类风湿关节炎 (rheumatoid arthritis) 是一种慢性、以滑膜炎为主的自身免疫性疾病,病理特征主要包括炎症反应、关节疼痛、肿胀、滑膜增生和血管翳形成,并伴随软骨和骨的破坏[1, 2]。另外,类风湿关节炎影响大约0.7%~1% 的成年人,并且女性的患病率是男性的两倍[3, 4]。人滑膜细胞是炎症细胞因子和基质金属蛋白酶 (MMP) 如IL-1β、IL-6、IL-8和MMP-1的一个主要来源,这些炎症介质都在类风湿关节炎的病理生理学中起到关键作用[5]。在病理情况下,炎症因子生产过剩会引起细胞因子失衡,唤起炎性损伤,甚至导致骨侵蚀、畸形和关节损坏[6, 7]。因此,对于类风湿关节炎的治疗研究主要集中在抑制炎症因子表达等方面。
在过去的20年中,植物药用化合物的广泛研究已经揭示了五环三萜类化合物具有极其重要的药理活性。五环三萜类化合物来源广泛,水果、蔬菜、谷类、豆类、植物和药用植物都是其主要来源,由于其抗炎和抗癌活性强,引起了人们极大的关注[8]。齐墩果酸 (oleanolic acid,图 1A) 又名士当归酸,是一种广泛分布于食品和药用植物中的五环三萜类化合物[9, 10],相对无毒,有保护肝脏的作用,并且表现出很强的抗肿瘤和抗炎特性[11, 12]。虽然齐墩果酸的抗炎作用已经通过大量的研究证实,但是尚未发现其在人滑膜细胞 (SW982) 中的研究,SW982细胞是研究类风湿关节炎中的炎性细胞因子或基质金属蛋白酶表达的有用工具。因此,本文主要研究齐墩果酸在SW982细胞中的抗炎作用的相关分子机制。
在本课题中,作者研究了在IL-1β刺激后,齐墩果酸对SW982细胞的抗炎作用,并分析了相关的信号通路,以确定其分子机制。
材料与方法材料 人滑膜肉瘤细胞SW982细胞从美国组织培养物保藏中心获得。齐墩果酸 (C30H48O3,HPLC ≥98%),购买于上海源叶生物科技有限公司; IL-1β、胎牛血清、细胞蛋白裂解液RIPA和MTT购自Sigma公司; 抗p-ERK、p-Akt、p-p38、p-JNK、p38、JNK2、ERK2、Akt、IκB-α和p65抗体购自于Cell Signaling Technology公司; 抗β-actin抗体购自北京中杉金桥生物技术有限公司。兔和小鼠的二抗购自Cell Signaling Technology公司; Bradford购自BIO-RAD公司; 化学发光液ECL购自Millipore公司; ELISA试剂盒购 自欣博盛生物科技有限公司。Trizol购自Invitrogen公司; 5×ALL-In-One RT Master Mix和Eva Green 2×qPCR Master Mix-Low ROX购自南京爱必梦生物材料有限公司; IL-6、IL-8、MMP-1和GAPDH的引物由苏州金唯智生物科技有限公司合成。
仪器 7500实时荧光定量PCR仪 (ABI公司); 荧光定量酶标仪 (BioTech公司); 电泳仪 (BIO-RAD公司); Amersham Imager 600化学发光成像仪 (GE Healthcare Bio-sciences AB公司)。
细胞培养和处理 SW982细胞用含15% 胎牛 血清的RPMI 1640培养基,在37 ℃、5% CO2饱和 湿度下培养。设未用任何药物处理的空白对照组、10 ng·mL-1 IL-1β组、5 μmol·L-1齐墩果酸 + 10 ng·mL-1 IL-1β组、10 μmol·L-1齐墩果酸 + 10 ng·mL-1 IL-1β组、20 μmol·L-1齐墩果酸 + 10 ng·mL-1 IL-1β组。当用药物处理细胞时,更换为1% 胎牛血清的培养基进行培养。
MTT法检测齐墩果酸对SW982细胞生长的影响 取对数生长期SW982细胞接种于96孔板,每孔100 μL,细胞数为每孔1×104个,在37 ℃、5% CO2培养箱中培养24 h后,分别设置对照组、0.1、1、10、40和80 μmol·L-1浓度的齐墩果酸组,每组设5个平行孔,处理24 h后每孔加入MTT (50 mg·mL-1) 10 μL,继续培养4 h。弃去上清液,每孔加入DMSO 100 μL,振荡10 min后,使用酶标仪在570 nm处检测吸光度值 (OD),对照组设置为100%,给药组与对照组相比,计算细胞抑制率。
ELISA检测实验 按照试剂盒的说明进行检测,以测得的OD为纵坐标,对应的标准物浓度为横坐标做标准曲线。采用10 ng·mL-1 IL-1β建立炎症模型,先用不同浓度的齐墩果酸 (5、10、20 μmol·L-1) 处理SW982细胞1 h后,然后用10 ng·mL-1 IL-1β刺激SW982细胞24 h后,提取其细胞上清液,测定IL-6、IL-8和MMP-1的OD。根据标准曲线及OD计算IL-6、IL-8和MMP-1的浓度变化情况。
Western blot分析 以不同浓度的齐墩果酸 (5、10、20 μmol·L-1) 处理SW982细胞30 min,加入10 ng·mL-1 IL-1β处理30 min,利用RIPA裂解液裂解细胞,提取总蛋白,并用Bradford测定总蛋白的浓度。之后采用聚丙烯酰胺凝胶电泳(SDS-PAGE) 分离,转膜,用5% 脱脂牛奶封闭1 h,一抗4℃孵育过夜,TBST漂洗3次后,二抗室温孵育40 min,洗膜后用ECL发光液以及全自动曝光仪曝光。用Image J软件对蛋白条带进行扫描,定量分析相应蛋白的表达变化情况。
实时荧光定量PCR分析 取SW982细胞,经不同浓度齐墩果酸 (5、10、20 μmol·L-1) 处理30 min后,加入10 ng·mL-1 IL-1β处理6 h,收集细胞,按照试剂盒的要求,采用Trizol试剂提取细胞总RNA。根据反转录试剂盒说明书逆转录合成cDNA的第一条链后,以此为模版,进行实时荧光定量PCR (real- time PCR) 检测IL-6、IL-8和MMP-1基因的表达变化,引物序列如表 1所示。PCR的反应程序: 95 ℃预变性30 s,95 ℃变性5 s,64 ℃退火延伸30 s,共40个循环。基因的相对表达量用2-ΔΔCt法计算,采用小鼠GAPDH作为内对照。
| Table 1 Primer sequences used for real-time PCR |
统计学分析 实验数据统计分析采用Graphpad Prism 5统计软件进行统计分析,结果以均数±标准差来表示,并用单因素方差分析进行处理,组间进行t检验,以P<0.05认为差异具有统计学意义。
结果 1 齐墩果酸对SW982细胞生长的影响MTT结果显示,采用不同浓度的齐墩果酸处理SW982细胞,与对照组相比,低浓度的齐墩果酸 (≤40 μmol·L-1) 对细胞几乎没有毒性; 当浓度增大到80 μmol·L-1时,对细胞有明显的毒性 (图 1B),说明低浓度的齐墩果酸对SW982细胞几乎无毒性,高浓度时会明显抑制细胞的存活率 (P<0.001)。
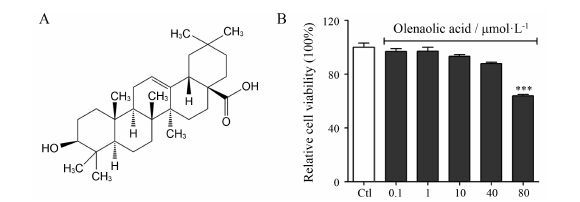
|
Figure 1 The structure of oleanolic acid (A). The effect of oleanolic acid on cell viability (B). MTT assay was performed to assess the cytotoxicity of oleanolic acidon SW982 cells. SW982 cells were treated with med ium only (control,Ctl),oleanolic acid (0.1,1,10,40,80 μmol·L-1). The cell viability was not affected after incubation for 24 h with oleanolic acid at concentrations up to 40 μmol·L-1; However,when the concentration was raised to 80 μmol·L-1,the survival rate of SW982 cells were inhibited. ***P<0.001 vs Ctl group |
ELISA结果显示,与空白对照组相比,在10 ng·mL-1 IL-1β刺激下,细胞炎症因子IL-6、IL-8和MMP-1的含量明显升高,当加入不同浓度的齐墩果酸 (5、10、20 μmol·L-1) 后,其相应的含量水平降低(图 2)。这些结果表明,齐墩果酸能抑制IL-1β刺激的炎症因子的产生。
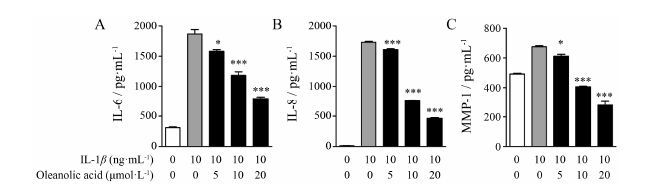
|
Figure 2 Effects of oleanolic acid on interleukin (IL)-1β induced productions of IL-6,IL-8 and matrix metalloproteinase (MMP-1). Cells were pretreated with oleanolic acid (5,10,20 μmol·L-1) for 1 h,then stimulated with IL-1β (10 ng·mL-1) for 24 h. Culture supernatants were collected,and the concentration of IL-6 (A),IL-8 (B) and MMP-1 (C) was measured using ELISA kits according to the instructions supplied by the manufacturer. n = 4,x± s. P<0.05,***P<0.001 vs IL-1β without oleanolic acid |
Real-time PCR结果显示,与对照组相比,当加入10 ng·mL-1 IL-1β后,炎症因子IL-6、IL-8和MMP-1的表达量明显上调,而加入不同浓度的齐墩果酸 (5、10、20 μmol·L-1) 后,炎症因子mRNA的表达量明显下调 (图 3)。这些结果说明,齐墩果酸能在mRNA水平上抑制炎症因子的表达。
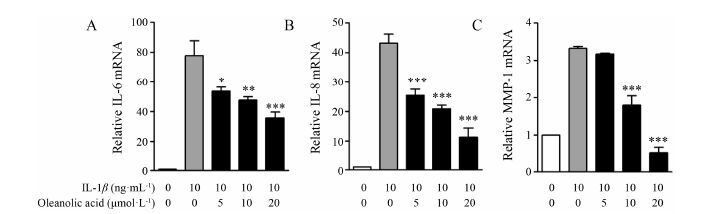
|
Figure 3 Effects of oleanolic acid on IL-1β induced mRNA expression of IL-6,IL-8 and MMP-1. SW982 cells were pretreated with various concentrations (5,10,20 μmol·L-1) of oleanolic acid for 1 h and then stimulated with IL-1β (10 ng·mL-1) for 6 h. Real-time PCR analysis was performed to analyze the expression level of mRNA of IL-6 (A),IL-8 (B) and MMP-1 (C). Relative expression levels of the genes were expressed with GAPDH as internal reference. n = 4,x± s. P<0.05,**P<0.01,***P<0.001 vs IL-1β without oleanolic acid |
Western blot结果发现,与空白对照组相比,在10 ng·mL-1 IL-1β刺激下,MAPK (ERK、p38和JNK) 信号通路有明显的激活作用; 当加入不同浓度齐墩果酸 (5、10、20 μmol·L-1) 后能明显地抑制p-ERK、p-p38、p-JNK的表达 (图 4)。这些结果说明齐墩果酸能显著地抑制IL-1β激活的MAPK信号通路。
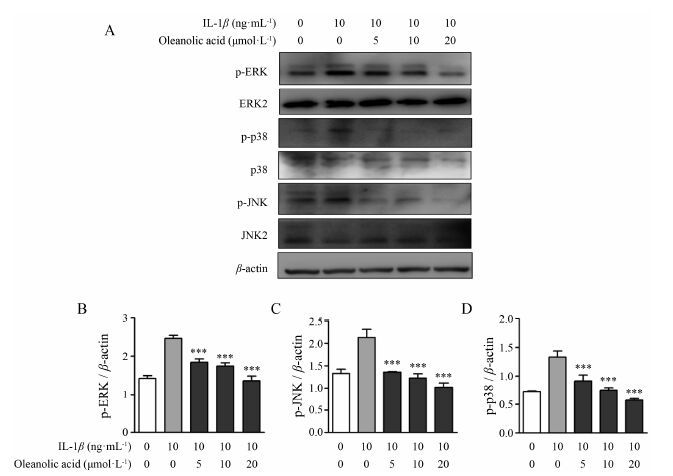
|
Figure 4 Effects of oleanolic acid on IL-1β-induced MAPK signal pathways in SW982 cells. SW982 cells were pretreated with various concentrations (5,10,20 μmol·L-1) of oleanolic acid for 1 h followed by 10 ng·mL-1 IL-1β treatment for 30 min. The protein expressions of p-ERK,p-P38,and p-JNK were determined by Western blot analysis (A). Histogram analysis for the expression levels of p-ERK (B),p-p38 (C) and p-JNK (D) as described above. β-actin was used as loading control. n = 3,x± s. ***P<0.001 vs IL-1β without oleanolic acid |
本研究结果发现,与空白对照组相比,在10 ng·mL-1 IL-1β作用下,p-Akt的表达量明显上调,IκB-α的表达量显著下调; 当加入不同浓度的齐墩果酸 (5、10、20 μmol·L-1) 后,IκB-α的表达量上调 (图 5A,B),p-Akt表达量下调 (图 5C,D),结果显示齐墩果酸能明显地抑制PI3K/Akt和NF-κB信号通路。
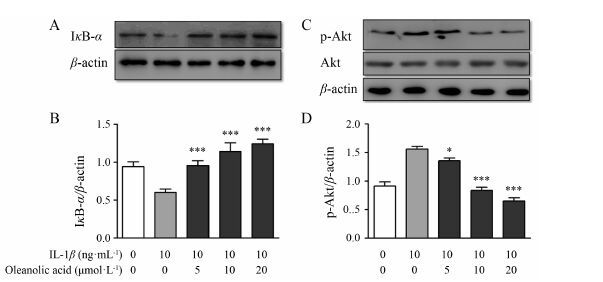
|
Figure 5 Effects of oleanolic acid on IL-1β-induced NF-κB activation and PI3K/Akt signal pathways in SW982 cells. Cells were pretreated with or without various concentrations (5,10,20 μmol·L-1) of oleanolic acid for 1 h and then induced by 10 ng·mL-1 IL-1β treatment for 30 min. The levels of IκB-α (A) and phosphorylated Akt (C) were analyzed by Western blot. Using β-actin was detected as a loading control. Histogram analysis for the expression levels of IκB-α (B) and phosphorylated Akt (D) as described above. n = 3,x± s. P<0.05,***P<0.001 vs IL-1β without oleanolic acid |
大量研究表明,含有五环三萜母核的皂苷化合物具有抗炎作用,并已应用于临床。五环三萜皂苷对炎症活性的影响可能与其结构变化有关。部分研究表明,五环三萜类化合物及其类似物C-3氧化后对5-脂氧化酶对炎症发生过程有较强的抑制作用[13]。桦 木酸及其衍生物通过抑制磷脂酶A2 (PLA2) 的活性从而影响炎症的发生[14]。在治疗组织型多肽抗原诱 导的炎症反应的实验中,科罗索酸也表现出了比抗炎药吲哚美辛 (市售的抗炎药) 更好的抗炎活性[15]。
齐墩果酸属于五环三萜类化合物,具有许多药理活性,包括抗炎、抗病毒、抗骨质疏松症和保肝作用[16]。在抗炎活性方面,Kang等[11]研究发现齐墩果 酸可以抑制脂多糖 (LPS) 刺激的腹腔巨噬细胞产生的促炎症因子TNF-α、IL-1β和IL-6的表达; Hwang等[17]研究证实在RAW264.7细胞中齐墩果酸通过抑制LPS诱导的一氧化氮 (NO) 和前列腺素E2 (PGE2) 具有明显的潜在抗炎活性; Li等[18]研究表明,在胰 岛素抵抗的HepG2细胞中齐墩果酸能下调IL-6和TNF-α的水平。基于以上研究结果,本研究以SW982细胞为研究对象,采用ELISA以及real-time PCR在蛋白和mRNA水平检测炎症因子的表达变化,结果显示齐墩果酸能明显地抑制IL-6、IL-8和MMP-1的表达,进一步在人滑膜细胞中证实了齐墩果酸的抗炎作用。
MAPK家族包括JNK、p38和ERK[19]。MAPK和PI3K/Akt信号通路与炎性介质的表达有关[20, 21]。Li[22]和Tao[23]等研究说明,在HeLa细胞中齐墩果酸能下调ERK1/2和p38的磷酸化,从而抑制MAPK信号通路; Shi等[16]研究发现在HepG2和SMC7721细胞中齐墩果酸能抑制PI3K/Akt1/mTOR信号通路,以上结果均与本研究结果一致。在本实验中,齐墩果酸可以明显地抑制ERK、p38、JNK和Akt的磷酸化,表明齐墩果酸可能是通过MAPK和PI3K/Akt信号 通路来抑制炎症因子的表达。
NF-κB已被公认为是炎症的关键调节因子,是一种多基因靶向的转录因子,控制多种细胞功能,包括免疫炎症反应、细胞黏附、分化、凋亡、应激反应、抗凋亡及多种慢性疾病的进程等[17, 24, 25]。在本研究 中,检测了齐墩果酸处理后IκB-α的表达情况,结果显示,当加入齐墩果酸后,IκB-α的降解被明显抑制,提示齐墩果酸可能是通过NF-κB信号通路来抑制炎症因子的表达。Hwang等[17]研究证明了齐墩果酸在LPS诱导的RAW264.7细胞中是通过NF-κB信号通路抑制炎症因子的表达,印证了本文的结论。
综上所述,本研究证明了齐墩果酸能显著抑制人滑膜细胞中炎症因子的表达,其相应的作用机制可能与MAPK (ERK、p38、JNK)、PI3K/Akt和NF-κB信号通路相关。这些结果为临床治疗类风湿关节炎提供了一定的理论依据。
| [1] | Parolini O, Souza-Moreira L, O'Valle F, et al. Therapeutic effect of human amniotic membrane-derived cells on experi-mental arthritis and other inflammatory disorders[J]. Arthritis Rheumatol , 2014, 66 :327–339. DOI:10.1002/art.v66.2 |
| [2] | Liu Q, Zhu XZ, Feng RB, et al. Crude triterpenoid sapon-ins from Anemone flaccida (Di Wu) exert anti-arthritic effects on type Ⅱ collagen-induced arthritis in rats[J]. Chin Med , 2015, 10 :20. DOI:10.1186/s13020-015-0052-y |
| [3] | Neovius M, Simard JF, Askling J, et al. Nationwide prevalence of rheumatoid arthritis and penetration of disease-modifying drugs in Sweden[J]. Ann Rheum Dis , 2011, 70 :624–629. DOI:10.1136/ard.2010.133371 |
| [4] | Widdifield J, Paterson JM, Bernatsky S, et al. The epidemic-ology of rheumatoid arthritis in Ontario, Canada[J]. Arthritis Rheumatol , 2014, 66 :786–793. DOI:10.1002/art.38306 |
| [5] | Firestein GS. Invasive fibroblast-like synoviocytes in rheu-matoid arthritis. Passive responders or transformed ag-gressors?[J]. Arthritis Rheumatol , 1996, 39 :1781–1790. DOI:10.1002/(ISSN)1529-0131 |
| [6] | Yin MC. Inhibitory effects and actions of pentacyclic triter-penes upon glycation[J]. Biomedicine , 2015, 5 :13. DOI:10.7603/s40681-015-0013-x |
| [7] | Choi EM, Lee YS. Luteolin suppresses IL-1β-induced cytokines and MMPs production via p38 MAPK, JNK, NF-κB and AP-1 activation in human synovial sarcoma cell line, SW982[J]. Food Chem Toxicol , 2010, 48 :2607–2611. DOI:10.1016/j.fct.2010.06.029 |
| [8] | Shanmugam MK, Nguyen AH, Kumar AP, et al. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: potential role in prevention and therapy of cancer[J]. Cancer Lett , 2012, 320 :158–170. DOI:10.1016/j.canlet.2012.02.037 |
| [9] | Liu J. Pharmacology of oleanolic acid and ursolic acid[J]. J Ethnopharmacol , 1995, 49 :57–68. DOI:10.1016/0378-8741(95)90032-2 |
| [10] | Meng YQ, Feng CQ, Zhang LF, et al. Synthesis and anti-tumor activity of oleanolic acid derivatives[J]. Acta Pharm Sin (药学学报) , 2015, 50 :469–474. |
| [11] | Kang GD, Lim S, Kim DH. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway[J]. Int Immunopharmacol , 2015, 29 :393–400. DOI:10.1016/j.intimp.2015.10.024 |
| [12] | Ma XH, Zhao YC, Yin L, et al. Studies on the effect of oleanolic acid on experimental liver injury[J]. Acta Pharm Sin (药学学报) , 1982, 17 :93–97. |
| [13] | Giner-Larza EM, Máñez S, Recio MC, et al. Oleanonic acid, a 3-oxotriterpene from Pistacia, inhibits leukotriene synthesis and has anti-inflammatory activity[J]. Eur J Pharmacol , 2001, 428 :137–143. DOI:10.1016/S0014-2999(01)01290-0 |
| [14] | Bernard P, Scior T, Didier B, et al. Ethnopharmacology and bioinformatic combination for leads discovery: application to phospholipase A2 inhibitors[J]. Phytochemistry , 2001, 58 :865–874. DOI:10.1016/S0031-9422(01)00312-0 |
| [15] | Taniguchi S, Imayoshi Y, Kobayashi E, et al. Production of bioactive triterpenes by Eriobotrya japonica calli[J]. Phytochemistry , 2002, 59 :315–323. DOI:10.1016/S0031-9422(01)00455-1 |
| [16] | Shi Y, Song Q, Hu D, et al. Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway[J]. Korean J Physiol Pharmacol , 2016, 20 :237–243. DOI:10.4196/kjpp.2016.20.3.237 |
| [17] | Hwang YJ, Song J, Kim HR, et al. Oleanolic acid regulates NF-κB signaling by suppressing MafK expression in RAW 264.7 cells[J]. BMB Rep , 2014, 47 :524–529. DOI:10.5483/BMBRep.2014.47.9.149 |
| [18] | Li M, Han ZY, Bei WJ, et al. Oleanolic acid attenuates insulin resistance via NF-κB to regulate the IRS1-GLUT4 pathway in HepG2 cells[J]. Evid Based Complement Alternat Med , 2015, 2015 :643102. |
| [19] | Schett G, Tohidast-Akrad M, Smolen JS, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-jun N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis[J]. Arthritis Rheum , 2000, 43 :2501–2512. DOI:10.1002/(ISSN)1529-0131 |
| [20] | Cohen S, Fleischmann R. Kinase inhibitors: a new ap-proach to rheumatoid arthritis treatment[J]. Curr Opin Rheumatol , 2010, 22 :330–335. DOI:10.1097/BOR.0b013e3283378e6f |
| [21] | Wu TF, Mohan C. The Akt axis as a therapeutic target in autoimmune diseases[J]. Endocr Metab Immune Disord Drug Targets , 2009, 9 :145–150. DOI:10.2174/187153009788452417 |
| [22] | Li Y, Lu X, Qi H, et al. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and suppression of ERK1/2 MAPK in HeLa cells[J]. J Pharmacol Sci , 2014, 125 :202–210. DOI:10.1254/jphs.14017FP |
| [23] | Tao J, Hou YY, Ma XY, et al. An integrated global chemomics and system biology approach to analyze the mechanisms of the traditional Chinese medicinal preparation Eriobotrya japonica-Fritillaria usuriensis dropping pills for pulmonary diseases[J]. BMC Complement Altern Med , 2016, 16 :4. |
| [24] | Guha M, Mackman N. LPS induction of gene expression in human monocytes[J]. Cell Signal , 2001, 13 :85–94. DOI:10.1016/S0898-6568(00)00149-2 |
| [25] | Yamamoto Y, Gaynor RB. Role of the NF-κB pathway in the pathogenesis of human disease states[J]. Curr Mol Med , 2001, 1 :287–296. DOI:10.2174/1566524013363816 |
 2016, Vol. 51
2016, Vol. 51


