草莓番石榴 (Psidum littorale Raddi) 为桃金娘科 (Myrtaceae) 番石榴属 (Psidium) 植物,在广东、广西及福建均有种植[1]。因果肉多汁,味如草莓故得名草莓番石榴。草莓番石榴具有抗氧化、抗肿瘤、止泻等多种功效[2, 3],而有关草莓番石榴的化学成分研究较少,目前报道了少数几个黄酮类成分[3-5]。据此,作者对草莓番石榴植物进行了系统的化学成分研究。之前作者曾报道从草莓番石榴90%乙醇提取物中分离得到4个四甲基环己烯单萜类及3个木脂素类化合物[6],此次报道从其中分离到16个黄酮类化合物,包括1个新化合物,10个首次在此植物中分离得到的化合物,以及1个首次报道其核磁共振碳氢谱数据化合物。
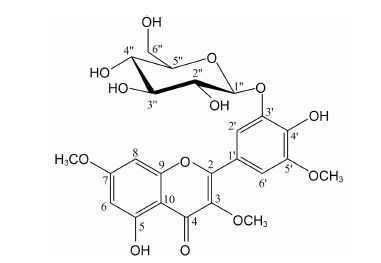
化合物10 黄色粉末 (甲醇),[α]D25 -29.3 (c0.032,MeOH); HR-ESI-MS 给出准分子离子峰m/z: 521.130 1 [M-H]- (计算值521.129 5,C24H25O13); UV λmax (MeOH) nm (logε): 362 (4.2),254 (3.2); 1H NMR和13C NMR数据见表 1。
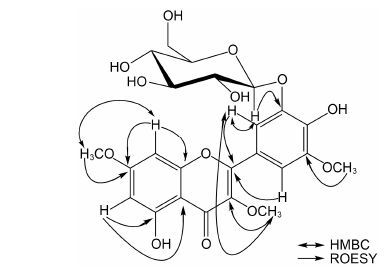
UV具有黄酮类化合物特征性的带Ⅰ和带Ⅱ吸收 (带Ⅰ = 362 nm,带Ⅱ = 254 nm),提示化合物为黄酮类 (图 1)。HR-ESI-MS 给出准分子离子峰m/z: 521.130 1 [M-H]- (计算值521.129 5),提示分子式为C24H26O13,不饱和度为12。1H NMR (表 1) 显示,A环具有1对邻位偶合质子: δH 6.68 (1H,d,J = 2.3 Hz,H-8) 和6.28 (1H,d,J = 2.3 Hz,H-6); B环具有1对邻位偶合质子: δH 7.73 (1H,br s,H-2') 和7.56 (1H,br s,H-6'); 3个甲氧基信号: δH 3.82 (3H,s),3.88 (3H,s),3.91 (3H,s)。另外,结合HSQC谱分析,化合物具有葡萄糖结构单元: δH 4.85 (1H,d,J = 7.6 Hz,H-1'' ),3.42~3.54 (4H,m,H-2'',3'',4'',5''),3.95 (1H,dd,J = 2.0,12.0 Hz,H-6'' a),3.76 (1H,dd,J = 5.6,12.0 Hz,H-6'' b); δC 104.7 (C-1''),75.0 (C-2''),77.8 (C-3'' ),71.6 (C-4''),78.6 (C-5''),62.8 (C-6'')。综合上述分析,提示化合物10为含有二个羟基和三个甲氧基的黄酮苷。再根据碳谱的化学位移及糖端基质子偶合常数 (J = 7.6 Hz),提示所连接的糖单元为β-葡萄糖。化合物10经酸 水解,气相色谱检测,保留时间与β-D-葡萄糖对照 品结果一致,提示化合物所连接的糖为β-D-葡萄糖。HMBC谱中 (图 2),两个间位偶合的芳香质子7.73 (br s) 和7.56 (br s) 均与158.2 (C-2) 有远程偶合,提示上述两个质子为B环上H-2和H-6,进一步提示化合物B环具有C-3',C-4',C-5' 三取代特征; HMBC谱显示,糖端基质子4.85 (d,J = 7.6 Hz,H-1'') 与147.9 (C-3') 具有远程偶合,提示葡萄糖连接在C-3' 位,并且在ROESY谱中 (图 2),葡萄糖端基质子4.85 (d,J = 7.6 Hz,H-1'') 与7.73 (s,H-2') 相关,7.73 (s,H-2') 与3.82 (s,3-OCH3) 相关,进一步证实了上述结论。 3个甲氧基3.82 (3H,s)、3.88 (3H,s)、3.91 (3H,s) 分别与139.8 (C-3)、167.4 (C-7) 和150.5 (C-5') 有远程偶合,提示化合物分别在C-3、C-7和C-5' 有甲氧基取代。综上分析,化合物10的结构鉴定为5,4'-二羟基-3,7,5'-三甲氧基黄酮-3'-O-β-D-葡萄糖苷,经Scifinder检索,化合物10为新化合物。
| Table 1 1H NMR (MeOH-d4,600 MHz) and 13C NMR (MeOH- d4,150 MHz) data of compound 10 |
|
Figure 1 Structure of compound 10 |
|
Figure 2 Key HMBC and ROESY of compound 10 |
AB Sciex 5600质谱仪; Bruker AVANCE Ⅲ HD 600 MHz型核磁共振波谱仪 (瑞士布鲁克公司),TMS为内标; DMSO-d6、MeOH-d4为溶剂。Finnigan Trace DSQ气质联用色谱仪 (美国Thermo公司); Perkin Elmer 343型旋光测定仪 (美国Perkin Elmer公司); Aglient 1100制备液相 (美国安捷伦公司),YMC-Actus ODS-A C18 (250 mm × 10 mm,5 µm) 半制备柱 (日本YMC公司)。柱色谱硅胶 (200~300目),薄层色谱硅胶 (青岛海洋化工),凝胶Sephadex LH-20 (日本三菱株式会社),显色剂为5% 浓硫酸乙醇溶液。常规分析纯化学试剂购自国药集团化学试剂有限公司。
草莓番石榴叶于2014年7月中旬采自广西省柳州,由江西中医药大学付小梅副教授鉴定,标本 (No.201407001) 现存于江西中医药大学现代中药制剂教育部重点实验室。
1 提取与分离自然干燥的草莓番石榴叶 (5.4 kg) 粉碎,经90%乙醇加热回流3次,每次1 h,提取液减压回收得浸膏1.2 kg,所得浸膏用适量水分散,然后依次用石油醚、乙酸乙酯萃取,剩余部分蒸干后,用甲醇溶解。回收溶剂得石油醚部分152.0 g、乙酸乙酯部分113.2 g、甲醇部分400.5 g。
取乙酸乙酯萃取部位 (150 g) 进行硅胶柱色谱分离,以二氯甲烷-甲醇 (100∶0,50∶1,25∶1,10∶1,5∶1,1∶1,0∶100) 梯度洗脱得到Fr.1~7。Fr.2 (20.7 g) 经硅胶柱色谱 (二氯甲烷-甲醇= 55∶1),得Fr.2-1~2-60。合并Fr.2-20~2-30,再经半制备柱色谱 (甲醇-水=55∶45,3 mL·min-1),得到化合物1 (7 mg,tR = 19.5 min)。合并Fr.2-36~2-40,再经半制备柱色谱 (甲醇-水 = 50∶50,3 mL·min-1),得到化合物14 (7 mg,tR = 17.6 min)、2 (8 mg,tR = 10.5 min)、3 (9 mg,tR = 13.8 min)。合并Fr.2-45~2-50,再经半制备柱色谱 (甲醇-水 = 40∶60,3 mL·min-1),得到化合物7 (8 mg,tR = 15.3 min)。Fr.4 (41.1 g) 经硅胶柱色谱 (二氯甲 烷-甲醇 = 20∶1),得Fr.4-1~4-190。合并Fr.4-12~4-15,再经Sephadex LH-20柱色谱 (CH2Cl2-MeOH = 1∶1),得到化合物15 (12 mg)。合并Fr.4-21~4-28,再经Sephadex LH-20柱色谱 (CH2Cl2-MeOH = 1∶1),得到化合物4 (12 mg)。合并Fr.4-36~4-42,再经Sephadex LH-20柱色谱 (CH2Cl2-MeOH = 1∶1),得到化合物5 (5 mg)、8 (10 mg)。合并Fr.4-49~4-56,再经Sephadex LH-20柱色谱 (CH2Cl2-MeOH = 1∶1),得到化合物6 (22 mg)、9 (9 mg)。合并Fr.4-101~4-121,再经Sephadex LH-20柱色谱 (CH2Cl2-MeOH = 1∶1),得到化合物10 (8 mg)。合并Fr.4-133~4-144,再经 半制备柱色谱 (甲醇-水 = 35∶65,3 mL·min-1),得到化合物11 (9 mg,tR = 11.7 min)。Fr.5 (19.8 g) 经MCI树脂 (GEL-CHP 20P,80 mm × 30 mm) 柱,用水- 甲醇 (100∶0,7∶3,4∶6,1∶9,0∶100) 梯度洗脱,得Fr.5-1~5-5。Fr.5-2再经Sephadex LH-20柱色谱 (CH2Cl2-MeOH = 1∶1),得到化合物12 (16 mg)。Fr.5-3再经Sephadex LH-20柱色谱 (MeOH),得Fr. 5-3-1~5-3-80,得到化合物13 (8 mg)。合并Fr.5-3- 63~5-3-75,再经半制备柱色谱 (甲醇-水 = 30∶70,3 mL·min-1),得到化合物16 (9 mg,tR = 12.3 min)。
2 结构鉴定化合物1 黄色粉末 (甲醇),分子式C15H10O6。1H NMR (CD3OD,600 MHz) δH: 6.15 (1H,d,J = 2.0 Hz,H-6),6.36 (1H,d,J = 2.0 Hz,H-8),6.87 (2H,m,H-3',5'),8.05 (2H,m,H-2',6'); 13C NMR (CD3OD,150 MHz) δC: 146.9 (C-2),131.8 (C-3),175.9 (C-4),160.8 (C-5),98.2 (C-6),163.8 (C-7),93.4 (C-8),156.3 (C-9),102.6 (C-10),121.4 (C-1'),129.6 (C-2'),115.7 (C-3'),159.3 (C-4'),115.4 (C-5'),129.2 (C-6')。以上1H NMR、13C NMR数据与文献[7]一致,故鉴定化合物1为山柰酚。
化合物2 黄色粉末 (二氯甲烷-甲醇),分子式C16H12O7。1H NMR (DMSO-d6,600 MHz) δH: 3.84 (3H,s,3'-OCH3),6.19 (1H,d,J = 2.1 Hz,H-6),6.47 (1H,d,J = 2.1 Hz,H-8) ,6.94 (1H,d,J = 8.5 Hz,H-5'),7.68 (1H,dd,J = 8.4,2.1 Hz,H-6'),7.75 (1H,d,J = 2.1 Hz,H-2'); 13C NMR (DMSO-d6,150 MHz) δC: 146.6 (C-2),135.8 (C-3),175.9 (C-4),160.7 (C-5),98.2 (C-6),164.0 (C-7),93.6 (C-8),156.2 (C-9),103.0 (C-10),122.0 (C-1'),111.7 (C-2'),148.8 (C-3'),147.4 (C-4'),115.5 (C-5'),121.7 (C-6'),55.8 (3'-OCH3)。以上1H NMR、13C NMR数据与文献[8]一致,故鉴定化合物2为isorhamnetin。
化合物3 黄色粉末 (甲醇),分子式C18H16O8。1H NMR (CD3OD,600 MHz) δH: 3.82 (3H,s,H-3),3.89 (3H,s,H-3'),3.93 (3H,s,H-7),6.33 (1H,d,J = 2.2 Hz,H-6),6.60 (1H,d,J = 2.2 Hz,H-8),7.36 (2H,s,H-2',6'); 13C NMR (CD3OD,150 MHz) δC: 158.3 (C-2),140.1 (C-3),180.2 (C-4),162.9 (C-5),99.1 (C-6),167.5 (C-7),93.3 (C-8),158.4 (C-9),106.9 (C-10),121.8 (C-1'),105.4 (C-2'),149.6 (C-3'),139.3 (C-4'),146.8 (C-5'),111.1 (C-6'),56.6 (7-OCH3),56.9 (3'-OCH3),60.7 (3-OCH3)。以上1H NMR、13C NMR数据与文献[9]一致,故鉴定化合物3为myricetin-3,7,3'-trimethyl ether。
化合物4 黄色粉末 (甲醇),分子式C16H12O8。1H NMR (CD3OD,600 MHz) δH: 3.92 (3H,s,3'-OMe),6.18 (1H,d,J = 2.1 Hz,H-6),6.40 (1H,d,J = 2.1 Hz,H-8),7.43 (1H,d,J = 2.0 Hz,H-6'),7.47 (1H,d,J = 2.0 Hz,H-2'); 13C NMR (CD3OD,150 MHz) δC: 149.5 (C-2),138.0 (C-3),177.5 (C-4),104.6 (C-4a),162.7 (C-5),99.4 (C-6),165.9 (C-7),94.6 (C-8),158.4 (C-8a),123.2 (C-1'),105.1 (C-2'),147.9 (C-3'),1 37.6 (C-4'),146.6 (C-5'),110.3 (C-6'),56.9 (-OCH3)。以上1H NMR、13C NMR数据与文献[10]一致,故鉴定化合物4为 laricitrin。
化合物5 黄色粉末 (甲醇),分子式C15H10O7。1H NMR (CD3OD,600 MHz) δH: 6.17 (1H,d,J = 2.0 Hz,H-6),6.38 (1H,d,J = 2.0 Hz,H-8),6.88 (1H,d,J = 8.5 Hz,H-5'),7.63 (1H,dd,J = 8.5,2.1 Hz,H-6'),7.73 (1H,d,J = 2.1 Hz,H-2'); 13C NMR (CD3OD,150 MHz) δC: 148.7 ( C-2),137. 1 (C-3),177.2 (C-4),162.7 ( C-5),99.2 (C-6),165.6 (C-7),94.4 (C-8),158.2 ( C-9),104.8 (C-10),124.1 (C-1'),115.9 (C-2'),146.1 (C-3'),147.9 (C-4'),116.1 (C-5'),121.6 (C-6')。以上1H NMR、13C NMR数据与文献[11]一致,故鉴定化合物5为槲 皮素。
化合物6 黄色粉末 (甲醇),分子式C15H10O8。1H NMR (CD3OD,600 MHz) δH: 6.08 (1H,s,H-6),6.27 (1H,s,H-8),7.25 (2H,s,H-2',6')。13C NMR (CD3OD,150 MHz) δC: 148.1 (C-2),137.0 (C-3),177.3 (C-4),162.6 (C-5),99.4 (C-6),165.8 (C-7),94.5 (C-8),158.3 (C-9),104.6 (C-10),123.2 (C-1'),108.6 (C-2',6'),146.8 (C-3',5'),137.5(C-4')。以上1H NMR、13C NMR数据与文献[12]一致,故鉴定化合物6为杨梅素。
化合物7 黄色粉末 (甲醇),分子式C17H14O7。1H NMR (CD3OD,600 MHz) δH: 3.80 (3H,s,OCH3),3.92 (3H,s,OCH3),6.20 (1H,d,J = 2.1 Hz,H-8),6.41 (1H,d,J = 2.1 Hz,H-6),7.08 (1H,d,J = 9.0 Hz,H-5'),7.33 (2H,d,J = 2.1 Hz,H-2',6'); 13C NMR (CD3OD,150 MHz) δC: 56.3 (OCH3),60.5 (OCH3),158.0 (C-2),139.2 (C-3),105.9 (C-10),180.1 (C-4),163.2 (C-5),100.0 (C-6),166.4 (C-7),94.9 (C-8),158.6 (C-9),125.5 (C-1'),115.9 (C-2'),146.9 (C-3'),149.6 (C-4'),114.8 (C-5'),121.9 (C-6')。以上1H NMR、13C NMR数据与文献[13]一致,故鉴定化合物7为quercein-3,4'-dimethyl ether。
化合物8 黄色粉末 (甲醇),分子式C20H18O11。1H NMR (CD3OD,600 MHz) δH: 3.44 (1H,br d,J = 10.5 Hz,glc-H-5),3.64 (1H,br d,J = 8.0 Hz,glc-H-3),3.84~3.81 (2H,m,glc-H-4,6),3.89 (1H,m,glc-H-2),5.16 (1H,d,J = 6.5 Hz,glc-H-1),6.20 (1H,br s,H-6),6.39 (1H,br s,H-8),6.86 (1H,d,J = 8.4 Hz,H-5'),7.55 (1H,d,J = 8.4 Hz,H-6'),7.75 (1H,s,H-2')。13C NMR (CD3OD,150 MHz) δC: 159.1 (C-2),136.1 (C-3),179.9 (C-4),163.4 (C-5),100.3 (C-6),166.4 (C-7),95.0 (C-8),158.8 (C-9),104.8 (C-10),123.3 (C-1'),117.9 (C-2'),146.5 (C-3'),150.1 (C-4'),116.6 (C-5'),123.3 (C-6'),106.1 (glc-C-1),74.5 (glc-C-2),73.3 (glc-C-3),69.5 (glc-C-4),67.1 (glc-C-5)。以上1H NMR、13C NMR数据与文献[14]一致,故鉴定化合物8为番石榴苷。
化合物9 黄色粉末 (甲醇),分子式C21H20O12。1H NMR (CD3OD,600 MHz) δH: 3.45~3.82 (2H,m,glc-H-2~6),5.14 (1H,d,J = 7.6 Hz,glc-H-1),6.16 (1H,br s,H-6),6.32 (1H,br s,H-8),6.85 (1H,d,J = 8.2 Hz,H-5'),7.56 (1H,d,J = 8.2 Hz,H-6'),7.70 (1H,br s,H-2')。13C NMR (CD3OD,150 MHz) δC: 159.5 (C-2),136.1 (C-3),180.1 (C-4),163.5 (C-5),100.4 (C-6),166.5 (C-7),95.0 (C-8),1 58.9 (C-9),105.8 (C-10),123.5 (C-1'),118.3 (C-2'),146.3 (C-3'),150.3 (C-4'),116.6 (C-5'),123.5 (C-6'),106.0 (glc-C-1),73.5 (glc- C-2),75.6 (glc-C-3),70.5 (glc-C-4),77.5 (glc-C-5),62.3 (glc-C-6)。以上1H NMR、13C NMR数据与文献[15]一致,故鉴定化合物9为金丝桃苷。
化合物11 无定形粉末 (甲醇),分子式C21H20O12。1H NMR (CD3OD,600 MHz) δH: 3.13 (1H,m,H-5''b),3.42 (1H,d,J = 8.7 Hz,H-3''),3.49 (1H,m,H-4''),3.52 (1H,m,H-5''a),3.81 (1H,dd,J = 11.6,5.2 Hz,H-2''),3.91 (3H,s,OMe),5.24 (1H,d,J = 7.3 Hz,H-1''),6.20 (1H,d,J = 2.1 Hz,H-6),6.40 (1H,d,J = 2.1 Hz,H-8),7.24 (1H,d,J = 2.0 Hz,H-2'),7.52 (1H,d,J = 2.0Hz,H-6'); 13C NMR (CD3OD,15MHz) δC: 158.6 (C-2),135.6 (C-3),179.5 (C-4),163.2 (C-5),100.2 (C-6),166.5 (C-7),94.9 (C-8),158.6 (C-9),105.7 (C-10),122.0 (C-1'),111.1 (C-2'),146.5 (C-3'),139.0 (C-4'),149.1 (C-5'),106.9 (C-6'),104.7 (C-1''),75.6 (C-2''),77.7 (C-3''),71.2 (C-4''),67.4 (C-5''),57.0 (OMe)。未查阅到文献报道此化合物的1H NMR和13C NMR数据,根据HSQC及HMBC二维图谱对此化合物进行归属,鉴定化合物11为laricitrin-3-O-xyloside。
化合物12 黄色粉末 (甲醇),分子式C21H20O12。1H NMR (CD3OD,600 MHz) δH: 0.96 (3H,d,J = 6.2 Hz,H-6''),3.49~4.20 (4H,m,H-2'',3'',4'',5''),5.31 (1H,s,H-1''),6.18 (1H,d,J = 2.1 Hz,H-6),6.34 (1H,d,J = 2.1 Hz,H-8),6.94 (2H,s,H-2',6'); 13C NMR (CD3OD,150 MHz) δC: 159.5 (C-2),136.4 (C-3),179.7 (C-4),163.3 (C-5),99.9 (C-6),165.9 (C-7),94.8 (C-8),158.6 (C-9),106.0 (C-10),122.0 (C-1'),109.7 (C-2',6'),146.9 (C-3',5'),138.0 (C-4'),103.7 (C-1''),72.0 (C-2''),72.1 (C-3''),73.5 (C-4''),72.2 (C-5''),17.8 (C-6'')。以上 1H NMR、13C NMR数据与文献[16]一致,故鉴定化合物12为杨梅素-3-O-α-L-鼠李吡喃糖苷。
化合物13 黄色粉末 (二氯甲烷-甲醇),分子式C20H18O12。1H NMR (DMSO-d6,600 MHz) δH: 2.96~3.65 (5H,m,H-2'',H-3'',H-4'',H-5''),5.35 (1H,d,J = 7.4 Hz,H-1''),6.20 (1H,d,J = 2.1 Hz,H-6),6.37 (1H,d,J = 2.0 Hz,H-8),7.16 (2H,s,H-2' ,6'),8.94 (1H,s,4'-OH),9.23 (2H,s,3'-OH,5'-OH),10.87 (1H,s,7-OH),12.63 (1H,s,5-OH); 13C NMR (DMSO-d6,150 MHz) δC: 156.2 (C-2),133.2 (C-3),177.3 (C-4),161.2 (C-5),98.6 (C-6),164.1 (C-7),93.3 (C-8),156.3 (C-9),103.9 (C-10),119.8 (C-1'),108.4 (C-2',6'),145.5 (C-3',5'),136.8 (C-4'),101.8 (C-1''),76.1 (C-2''),73.4 (C-3''),69.3 (C-4''),66.1 (C-5'')。以上1H NMR、13C NMR数据与文献[17]一致,故鉴定化合物13为杨梅素-3-O-β-D-吡喃木糖苷。
化合物14 浅黄色固体 (甲醇),分子式C16H14O7。1H NMR (CD3OD,600 MHz) δH: 3.87 (3H,s,4'-OCH3),4.56 (1H,d,J = 11.6 Hz,H-3),4.97 (1H,d,J = 11.6 Hz,H-2),5.92 (1H,d,J = 2.0 Hz,H-8),6.82 (1H,d,J = 8.1 Hz,H-5'),6.96 (1H,dd,J = 8.1,2.0 Hz,H-6'),7.10 (1H,d,J = 2.0 Hz,H-2'); 13C NMR (CD3OD,150 MHz) δC: 85.4 (C-2),73.8 (C-3),198.5 (C-4),164.6 (C-5),168.2 (C-7),96.5 (C-8),165.4 (C-9),101.9 (C-10),112.6 (C-1'),122.3 (C-2'),148.5 (C-3'),149.1 (C-4'),116.1 (C-5'),130.0 (C-6'),56.6 (4'-OCH3)。以上1H NMR、13C NMR数据与文献[18]一致,故鉴定化合物14为4'-甲氧基二氢槲皮素。
化合物15 浅黄色粉末 (甲醇),分子式C15H12O5。1H NMR (CD3OD,600 MHz) δH: 3.11 (1H,d,J = 13.0 Hz,H-3),5.34 (1H,d,J = 13.0 Hz,H-2),5.89 (1H,s,H-8),6.81 (2H,d,J = 8.6 Hz,H-3',5'),7.31 (2H,d,J = 8.6 Hz,H-2',6'); 13C NMR (CD3OD,150 MHz) δC: 80.6 (C-2),44.2 (C-3),197.9 (C-4),165.6 (C-5),168.7 (C-7),165.0 (C-9),103.4 (C-10),131.3 (C-1'),129.2 (C-2',6'),116.5 (C-3',5'),159.2 (C-4')。以上1H NMR、13C NMR数据与文献[19]一致,故鉴定化合物15为二氢芹菜素。
化合物16 淡黄色粉末 (甲醇),分子式C21H22O13。1H NMR (CD3OD,600 MHz) δH: 4.43 (1H,d,J = 11.3 Hz,H-3),4.60 (1H,d,J = 7.8 Hz,H-1''),5.87 (1H,s,H-8),6.58 (2H,s,H-2',6'); 13C NMR (CD3OD,150 MHz) δC: 84.9 (C-2),73.8 (C-3),197.9 (C-4),165.4 (C-5),169.7 (C-7),96.7 (C-8),164.4 (C-9),101.7 (C-10),136.3 (C-1'),108.4 (C-2'),151.6 (C-3'),135.0 (C-4'),151.6 (C-5'),108.4 (C-6'),107.9 (C-1''),75.2 (C-2''),77.8 (C-3''),70.8 (C-4''),78.7 (C-5''),62.0 (C- 6''),以上1H NMR、13C NMR数据与文献[20]一致,故鉴定化合物16为蛇葡萄素4'-O-β-D-吡喃葡萄糖苷。
3 化合物10酸水解参照文献[21],称取化合物10 2 mg,加入3 mol·L-1三氯乙酸5 mL,120 ℃加热2 h,蒸干,干燥过夜。依次加入20 μL (2S)-1-氨基-2-丙醇/甲醇 (1/8) 混合液,17 μL乙酸/甲醇 (1∶4) 及13 μL 3% 氰基硼氢化钠甲醇溶液。上述混合液放置65 ℃水浴加热2 h。冷却后缓慢加入3 mol·L-1三氟乙酸至pH为1,减压回收,残渣真空干燥过夜,再加入吡啶和乙酸酐各0.2 mL,置100 ℃水浴加热1 h。冷却后,加适量水,用三氯甲烷萃取,用0.5 mol·L-1 Na2CO3和水各洗滤3次。三氯甲烷层加入无水硫酸钠干燥后过滤,注入气质联用色谱仪。
| [1] | Delectis florae republicae popularis sinicae agendae acade-miae sinicaen. Flora Republicae Popularis Sinicae (中国植物志)[M]. Beijing: Science Press, 1984 : 123 . |
| [2] | Moon JY, Mosaddik A, Kim H, et al. The chloroform fraction of guava (Psidium cattleianum Sabine) leaf extract inhibits human gastric cancer cell proliferation via induction of apop-tosis[J]. Food Chem , 2011, 125 :369–375. DOI:10.1016/j.foodchem.2010.09.007 |
| [3] | Ho R, Violette A, Cressend D, et al. Antioxidant potential and radical-scavenging effects of flavonoids from the leaves of Psidium cattleianum grown in French Polynesia[J]. Nat Prod Res , 2012, 26 :274–277. DOI:10.1080/14786419.2011.585610 |
| [4] | Pino JA, Marbot R, Vázquez C. Characterization of volatiles in strawberry guava (Psidium cattleianum Sabine) fruit[J]. J Agric Food Chem , 2001, 49 :5883–5887. DOI:10.1021/jf010414r |
| [5] | Dachriyanus D, Salni S, Sargent MV, et al. Rhodomyrtone, an antibiotic from Rhodomyrtus tomentosa[J]. Aust J Chem , 2002, 55 :229–232. DOI:10.1071/CH01194 |
| [6] | Peng CY, Liu JQ, Shu JC, et al. Study on megastigmane glycosides and lignan constituents from leaves of Psidium littorale[J]. J Chin Med Mater (中药材) , 2014, 37 :2201–2203. |
| [7] | Qin LH, Guo XY, Fan M, et al. Anti-anoxic constituents from Mesona chinensis Benth[J]. J Shenyang Pharm Univ (沈阳药科大学学报) , 2006, 23 :633–636. |
| [8] | Shi TX, Li YG. Isolation of flavonoids from the aerial parts of Polygala tenuifolia Willd. and their antioxidant activities[J]. J Chin Pharm Sci , 2013, 22 :36–39. |
| [9] | de Carvalho Correia AC, Macêdo CL, de Souza Monteiro F, et al. Aerial parts of Solanum agrarium Sendtn. (Solanaceae) present the flavonoid myricetin 3,7,3' trimethyl ether and anti-spasmodic effect on guinea-pig ileum by blockade of voltage-gated calcium channels[J]. J Med Plants Res , 2013, 31 :2293–2299. |
| [10] | Zhu CF, Liu HX, He L, et al. Chemical constituents from leaves of Rhodomyrtus tomentosa[J]. J Trop Subtrop Bot (热带亚热带植物学报) , 2015, 23 :103–108. |
| [11] | Huang FJ, Song JX, Liu JJ, et al. Chemical constituents in Thunbergia from Africa[J]. China J Chin Mater Med (中国中药杂志) , 2013, 38 :1183–1187. |
| [12] | Zhou XL, Qin CH, Mei Y, et al. Chemical constituents from leaves of Rhododendron anthopogon D. Don[J]. Chin Tradit Herb Drugs (中草药) , 2010, 41 :206–208. |
| [13] | Kwon YS, Kim MC. Antioxidant constituents from the stem of Sorghum bicolor[J]. Arch Pharm Res , 2003, 26 :535–539. DOI:10.1007/BF02976877 |
| [14] | Hidetoshi A, Gen-ichi D. Isolation of antimicrobial com-pounds from guava (Psidium guajauv L.) and their structural elucida-tion[J]. Biosci Biotechnol Biochem , 2002, 66 :1727–1730. DOI:10.1271/bbb.66.1727 |
| [15] | Yang CX, Jia ZJ. Flavonoids from Heteropappus semiprost Griers[J]. Nat Prod Res Dev, (天然产物研究与开发) , 2007, 19 :430–432. |
| [16] | Li YL, Li KM, Su MX, et al. Studies on antiviral constitu-ents in stems and leaves of Pithecellibium clypearia[J]. China J Chin Mater Med (中国中药杂志) , 2006, 31 :397–400. |
| [17] | Sun CL, Tang XL, Zhou JF, et al. Chemical components of flavonoids from leaves of Zelkova serrata[J]. Chin Pharm J (中国药学杂志) , 2015, 50 :1803–1805. |
| [18] | Zhang N, Wei XY, Lin LD. Chemical constituents from the leaves of Siraitia grosvenorii (Swingle) C. Jeffrey[J]. J Trop Subtrop Bot (热带亚热带植物学报) , 2014, 22 :96–100. |
| [19] | Bai LM, Gao HY, Ma YK, et al. Study on chemical constituents from Gnaphalium affine and their anti-oxidative activities[J]. Chin Tradit Herb Drugs (中草药) , 2016, 47 :549–553. |
| [20] | Gao Y, Yuan JZ, Wang YX, et al. Isolation and identifica-tion of flavonoids from pine needle of Pinus koraiensis Sieb. et Zucc[J]. J Shenyang Pharm Univ (沈阳药科大学学报) , 2010, 27 :539–543. |
| [21] | Xin W, Chou G, Wang Z. Triterpenoids and saponins from the leaves of Uncaria hirsute[J]. Helv Chem Acta , 2009, 92 :638–644. DOI:10.1002/hlca.v92:4 |
 2016, Vol. 51
2016, Vol. 51


