2. 军事医学科学院毒物药物研究所, 抗毒药物与毒理学国家重点实验室, 北京 100850
2. State Key Laboratory of Toxicology and Medical Countermeasures, Institute of Pharmacology and Toxicology, Academy of Military Medical Science, Beijing 100850, China
脑缺血再灌注 (cerebral ischemia-reperfusion,CIR) 损伤是指脑缺血一定时间后恢复血液供应,脑功能不但不能恢复,却出现更加严重的功能障碍。CIR后引起一系列病理生理损伤,包括局部神经元蛋白质合成停止,神经元去极化,钙离子内流,兴奋性氨基酸大量释放,导致线粒体功能改变,诱发神经元凋亡等。在引起CIR损伤的诸多因素中,备受重视的是氧自由基的产生[1]。氧自由基过量产生可导致脂质过氧化、DNA损伤等。因此,减少自由基的生成,清除氧自由基,抑制氧自由基引起的过氧化反应,是减轻氧化应激损伤的重要途径[2, 3]。
研究表明,氧化应激与磷酸化Akt有密切关系,可有效影响CIR病理进展[4]。Akt可以激活核转录 因子E2相关因子2 (nuclear factor erythroid 2 related factor 2,Nrf2),在抗氧化应激中发挥重要作用。Nrf2是细胞发生氧化应激反应的关键因子及中枢调节 者[5, 6],诱导多种抗氧化酶及解毒酶,加速酶促反应,提高谷胱甘肽及超氧化物质,维持细胞内的氧化还原水平,发挥细胞保护作用[7-9]。血红素氧合酶 (heme oxygenase-1,HO-1) 作为一种重要的Ⅱ相解毒酶,可以通过催化血红素代谢为一氧化碳、铁、胆绿素等发挥其抗氧化能力[10]。
丹酚酸A (salvianolic acid A,SAA) 是唇形科植物丹参的干燥根及根茎中含有的一种水溶性酚酸类化合物,具有多种药理学活性,尤其具有很强的抗氧化作用,能清除氧自由基,降低细胞内钙[11-14]。最近有研究显示SAA可以通过Nrf2/HO-1信号通路减轻H2O2诱导的细胞氧化损伤[15],还可以通过靶向Nrf2治疗糖尿病并发症及离子辐射损伤[16],但其能否通过Nrf2/HO-1途径减轻大鼠CIR损伤有待进一步研究,本文对这一作用机制进行了考察。
材料与方法动物 SPF级雄性SD大鼠,体重240~260 g,购自北京维通利华动物技术有限公司,合格证号: SCXK (京) 2014-0004。
试剂 RPMI 1640培养基、胎牛血清、马血清均购自Gibco; Triton-100购自Amresco; Nrf2 siRNA®、siRNA Reagent System以及Nrf2、HO-1、histone H3抗体均购自Santa Cruz; SAA,中国医学科学院药物研究所自制; 依达拉奉 (edaravone) 购自吉林省辉 南长龙生化药业股份有限公司; 水合氯醛购自国药集团化学试剂有限公司; Hoechst 33258、细胞核蛋白与细胞浆蛋白抽提试剂盒购自碧云天生物技术有限公司; TTC、PMSF均购自Sigma-Aldrich; 蛋白裂解液、β-actin、GAPDH、Anti-rabbit IgG (H+L),F(ab')2 fragment (Alexa Fluor® 488 Conjugate) 抗体均购自Cell Signaling Technology; BCA蛋白定量试剂盒、高灵敏度化学发光检测试剂盒、goat anti-rabbit IgG、goat anti-mouse IgG均购自康为世纪; 蛋白marker购自Thermo Scientific。
仪器 MCAO线栓,购自北京西浓科技有限公司; Cannon E05 450D照相机,购自佳能公司; Eclipse Ti-U荧光显微镜,购自尼康公司; 蛋白电泳仪及转膜仪、Molecular Imager ChemiDoc XRS+System曝光仪购自Bio-Rad; Thermo Scientific ArrayScan Infinity高内涵分析仪,购自Thermo Scientific。
动物模型建立 10% 水合氯醛 (380 mg·kg-1) 腹腔注射麻醉大鼠。仰卧位固定,剪去颈前鼠毛,碘伏消毒,颈正中线切口,钝性分离,沿胸锁乳突肌内缘分离肌肉和筋膜,分离右侧颈总动脉 (CCA)、颈外动脉 (ECA) 和颈内动脉 (ICA)。用1号缝合线勒紧CCA近心端及ICA。结扎ECA远心端,并于ECA下埋1号缝合线用于固定线栓,在ECA上剪出斜行切口,插入线栓,进入18 mm左右,固定线栓。用蘸生理盐水的棉花保护颈部伤口。术后用电热毯为大鼠保持体温。缺血1.5 h后,拔出线栓,实现再灌注[17]。逐层缝合伤口,并用碘伏处理伤口。大鼠归笼饲养。
行为学检测 再灌注24 h,处死动物前进行神经行为学评分,采用Bederson’s评分标准[18]。具体操作: 提起鼠尾离开地面,观察大鼠两前肢伸展状况,然后将大鼠置于水平地面,观察其爬行情况,推动其双肩,观察两侧抵抗力有无差异。采用四级评分法 (0~4分),分数越高,说明其神经行为损伤越严重。
脑梗死体积测定 神经行为评分后,断头处死大鼠,迅速取出脑组织并置于 -20 ℃冰箱,15 min后取出置于大鼠脑切片模具中,切除嗅球、小脑和低位脑干后,间隔2 mm冠状切片。然后迅速将脑切片置于0.5% TTC溶液中,于37 ℃避光孵育20 min。经TTC染色后,正常组织呈玫瑰红色,而缺血梗死组织呈苍白色。取出脑片,置于4% 的多聚甲醛溶液中固定。最后将每只大鼠的脑片排列整齐,拍照保存[19]。采用Image J图像分析软件计算每张脑片的梗死面积。
脑水肿检测 断头处死大鼠后,迅速取出脑组织,称湿重,然后将脑组织置于100 ℃恒温干燥箱中,烘干24 h至恒重即为干重。脑水肿百分比 = (湿重- 干重) / 湿重× 100%[20]。
细胞培养 PC12细胞用含10% 马血清和5% 胎牛血清的RPMI 1640培养液,37 ℃、5% CO2培养,0.25% 胰酶消化传代。
MTT法检测细胞存活率 采用MTT法测定SAA对缺糖缺氧复糖复氧 (OGD/R) 损伤的PC12细胞存活率的影响。将PC12细胞以8000 cells/100 L/ well接种于96孔板,24 h后撤血清,换低糖RPMI 1640培养基,置于三气培养箱 (1% O2,5% CO2,94% N2) 37 ℃培养6 h,之后更换正常RPMI 1640培养基,同时加药,在CO2培养箱 (95% 空气,5% CO2) 37 ℃继续培养24 h。然后每孔加入0.5 mg·mL-1 MTT溶液100 L,4 h以后DMSO溶解MTT,并于570 nm处测定吸光度。
免疫荧光检测Nrf2和HO-1 采用免疫荧光法测定SAA对OGD/R损伤的PC12细胞Nrf2和HO-1表达的影响。同“MTT法检测细胞存活率”接种细胞并加药处理,弃去原培养液,用生理盐水洗一次,加入4% 多聚甲醛37 ℃固定15 min; 生理盐水洗一 次,加入0.3% Triton-100 37 ℃孵育10 min; 用生理盐水洗一次,加入5% BSA 37 ℃封闭1 h; 之后加入稀释后的一抗,4 ℃孵育过夜; 生理盐水洗3次,每次10 min; 加入相应的荧光二抗稀释液,37 ℃孵育1.5 h,生理盐水洗3次,每次10 min; 加入Hoechst于37 ℃孵育30 min; 生理盐水洗一次,使用Thermo Scientific ArrayScan Infinity高内涵分析仪进行检测。
Nrf2 siRNA转染PC12细胞 将PC12细胞以2×105 cells/2 mL/well接种于6孔板,24 h后细胞密 度达到约60%; 每孔加入siRNA transfection medium 0.8 mL,轻轻混匀,覆盖细胞。37 ℃、CO2培养箱培养6 h,舍弃含有转染试剂的培养液,加入正常培养基,继续培养24 h,提取蛋白进行Western blot检测。
Western blot分析 Western blot法检测Nrf2、HO-1蛋白表达变化,提取组织、细胞总蛋白或用细胞核与细胞浆蛋白抽提试剂盒分别提取细胞核蛋白和细胞浆蛋白,用BCA蛋白定量试剂盒进行定量。加入SDS上样缓冲液,沸水浴10 min,以同等蛋白含量进行SDS-PAGE电泳后转PVDF膜,然后用5% BSA室温封闭2 h,加入稀释后的一抗,4 ℃孵育过夜; TBST洗3次,每次10 min; 加入相应的二抗稀释液,室温孵育1 h,TBST洗3次,每次10 min; 用ECL发光法检测不同样品各种蛋白表达情况。
统计学分析 实验结果以means ± SD表示,应用SPSS 19.0统计软件对各组数据进行处理,采用单因素方差分析 (one-way ANOVA),各组间差异采用Tukey’s post hoc test计算,P<0.05为具有统计学意义。
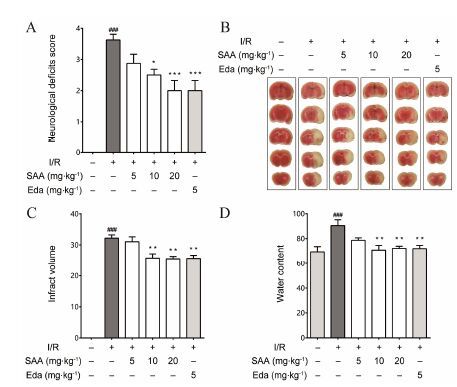
结果 1 SAA改善脑缺血再灌注大鼠神经行为学变化由于大脑中动脉阻塞伤及调控机体运动的脑区,动物会表现出严重的运动功能失调的神经行为学损伤症状。大脑中动脉闭塞/再灌注 (MCAO/R) 造成某一侧调控机体运动的脑区发生损伤,则导致一侧肢体肌无力,运动时向无力侧旋转,表现出典型的追尾或倾倒等症状。结果如图 1A所示,大鼠MCAO后1.5 h,再灌注24 h可造成明显的神经功能障碍,多数动物出现追尾或向一侧倾倒。SAA可剂量依赖性地改善MCAO/R引起的神经功能损伤症状,显著降低神经行为学评分。阳性药依达拉奉也可显著降低MCAO/R大鼠神经行为学评分。
|
Figure 1 SAA protects against middle cerebral artery occlusion and reperfusion (MACO/R) injury in rats. (A) Salvianolic acid A (SAA) improves the neurological deficit scores in MCAO/R rats (n = 8); (B) Illustrative coronal sections showing infarct area detected by TTC staining; (C) Effects of SAA on brain infarct volume in MCAO/R rats (n = 6); (D) SAA attenuates brain water content in rats subjected to 1.5 h of MCAO followed by 24 h repe rfusion (n = 6). Eda: Edaravone. Data are expressed as means ± SD and analyzed by one-way ANOVA. ###P<0.001 vs Sham group; P<0.05,**P<0.01,***P<0.001 vs I/R group |
脑梗死体积是反映抗MCAO/R损伤药物药效最直接指标。MCAO手术1.5 h,再灌注24 h,大鼠缺血脑半球出现梗死,梗死体积达32%。SAA中高剂量组和阳性药依达拉奉治疗组可显著减小MCAO/R大鼠脑梗死体积,其降低百分比为21% (图 1B,1C)。
3 SAA降低脑缺血再灌注大鼠脑水肿程度MCAO/R因炎症反应和血脑屏障破坏及细胞膜损伤,会产生血管源性及细胞毒性脑水肿。如图 1D显示,大鼠MCAO 1.5 h,再灌注24 h,缺血半球出 现明显水肿,与正常组相比水肿程度增加30%。而SAA中高剂量组和阳性药依达拉奉治疗组均可显著减小MCAO/R引起的水肿程度,与模型组相比,其减小百分比分别为22%、20% 和21%。
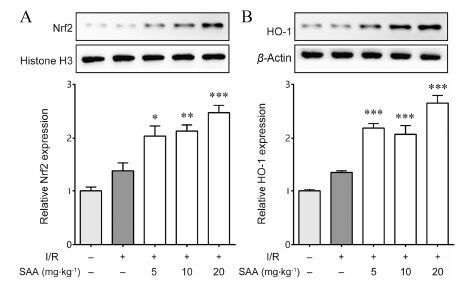
4 SAA对脑缺血再灌注大鼠Nrf2通路关键蛋白表达的影响如图 2显示,与Sham组相比,MCAO/R组Nrf2总蛋白的表达没有明显变化,但与Sham组及MCAO/R组相比,SAA治疗组能够剂量依赖地增加Nrf2因子总蛋白的表达 (图 2A)。同时,与Sham组相比,MCAO/R组HO-1总蛋白的表达没有明显变化,但与Sham组及MCAO/R组相比,SAA各剂量组都能够显著增加HO-1总蛋白的表达 (图 2B)。
|
Figure 2 Effects of SAA on Nrf2 and HO-1 levels in rat brain of rats subjected to 1.5 h of MCAO followed by 24 h reperfusion. The nuclear factor erythroid 2 related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) levels were determined by Western blot. (A) The effects of SAA on Nrf2 levels in rat brain,histone H3 served as loading control; (B) The effects of SAA on HO-1 levels in rat brain,β-actin served as loading control. n = 6,x± s. P<0.05,**P<0.01,***P<0.001 vs I/R group |
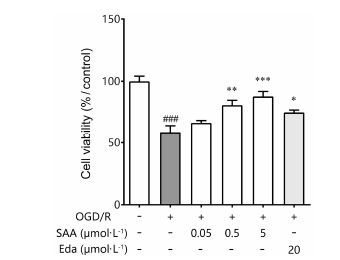
MTT检测结果显示,缺糖缺氧6 h,复糖复氧 24 h后,PC12细胞存活率显著降低,只有正常对照组的58%。中高浓度的SAA (0.5和5 μmol·L-1) 可以降低OGD/R对PC12细胞造成的损伤,提高PC12细胞存活率。随着药物浓度的增加,PC12细胞的存活率逐渐升高,0.5 μmol·L-1 SAA治疗组细胞存活率为80%,5 μmol·L-1 SAA治疗组细胞存活率为87%,其作用呈浓度-效应依赖性。20 μmol·L-1阳性药依达拉奉治疗组细胞存活率为75% (图 3)。
|
Figure 3 Protection effects of SAA on cell viability. n = 6,x± s. ###P<0.001 vs Sham group; P<0.05,**P<0.01,***P<0.001 vs oxygen and glucose deprivation/reoxygenation (OGD/R) group |
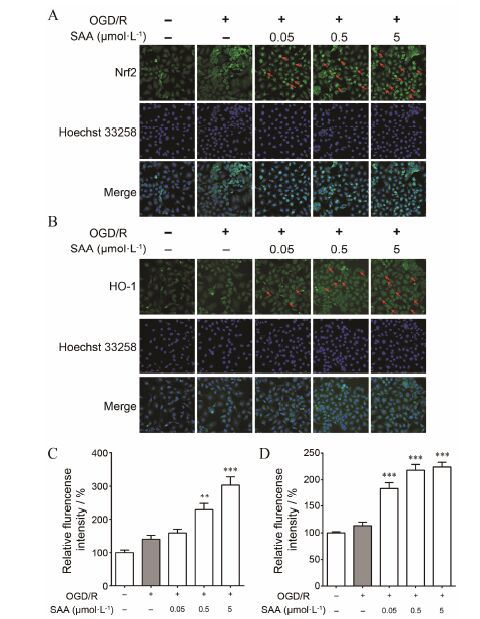
细胞免疫荧光化学检测结果显示,正常PC12细胞内Nrf2表达量较低,细胞核内Nrf2绿色荧光强度很低,OGD/R处理后,Nrf2表达没有显著性变化,细胞核内Nrf2绿色荧光强度依然很低。而0.5和5 μmol·L-1的SAA,能够显著增加Nrf2表达,且主 要表达在细胞核 (图 4A,如红色箭头所示)。正常和OGD/R处理后PC12细胞内HO-1表达很低,而0.05、0.5和5 μmol·L-1 SAA给药组均能够显著增加HO-1表达 (图 4B)。提示,SAA对OGD/R诱导的PC12细胞损伤的保护作用,可能是通过促进Nrf2合成和核转位、促进HO-1表达产生效应。
|
Figure 4 Effects of SAA on expression of Nrf2 and HO-1 in PC12 cells after OGD/R injury. Representative double immunofluorescent staining for (A) Nrf2/Hoechst and (B) HO-1/Hoechst are shown in OGD/R injury. Quantitative analysis of the expression of (C) Nrf2 and (D) HO-1. n = 6,x± s. **P<0.01,***P<0.001 vs OGD/R group |
利用Western blot方法检测Nrf2 siRNA和转染PC12细胞后,Nrf2和HO-1的表达情况。如图 5显 示,5 μmol·L-1 SAA能够促进正常和空白siRNA干扰组的Nrf2核转位,但是对Nrf2 siRNA干扰组没有明显作用 (图 5A)。对Nrf2 siRNA干扰组HO-1的表达进行检测,结果表明Nrf2 siRNA转染PC12细胞能 够降低SAA引起的HO-1表达增加 (图 5B)。进一步的MTT检测结果表明,在Nrf2 siRNA存在的情况 下,SAA并不能减轻OGD/R对PC12细胞的损伤 (图 5C)。以上结果表明,SAA对PC12细胞损伤的保护作用,确实是通过激活Nrf2/HO-1信号途径、促进Nrf2合成和核转位、进而促进下游HO-1表达产生效应。
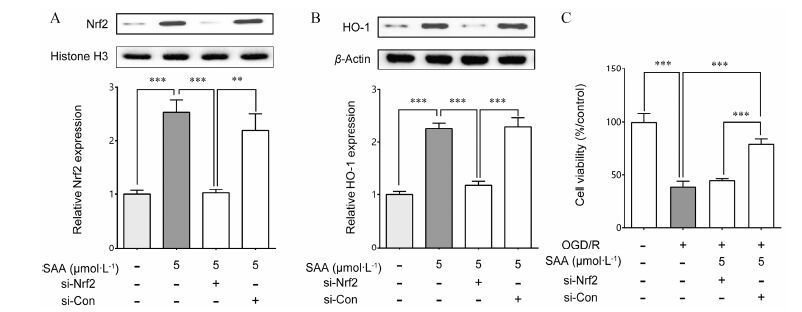
|
Figure 5 SAA induces expression of Nrf2 and HO-1 in PC12 cells. (A) PC12 cells were transfected with control or Nrf2 siRNA,followed by treatment with SAA. Nuclear extracts were analyzed for Nrf2 levels,histone H3 served as loading control; (B) PC12 cells were transfected with control or Nrf2 siRNA,followed by treatment with SAA,analyzed HO-1 levels,β-actin served as loading control; (C) Inhibition effects of Nrf2 siRNA on cell viability. n = 6,x± s. **P<0.01,***P<0.001 vs other groups |
本实验在整体动物水平选用SD大鼠建立MCAO/R模型,以此探讨SAA对MCAO/R大鼠脑组织损伤的保护作用。实验结果表明,SAA可以改善MCAO/R导致的大鼠左侧肢体偏瘫,降低神经行为学评分[14, 21],缩小脑梗死体积,降低脑水肿程度。SAA对MCAO/R大鼠的脑保护作用[22, 23],可能是通过上调Nrf2及HO-1的表达水平实现的。
Nrf2作为亮氨酸拉链家族调节抗氧化应激反应的重要转录因子,具有协同调节编码药物代谢酶与抗氧化蛋白的作用[24-26],通过与细胞核中ARE应答元件结合,从而上调HO-1基因表达,抗氧化酶HO-1具有强大的细胞防护功能[27, 28]。因此本研究检测了脑组织中Nrf2和HO-1的蛋白表达水平。在正常和MCAO/R组大鼠中,Nrf2和HO-1蛋白表达量很低,SAA给药组能够剂量依赖地增加Nrf2和HO-1蛋白表达。
为了进一步证实体内实验结果,确定OGD/R对细胞氧化应激损伤的影响。本实验选用大鼠肾上腺嗜铬细胞瘤细胞PC12建立OGD/R损伤模型,以此来模拟MCAO/R损伤的病理过程,探讨SAA对OGD/R损伤处理的神经元细胞的保护作用[29, 30]。实验结果显示,对正常和OGD/R损伤PC12细胞,SAA均可剂量依赖性地诱导Nrf2蛋白表达增加。在正常PC12细胞中,SAA可诱导HO-1蛋白表达上升; 当OGD/R刺激导致氧化应激时,活性氧会诱导抗氧化酶HO-1上调,SAA给药组使HO-1的表达达到最高,证明由SAA引起的氧化应激减少从而对细胞发挥抗氧化保护作用与其上调抗氧化酶HO-1的表达有关。
为了进一步明确Nrf2基因的作用,SAA诱导HO-1表达增加是否与Nrf2的激活有关,本文探索了SAA对siRNA干扰Nrf2的PC12细胞中Nrf2和HO-1表达的影响。RNA干扰是指由靶基因序列同源的双链RNA启动的一种序列特异性的转录后基因沉默现象。采用化学合成的siRNA特异性的封闭靶基因Nrf2,结果显示,SAA在正常情况下上调Nrf2和HO-1的现象,在转染siRNA Nrf2后受到明显的抑制。证明Nrf2转导通路是SAA发挥抗氧化作用的关键机制,SAA正是通过激活Nrf2的信号转导,进一步促进下游HO-1表达上升,实现细胞保护作用。
综上所述,SAA具有抗脑缺血再灌注损伤的作用,其机制与激活Nrf2/HO-1信号途径、促进Nrf2合成和核转位、从而促进下游抗氧化蛋白HO-1的表达有关。
| [1] | Vallon M, Chang J, Zhang H, et al. Developmental and pathological angiogenesis in the central nervous system[J]. Cell Mol Life Sci , 2014, 71 :3489–3506. DOI:10.1007/s00018-014-1625-0 |
| [2] | Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention[J]. Trends Pharmacol Sci , 2013, 34 :313–319. DOI:10.1016/j.tips.2013.03.007 |
| [3] | del Zoppo GJ. Stroke and neurovascular protection[J]. N Engl J Med , 2006, 354 :553–555. DOI:10.1056/NEJMp058312 |
| [4] | Tang H, Pan CS, Mao XW, et al. Role of NADPH oxidase in total salvianolic acid injection attenuating ischemia-reperfusion impaired cerebral microcirculation and neurons: implication of AMPK/Akt/PKC[J]. Microcirculation , 2014, 21 :615–627. DOI:10.1111/micc.2014.21.issue-7 |
| [5] | Abed DA, Goldstein M, Albanyan H, et al. Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents[J]. Acta Pharm Sin B , 2015, 5 :285–299. DOI:10.1016/j.apsb.2015.05.008 |
| [6] | Zhao CY, Wang XL, Peng Y. Role of Nrf2 in neurodegenera-tive diseases and recent progress of its activators[J]. Acta Phram Sin (药学学报) , 2015, 50 :375–384. |
| [7] | Hybertson BM, Gao B, Bose SK, et al. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activa-tion[J]. Mol Aspects Med , 2011, 32 :234–246. DOI:10.1016/j.mam.2011.10.006 |
| [8] | Shah ZA, Li RC, Thimmulappa RK, et al. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury[J]. Neuroscience , 2007, 147 :53–59. DOI:10.1016/j.neuroscience.2007.02.066 |
| [9] | Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo[J]. J Neurosci , 2005, 25 :10321–10335. DOI:10.1523/JNEUROSCI.4014-05.2005 |
| [10] | Li M, Zhang X, Cui L, et al. The neuroprotection of oxyma-trine in cerebral ischemia/reperfusion is related to nuclear factor erythroid 2-related factor 2(Nrf2)-mediated antioxidant response: role of Nrf2 and hemeoxygenase-1 expression[J]. Biol Pharm Bull , 2011, 34 :595–601. DOI:10.1248/bpb.34.595 |
| [11] | Lin TJ, Zhang KJ, Liu GT. Effects of salvianolic acid A on oxygen radicals released by rat neutrophils and on neutrophil function[J]. Biochem Pharmacol , 1996, 51 :1237–1241. DOI:10.1016/0006-2952(96)00067-6 |
| [12] | Li YJ, Duan CL, Liu JX. Salvianolic acid A promotes the acceleration of neovascularization in the ischemic rat myocar-dium and the functions of endothelial progenitor cells[J]. J Ethnopharmacol , 2014, 151 :218–227. DOI:10.1016/j.jep.2013.10.019 |
| [13] | Xu H, Li Y, Che X, et al. Metabolism of salvianolic acid A and antioxidant activities of its methylated metabolites[J]. Drug Meta Dispos , 2014, 42 :274–281. |
| [14] | Du GH, Zhang JT. Protective effects of salvianolic acid A against impairment of memory induced by cerebral ische-mia-reperfusion in mice[J]. Acta Phram Sin (药学学报) , 1995, 30 :184–190. |
| [15] | Zhang H, Liu YY, Jiang Q, et al. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling[J]. Free Radic Med , 2014, 69 :219–228. DOI:10.1016/j.freeradbiomed.2014.01.025 |
| [16] | Wu P, Yan Y, Ma LL, et al. Effects of the Nrf2 modulator salvianolic acid A alone or combined with metformin on diabetes-associated macrovascular and renal injury[J]. J Biol Chem , 2016, 291 :22288–22301. DOI:10.1074/jbc.M115.712703 |
| [17] | Mhairi MI. New models of focal cerebral ischaemia[J]. Br J Clin Pharmacol , 1992, 34 :302–308. DOI:10.1111/j.1365-2125.1992.tb05634.x |
| [18] | Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats[J]. Stroke , 1989, 20 :84–91. DOI:10.1161/01.STR.20.1.84 |
| [19] | Clark WM, Lessov NS, Dixon MP, et al. Monofilament intraluminal middle cerebral artery occlusion in the mouse[J]. Neurol Res , 1997, 19 :641–648. DOI:10.1080/01616412.1997.11740874 |
| [20] | Mdzinarishvili A, Kiewert C, Kumar V, et al. Bilobalide prevents ischemia-induced edema formation in vitro and in vivo[J]. Neuroscience , 2007, 144 :217–222. DOI:10.1016/j.neuroscience.2006.08.037 |
| [21] | Du G, Zhang J. Protective effects of salvianolic acid A against impairment of memory induced by cerebral ische-mia-reperfusion in mice[J]. Chin Med J (Engl) , 1997, 110 :65–68. |
| [22] | Ding Y, Chen M, Wang M, et al. Neuroprotection by ace-tyl-11-keto-β-boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway[J]. Sci Rep , 2014, 4 :7002–7010. DOI:10.1038/srep07002 |
| [23] | Lee H, Park YH, Jeon YT, et al. Sevoflurane post-condi-tioning increases nuclear factor erythroid 2-related factor and haemoxygenase-1 expression via protein kinase C pathway in a rat model of transient global cerebral ischaemia[J]. Br J Anaesth , 2015, 114 :307–318. DOI:10.1093/bja/aeu268 |
| [24] | Kaspar JW, Niture SK, Jaiswal AK. Nrf2: INrf2(Keap1) signaling in oxidative stress[J]. Free Radic Biol Med , 2009, 47 :1304–1309. DOI:10.1016/j.freeradbiomed.2009.07.035 |
| [25] | Wakabayashi N, Skoko JJ, Chartoumpekis DV, et al. Notch-Nrf2 axis: regulation of Nrf2 gene expression and cytoprotection by notch signaling[J]. Mol Cell Biol , 2014, 34 :653–663. DOI:10.1128/MCB.01408-13 |
| [26] | Han J, Xiao Q, Lin YH, et al. Neuroprotective effects of salidroside on focal cerebral ischemia/reperfusion injury involve the nuclear erythroid 2-related factor 2 pathway[J]. Neural Regen Res , 2015, 10 :1989–1996. DOI:10.4103/1673-5374.172317 |
| [27] | Hu T, Wei G, Xi M, et al. Synergistic cardioprotective effects of Danshensu and hydroxysafflor yellow A against myocardial ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1 pathway[J]. Int J Mol Med , 2016, 38 :83–94. |
| [28] | Huang XP, Qiu YY, Wang B, et al. Effects of astragaloside IV combined with the active components of Panax notogin-seng on oxidative stress injury and nuclear factor-erythroid 2-related factor 2/heme oxygenase-1 signaling pathway after cerebral ischemia-reperfusion in mice[J]. Pharmacogn Mag , 2014, 10 :402–409. DOI:10.4103/0973-1296.141765 |
| [29] | Soane L, Li Dai W, Fiskum G, et al. Sulforaphane protects immature hippocampal neurons against death caused by exposure to hemin or to oxygen and glucose deprivation[J]. J Neurosci Res , 2010, 88 :1355–1363. |
| [30] | Qi HY, Li L, Yu J, et al. Proteomic identification of Nrf2-mediated phase Ⅱ enzymes critical for protection of tao hong si wu decoction against oxygen glucose deprivation injury in PC12 cells[J]. Evid Based Complement Alternat Med , 2014, 2014 :945814. |
 2016, Vol. 51
2016, Vol. 51


