ArrayScan XTI HCA Infinity Configuration高内涵分析仪观察并采集图像。每组随机选取3张切片, 每张切片选择观察400倍×5个视野, NF-κB (p65) 标记为绿色荧光, 细胞核的DAPI染色为蓝色荧光, 计数绿色荧光总数及蓝色细胞核中绿色荧光数, 两者之差即为细胞浆NF-κB (p65) 表达数。入核率 = 细胞核中绿色荧光数/绿色荧光总数 (即细胞核与细胞浆中绿色荧光数之和)。
统计学方法 采用SPSS 19.0统计软件, 实验数据以x± s表示, 组间比较采用单因素方差分析, 组间两两比较采用LSD-t检验, 显著性结果以*P < 0.05, **P < 0.01, ***P < 0.001表示。
结果 1 五味子甲素对BMDM细胞的细胞毒性作用通过CCK-8法检测五味子甲素对BMDM细胞的细胞毒性作用, 确定五味子甲素的给药浓度。如图 1所示, 与正常组相比, 五味子甲素 (6.25~400 μmol·L-1) 可提高BMDM细胞的生存率, 高浓度的五味子甲素 (800、1 600 μmol·L#8722;1) 会显著降低细胞的生存率 (P < 0.01) 。因此, 五味子甲素的给药浓度确定在6.25~400 μmol·L-1内, 根据实验选择给药浓度为25、50、100和200 μmol·L-1。
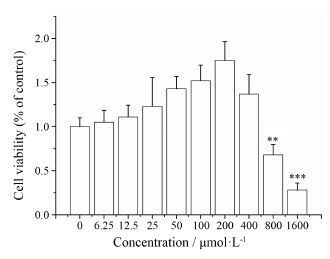
|
Figure 1 Effect of deoxyschizandrin (6.25, 12.5, 25, 50, 100, 200, 400, 800, 1 600 μmol·L-1) on cell viability in BMDM cells. The cells were treated with various of concentrations of deoxy-schizandrin for 24 h. The cytotoxicity of deoxyschizandrin for BMDM cells was analyzed by CCK-8 assay. n = 5, x± s. **P < 0.01, ***P < 0.001 vs control group |
结果显示 (图 2) , 在外源性刺激Nig诱导的BMDM细胞中, 五味子甲素能够降低上清中caspase-1和IL-1β的表达, 而胞质中pro-caspase-1、pro-IL-1β、ASC、NLRP3等蛋白的表达水平基本没有差异。结果表明, 五味子甲素能够抑制Nig引起的NLRP3炎症小体活化, 抑制pro-caspase-1剪切, 进而抑制IL-1β的分泌, 但不影响NLRP3、ASC、pro-caspase-1和pro-IL-1β等炎症小体组成蛋白及前体蛋白的表达。
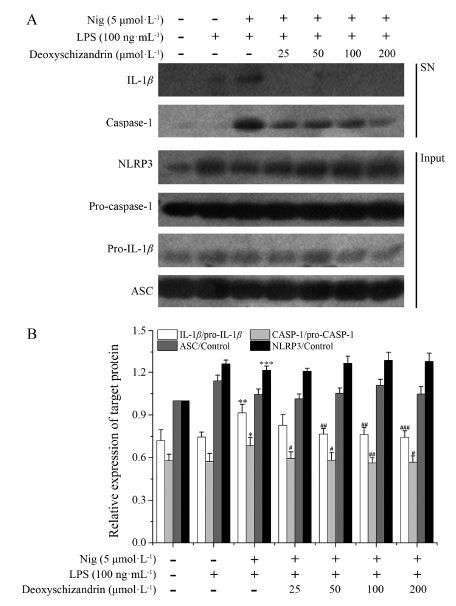
|
Figure 2 Effects of deoxyschizandrin on inflammasome-induced interleukin (IL)-1β secretion in vitro. LPS-primed bone marrow-derived macrophages (BMDMs) were pretreated with deoxyschizandrin for 3 h and stimulated with nigericin (Nig, 5 μmol·L-1) for 45 min, and supernatants (SN) were used to detect cleaved IL-1β (p17) and caspase-1 (p20) . Cell extracts (Input) were used to detect internal controls, such as pro-IL-1β (p31) , ASC, pro-caspase-1 (p45) , and NLRP3. The protein expres-sions of IL-1β, caspase-1, NLRP3, pro-caspase-1, pro-IL-1β, ASC were analyzed by Western blot. n = 3, x± s. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs LPS & stimulation group |
结果显示 (图 3) , 在内源性刺激ATP诱导的BMDM细胞中, 五味子甲素能够降低上清中caspase-1和IL-1β的表达, 呈剂量依赖性, 而胞质中pro-caspase-1、pro-IL-1β、ASC、NLRP3等蛋白的表达水平基本没有差异。结果表明, 五味子甲素能够抑制ATP引起的NLRP3炎症小体活化, 抑制pro-caspase-1剪切, 进而抑制IL-1β的分泌, 但不影响NLRP3、ASC、pro-caspase-1和pro-IL-1β等炎症小体组成蛋白及前体蛋白的表达。

|
Figure 3 Effects of deoxyschizandrin on inflammasome-induced IL-1β secretion in vitro. LPS-primed BMDMs were pretreated with deoxyschizandrin for 3 h and stimulated with adenosine triphosphate (ATP, 1 mmol·L-1) for 45 min, and SN were used to detect cleaved IL-1β (p17) and caspase-1 (p20) . Input were used to detect internal controls, such as pro-IL-1β (p31) , ASC, pro-caspase-1 (p45) , and NLRP3. The protein expressions of IL-1β, caspase-1, NLRP3, pro-caspase-1, pro-IL-1β, ASC were analyzed by Western blot. n = 3, x± s. ***P < 0.001 vs control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs LPS & stimulation group |
高内涵数据分析如图 4、5所示, 在静息状态下NF-κB nuc/cyt荧光强度比值<1, 表明此时NF-κB荧光信号主要分布在胞浆, 即NF-κB信号通路处于灭活状态; 在LPS刺激0.5 h后, NF-κB nuc/cyt荧光强度比值增大, 表明NF-κB荧光信号在胞核分布增强, 即LPS诱导NF-κB发生核转位, NF-κB信号通路处于激活状态。随着五味子甲素浓度的增加, NF-κB nuc/cyt荧光强度比值与LPS刺激组相比并没有明显减弱, 说明五味子甲素在50~200 μmol·L-1剂量内对LPS刺激导致的NF-κB核转位没有明显影响。
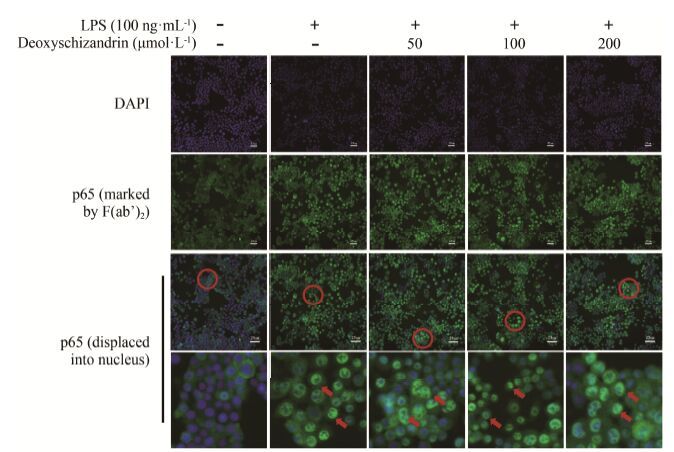
|
Figure 4 Effects of deoxyschizandrin on NF-κB (p65) transportation to the nucleus in vitro. The cells were pretreated with deoxy-schizandrin (50, 100, 200 μmol·L-1) for 4 h and then incubated with LPS (100 ng·mL-1) for 0.5 h. NF-κB (p65) protein expression and distribution in BMDM were observed by HCA Infinity Configuration. From top to bottom: nuclear region stained by DAPI; NF-κB (p65) marked by FITC; NF-κB (p65) displaced into nucleus |
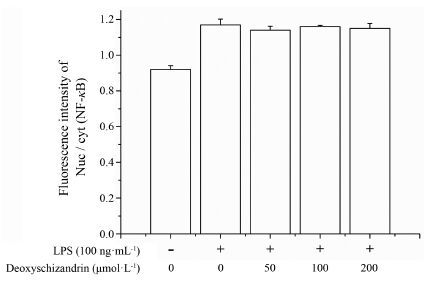
|
Figure 5 Effect of deoxyschizandrin on the expression of NF-κB (p65) protein in LPS (100 ng·mL-1)-induced BMDM (fluorescent number/each visual field). The cells were pretreated with deoxyschizandrin (50, 100, 200 μmol·L-1) for 4 h and then incu-bated with LPS (100 ng·mL-1) for 0.5 h. NF-κB (p65) protein expression in BMDM were analyzed by HCA Infinity Configura-tion. n = 5, x± s |
NLRP3炎性小体是一种由NLRP3、ASC以及pro-caspase-1组成、存在于细胞质中的多蛋白复合体[14, 15], 依赖caspase-1的分子平台促进白细胞介素IL-1β和IL-18的剪切、成熟和释放, 引起一系列炎症反应, 在免疫细胞 (Kuffer、T细胞等) 的激活与肝实质细胞的凋亡扮演着重要的角色[16, 17]。NLRP3炎性小体能被多种内源性、外源性刺激物激活, 包括内源性危险信号如ATP、尿酸钠, 微生物成分如细菌、病毒及真菌的DNA或RNA, 细菌成孔毒素如Nig, 晶体物质如胆固醇、二氧化硅, 环境刺激物如石棉、紫外线等[18-20]。其中, 在LPS预处理的基础上, 给予Nig、ATP是经典的NLRP3激活评价模型, 被广泛应用于NLRP3炎性小体相关机制研究及抑制剂筛选实验中[21]。本实验结果首次表明, 五味子甲素能够抑制Nig和ATP引起的NLRP3炎性小体活化以及pro-caspase-1剪切, 进而抑制IL-1β的分泌。同时, 五味子甲素对胞内NLRP3、ASC、pro-caspase-1等蛋白的表达没有影响, 表明五味子甲素并不影响NLRP3炎性小体的组成蛋白及前体蛋白的表达。
NLRP3炎症小体活化受Toll样受体 (Toll-like receptor, TLR)、NOD样受体 (NOD-like receptor, NLR) 介导的双信号通路调控[20-22]。通常胞内IL-1β前体等炎症小体相关蛋白表达量较低, 当受到LPS等刺激时, 激活的TLR促进NF-κB通路活性, 转录翻译产生pro-IL-1β等, 形成第一条信号通路。在此基础上给予Nig和ATP刺激时, 引起caspase-1活化, 活化的caspase-1将pro-IL-1β剪切, 形成成熟的IL-1β等, 形成第二条信号通路, 导致炎症小体活化[23]。其中, NF-κB信号通路中的主要蛋白NF-κB (p65) 的核位移是信号通路被激活的关键[24]。因此, 为了探讨五味子甲素抑制NLRP3炎症小体活化的生物学机制, 本研究基于高内涵成像系统和免疫荧光技术, 采用单独LPS刺激实验研究五味子甲素对NF-κB通路活性 (p65入核) 的影响。结果表明, 五味子甲素在50~200 μmol·L-1浓度内对LPS刺激导致的NF-κB核转位没有明显影响, 提示五味子甲素不通过下调NF-κB通路活性发挥抑制NLRP3炎性小体活化的作用, 而是通过抑制NLRP3炎性小体活化的第二条信号通路, 阻断caspase-1的活化, 抑制caspase-1对pro-IL-1β的剪切活化。但五味子甲素抑制caspase-1活化的直接作用靶点还有待进一步研究, 可以考虑后续实验探讨五味子甲素对NLRP3上、下游的影响来进一步明确药物作用的靶位。
综上, 本实验首次证实五味子甲素能够在一定浓度范围内 (25~200 μmol·L-1) 抑制NLRP3炎性小体活化。免疫荧光和Western blot实验同时表明, 五味子甲素对NLRP3炎症小体活性的抑制作用不依赖于NF-κB通路活性及其诱导的NLRP3、ASC、pro-IL-1β等炎性小体蛋白的表达, 而是通过阻断caspase-1活化, 抑制caspase-1对pro-IL-1β的剪切活化, 进而抑制NLRP3炎性小体的活性, 减轻免疫炎症反应。本研究为五味子甲素防治NLRP3炎性小体相关的疾病提供了参考依据。
| [1] | Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes[J]. Nat Rev Immunol, 2013, 13: 397–411. DOI:10.1038/nri3452 |
| [2] | Franchi L, Eigenbrod T, Muñoz-Planillo R, et al. The inflammasome:a caspase-1-activation platform that regulates immune responses and disease pathogenesis[J]. Nat Immunol, 2009, 10: 241–247. |
| [3] | Qing Y, Zhang Q, Zhou JG. Innate immunity functional gene polymorphisms and gout susceptibility[J]. Gene, 2013, 524: 412–414. DOI:10.1016/j.gene.2013.04.039 |
| [4] | Jourdan T, Godlewski G, Cinar R, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes[J]. Nat Med, 2013, 19: 1132–1140. DOI:10.1038/nm.3265 |
| [5] | Tan MS, Yu JT, Jiang T, et al. The NLRP3 inflammasome in Alzheimer's disease[J]. Mol Neurobiol, 2013, 48: 875–882. DOI:10.1007/s12035-013-8475-x |
| [6] | Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China (中华人民共和国药典)[M]. Beijing:China Medical Science Press, 2015. |
| [7] | Wu J, Cao Y, Zhang Y, et al. Deoxyschizandrin, a naturally occurring lignan, is a specific probe substrate of human cytochrome P4503A[J]. Drug Metab Dispos, 2014, 42: 94–104. |
| [8] | Chang R, Li Y, Yang X, et al. Protective role of deoxyschizandrin and schisantherin A against myocardial ischemiareperfusion injury in rats[J]. PLoS One, 2013, 8: e61590. DOI:10.1371/journal.pone.0061590 |
| [9] | Liu C, Cao YF, Fang ZZ, et al. Strong inhibition of deoxyschizandrin and schisantherin A toward UDP-glucuronosyltransferase (UGT) 1A3 indicating UGT inhibition-based herbdrug interaction[J]. Fitoterapia, 2012, 83: 1415–1419. DOI:10.1016/j.fitote.2012.08.004 |
| [10] | Lu Y, Wang WJ, Song YZ, et al. The protective mechanism of schisandrin A in d-galactosamine-induced acute liver injury through activation of autophagy[J]. Pharm Biol, 2014, 52: 1302–1307. DOI:10.3109/13880209.2014.890232 |
| [11] | Yang D, Liu Z, Chen Y, et al. Effect of deoxyschizandrin on inflammatory factors in mononuclear phagocyte[J]. Chin J Gerontol (中国老年学杂志), 2013, 23: 5911–5913. |
| [12] | Yan Y, Jiang W, Spinetti T, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation[J]. Cell, 2013, 27: 1154–1163. |
| [13] | Mu R, Li T, Gao Y, et al. The technical system establishment of NF-κB signaling screen by HCS[J]. Sci Technol Engin, 2011, 14: 1671–1815. |
| [14] | Martinon F, Burns K, Tschopp J. The inflammasome:a molecular platform triggering activation of inflammatory caspases and processing of pro-IL-β[J]. Mol Cell, 2002, 10: 417–426. DOI:10.1016/S1097-2765(02)00599-3 |
| [15] | Bauernfeind F, Hornung V. Of inflammasomes and pathogens-sensing of microbes by the inflammasome[J]. EMBO Mol Med, 2013, 5: 814–826. DOI:10.1002/emmm.201201771 |
| [16] | Franchi L. The inflammasome:a caspase-1-activation platform that regulates immune responses and disease pathogenesis[J]. Nat Immunol, 2009, 10: 241–247. |
| [17] | Guarda G, Zenger M, Yazdi AS, et al. Differential expression of NLRP3 among hematopoietic cells[J]. J Immunol, 2011, 186: 2529–2534. DOI:10.4049/jimmunol.1002720 |
| [18] | Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes[J]. Nat Immunol, 2012, 13: 325–332. DOI:10.1038/ni.2231 |
| [19] | Bergsbaken T, Fink SL, Cookson BT. Pyroptosis:host cell death and inflammation[J]. Nat Rev Microbiol, 2009, 7: 99–109. DOI:10.1038/nrmicro2070 |
| [20] | Yang CS, Shin DM, Jo EK. The role of NLR-related protein 3 inflammasome in host defense and inflammatory diseases[J]. Int Neurourol J, 2012, 16: 2–12. DOI:10.5213/inj.2012.16.1.2 |
| [21] | Yan Y, Jiang W, Liu L, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome[J]. Cell, 2015, 15: 62–73. |
| [22] | Menu P, Vince JE. The NLRP3 inflammasome in health and disease:the good, the bad and the ugly[J]. Clin Exp Immunol, 2011, 166: 1–15. DOI:10.1111/j.1365-2249.2011.04440.x |
| [23] | Tsutsui H, Imamura M, Fujimoto J, et al. The TLR4/TRIFmediated activation of NLRP3 inflammasome underlies endotoxin-induced liver injury in mice[J]. Gastroenterol Res Pract, 2010, 2010: 641865. |
| [24] | Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases[J]. Annu Rev Immunol, 2011, 29: 707–735. DOI:10.1146/annurev-immunol-031210-101405 |
 2017, Vol. 52
2017, Vol. 52


