2. Department of Plant Science, Jilin Agricultural Science and Technology College, Jilin 132101, China ;
3. Department of Pharmacy, Shanghai Putuo District Liqun Hospital, Shanghai 200333, China
2. 吉林农业科技学院植物科学学院, 吉林 吉林 132101 ;
3. 上海普陀区利群医院药学部, 上海 200333
Petasites species(Asteraceae) are distributed mainly in the northern Eurasian and North American continents. The reported characteristic chemical components of Petasites species include sesquiterpenes (bakkenolides) and triterpenoids[1-12]. P. japonicus (Sieb. et Zucc.) Maxim. is distributed widely in central,southern and southwest China. Russian Far-East,Japan,Korea are also distributed. Its rhizomes have been applied as a folk medicine for the treatment of traumatic injury,parotitis and snakebite[13]. In our previous study,we reported the structural elucidation of three known bakkenolides and five novel bakkenolides (bakkenolide-Ⅰa,Ⅱa,Ⅲa,Ⅳa and Ⅴa) from P. tricholobusFranch.[9, 14-16]. We also reported three known bakkenolides from P. japonicus[17]. In the present study,we isolated and identified a novel sesquiterpene from the rhizome of P. japonicus. Its anti-hypoxic activitywas tested in vivo and in vitro.
Results and discussion 1 Bakkenolide-ⅥaHPLC data suggested that the purity of compound 1 was 99.3%. 1 was obtained as colorless needles (in MeOH),mp 120-123 ℃; UV (MeOH) λmax (204,230,289 nm). The IR spectrum indicated the presence of ketone (1 763 cm-1). Its HR-ESI-MS exhibited a quasi molecular ion peak at m/z 455.203 0 [M+Na]+ (Calcd. 455.204 6),which was compatible with the molecular formula C24H32O7. The 13C and DEPT NMR spectra of 1 indicated 24 carbon signals,belonging to six methyl (δ 8.4,8.5,15.7,19.1,19.8 and 20.6),three methylene (δ 25.7,25.9 and 38.8),seven methine (δ35.8,36.3,68.2,72.2,113.5,138.3 and 151.1),and eight quaternary carbons (δ 41.6,115.3,119.7,127.7,150.4,165.6,167.4 and 174.3). Referring to our previous study,these findings above suggested an eremophilenolide skeleton of the molecule. Careful analysis of the NMR spectra of 1,including 2D-NMR,allowed the 1H and 13C NMR signals (Table 1) being assigned. In the HMBC spectrum,the methyl proton CH3-14 at δ 0.98 correlated with the carbon signals at δ 35.8 (C-4),41.6 (C-5),72.2 (C-3); the methyl proton CH3-15 at δ 1.05 correlated with the carbon signals at δ 36.3 (C-10),41.6 (C-5). Thus,the two methyl groups were located at C-4 and C-5,respectively. The position of the side chain was determined by HMBC spectrum. The H-3 at δ 5.38 had a HMBC correlation with the carbon signal at δ167.4 (C-1″) and the H-6 at δ 6.45 had a HMBC correlation with the carbon signal at δ 165.6 (C-1′). Some other key HMBC correlations were observed and listed in Table 1.
| Table 1 The 1H NMR (500 MHz),13C NMR (125 MHz) and HMBC spectral data of compound 1 in CDCl3 (J in Hz) |
The relative stereochemistry of 1 was determined by NOESY experiment,in which the correlations between H-14 and H-6,H-10,H-15; H-6 and H-3 were observed. Thus H-3,H-6,H-10,H-14 and H-15 adopted the same orientation and were arbitrarily designated as the β-orientation (Figure 1). Therefore,1 was a novel sesquiterpene,named as bakkenolide-Ⅵa.
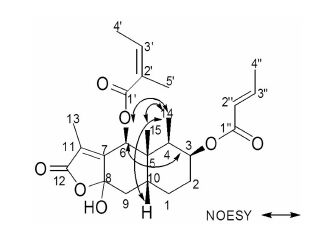
|
Figure 1 The main NOESY correlations of compound 1 |
Compound 1 significantly increased the survival time and gasping time of the mice after decapitation. 1 also increased creatine kinase (CK) content and decreased lactic acid content in brain tissues of mice at the same time (Table 2 and 3). These results indicated that 1 possess potent cerebral hypoxia-ischemia activity in mice.
| Table 2 Effects of compound1 on survival time and gasping time by decapitation in mice. n = 10,x± s. P < 0.05 vs vehicle group |
| Table 3 Effects of compound1 on creatine kinase (CK) activity and lactic acid content in brain tissues of mice. n = 10,x± s. P < 0.05,**P < 0.01 vs vehicle group |
Compound 1 significantly increased the cell viability of the PC12 cells after anaerobic cultivation (Table 4). 1 also increased lactate dehydrogenase (LDH) content in PC12 cells (Table 5). These results indicated that 1 can effectively protect PC12 cells from hypoxia injury.
| Table 4 Effects of compound1 on cell viability in PC12 cells. n = 5,x± s. **P < 0.01 vs hypoxia group. Data are expressed as percentages of normals |
| Table 5 Effects of compound1 on lactate dehydrogenase (LDH) activity in PC12 cells. n = 5,x± s. **P < 0.01 vs normal group; ΔP < 0.05,ΔΔP < 0.01 vs hypoxia group |
The above results confirmed compound 1 possessed the anti-hypoxic activity.
ExperimentalUV spectra were recorded on a WFH-203 UV spectrophotometer (Shanghai Jingke Industrial Co. Ltd). EI-MS and HR-ESI-MS spectra were obtained on a MAT-212 mass spectrometer (Finnigan) and a micromass Q-TOF spectrometer (Waters),respectively. Melting points were measured with a Yanaco MS-S3 (Yanaco Co. Ltd) micro-melting point apparatus (uncorrected). The 1H NMR,13C NMR,and 2D-NMR spectra were recorded on a Bruker AVANCE-500 FT- NMR spectrometer with superconducting,ultra shielded magnet (Bruker) using TMS as an internal standard. Silica gel H (200-300 mesh) and TLC plates (HSGF254) were made by Qingdao Haiyang Chemical Corp. Model 1029 anaerobic system (Thermo Forma),CO2 cell incubator (Thermo Forma),IX50 Inverted phase- contrast microscope (Olympus Company) and ELx800 enzyme sign meter (Bio-Rad) were used during PC12 cells culture experiment.
Ethanol (AR) and petroleum ether (60-90 ℃,AR) were made by Sinopharm Chemical Reagent Co. Ltd. DMEM medium,sugar-free DMEM medium,fetal bovine serum and horse serum were purchased from Gibco Company. Nimodipine was provided by Bayer Healthcare. Methyl thiazolyl tetrazolium salt (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide,MTT assay) and dimethyl sulfoxide (DMSO) were purchased from Pik-Day. Lactate dehydrogenase (LDH) test kit was provided by Nanjing Jiancheng Bioengi-neering Institute.
The rhizome of P. japonicas was collected from Huzhou,Zhejiang Province of China in September 2006. It was identified by professor Mei-li Guo from Department of Pharmacognosy,Second Military Medical University. A voucher specimen (PJ. 20060905) was stored in our lab.
6-week-old ICR mice (SPF) with half male and half female weighing (21 ± 1) g were provided by Shanghai SLACOM Experimental Animal Company. Animal permit number: SCXK (Hu) 2007-0005. PC12 cells were provided by Shanghai Institute of Cell Biology,Chinese Academy of Sciences.
1 Extraction and isolationDry rhizomes of P. japonicus (10.0 kg) were ground and extracted with 95% ethanol (30 L×6) by percolation. The solvent was evaporated under reduced pressure to give an extract (625.4 g),equivalent to 6.3% of the weight of the dry sample. This extract were suspended in H2O (6 L) and extracted with petroleum ether (6 L×3). The petroleum ether extract (315.2 g) was subjected to column chromatography packed with 3.6 kg of silica gel H and gradiently eluted with mixed petroleum ether and EtOAc (10∶1,8∶1,5∶1,2∶1 and 1∶1; 15 L of eluent for each step) to give six fractions according to TLC analysis. The fifth fraction (eluted with petroleum ether and EtOAc 2∶1,3.8 g) was loaded on a silica gel H column (250 g,2.5 cm × 80 cm),eluted with petroleum ether-EtOAc 5∶1,filtered and crystallized repeatedly to give compound 1 (350 mg).
2 Animal experimentFifty healthy ICR mice were partitioned into five groups (n = 10 ineach group,5 female and 5 male) at random,three test groups for compound 1 were low dose group,middle dose group,high dose group with dose of 7.5,15 and 30 mg·kg-1,bw,respectively,a nimodipine positive group (18 mg·kg-1,bw),and a vehicle group. All the test groups were treated as aboveby intragastric administration. The vehicle group was treated with an equal volume of vehicle. After administration for 5 days,fifty mice were subjected to decapitation. The effect of 1 on the survival time and gasping time of the mice were observed. The content of creatine kinase (CK) and lactic acid in brain tissues homogenate were measured.
3 PC12 cells culture experimentPC12 cells were cultured in medium of 10% fetal bovine serum,10% horse serum and 80% sugar-free DMEM,and grown in a 5% CO2 incubator at 37 ℃. They were digested by 0.25% trypsin to passage once per 2-3 days. Medication was during logarithmic phase.
Hypoxia-reoxygenation model was established as follow. PC12 cells were washed in sugar-free DMEM twice and randomly grouped. The hypoxia group was added sugar-free DMEM medium 200 μL,and subjected to anaerobic cultivation at 37 ℃ in an incubator of 5% CO2 and 95% N2. Test groups were added sugar-free DMEM medium supplemented with different concentrations of compound 1 200 μL at 37 ℃ in a 5% CO2 incubator 30 min before anaerobic cultivation. The normal group was out of anaerobic cultivation,while other treatments were the same as the hypoxia group. 1 h after anaerobic cultivation,all groups were turned into a 5% CO2 incubator at 37 ℃. 24 h later,cell viability and LDH content of each group were determined using methods below.
Quantification of cell viability was made using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. 96-well microassay culture plates were inoculated with PC12 cells in logarithmic phase (1×105 cells per well). These cells were grown at 37 ℃ in a 5% CO2 incubator overnight and partitioned into ten groups. They got different treatments ut supra as compound 1 was added to the wells to get eight test groups (16,31,63,125,250,500,1 000 and 2 000 ng·mL-1). Wells containing culture medium without cells were used as blanks. After treatments,stock MTT dye solution (20 μL,5 mg·mL-1) was added to each well. After 4 h incubation at 37 ℃ in a 5% CO2 incubator,100 μL DMSO was added to solubilize the MTT formazan. The absorbance intensity of each well was measured on the enzyme sign meter at a wavelength of 570 nm. Reference wavelength was 630 nm. Each experiment was repeated five times to get the mean value s.
LDH content was measured with the test kit. 96-well microassay culture plates were inoculated with PC12 cells in logarithmic phase (5×104 cells per well). They were grouped and treated with different conditions ut supra as compound 1 was added to the wells to get three test groups (31,250 and 2 000 ng·mL-1). After treatments,supernatant 100 μL was taken from each well to operate following the instruct-tions of the test kit strictly. The absorbance intensity was measured on the UV spectrophotometer at a wave-length of 440 nm and LDH content was calculated by formula. Each experiment was repeated five times to get the mean values.
4 Statistical analysisThe results were presented as Mean ± SD. The differences among the means were analyzed using one- way analysis of variance (ANOVA) followed by post hoc Dunnett’s multiple comparison test at the 95% (P < 0.05) and 99% (P < 0.01) confidence levels.
| [1] | Nobuo A, Ryoichi O, Katsuo R, et al. The structures of bakkenolides-B,-C and-D as determined by the use of a nuclear overhauser effect[J]. Tetrahedron Lett , 1968, 9 :1993–1997. DOI:10.1016/S0040-4039(01)99073-2 |
| [2] | Wu TS, Kao MS, Wu PL, et al. The bakkenolides from the root of Petasites formosanus and their cytotoxicity[J]. Chem Pharm Bull , 1999, 47 :375–382. DOI:10.1248/cpb.47.375 |
| [3] | Wu TS, Kao MS, Wu PL, et al. Antiplatelet principles from the root of Petasites formosanus[J]. Phytochemistry , 1999, 52 :901–905. DOI:10.1016/S0031-9422(99)00333-7 |
| [4] | Yasunori Y, Masao K. Structures of new eremophilane derivatives from the rhizomes of Petasites japonicas Maxim[J]. Chem Pharm Bull , 1995, 43 :1738–1743. DOI:10.1248/cpb.43.1738 |
| [5] | Motoo T, Makiko K, Masakazu S. Eremophilane-type ses quiterpenes from fresh rhizomes of Petasites japonicus[J]. Phytochemistry , 1998, 47 :401–409. DOI:10.1016/S0031-9422(97)00581-5 |
| [6] | Markus N, Andreas N, Ernst S, et al. Structure of sesquiter penes of Petasites hybridus (L.) G.M.et SCH.:petasol and isopetasol derivatives[J]. Helv Chim Acta , 1979, 62 :609–626. DOI:10.1002/hlca.19790620228 |
| [7] | Keizo N, Ichiro T. The structure of petasitin,a new ses quiterpene from Petasites japonicus Maxim[J]. Tetrahedron Lett , 1968, 9 :629–632. DOI:10.1016/S0040-4039(01)98819-7 |
| [8] | Xie WD, Li RJ, Gao X, et al. Bakkenolides from Petasites tatewakianus[J]. Fitoterapia , 2010, 81 :153–156. DOI:10.1016/j.fitote.2009.08.013 |
| [9] | Xie WD, Zhang Q, Li PL, et al. Two triterpenoids and other constituents from Petasites tricholobus[J]. Phytochemistry , 2005, 66 :2340–2345. DOI:10.1016/j.phytochem.2005.03.032 |
| [10] | Wang S, Jin DQ, Xie C, et al. Isolation,characterization,and neuroprotective activities of sesquiterpenes from Petasites japonicus[J]. Food Chem , 2013, 141 :2075–2082. DOI:10.1016/j.foodchem.2013.04.116 |
| [11] | He J, Wang Q, Wang Y, et al. Simultaneous determination of eight bioactive bakkenolides of Petasites tatewakianus Kitam by HPLC-UV[J]. Acta Pharm Sin B , 2013, 3 :354–360. DOI:10.1016/j.apsb.2013.08.003 |
| [12] | Zhang Y, Gao YY, Jia Q, et al. Two new sulfated sesquiterpenoids from Petasites tricholobus[J]. Acta Pharm Sin (药学学报) , 2014, 49 :1433–1437. |
| [13] | Chen YL.Compositae (Five)[M]//Editoria Committee of Flora of China.Flora of China:Vol 77(1)(中国植物志:第77(1)卷).Beijing:Science Press,1999:99. |
| [14] | Wang YL, Guo ML, Zhang G, et al. Chemical constituents in root of Petasites tricholobus Franch.and their anti-inflammatory activity[J]. Acad J Second Mil Med Univ (第二军医大学学报) , 2006, 27 :1210–1213. |
| [15] | Wang YL, Li RP, Guo ML, et al. Bakkenolides from Petasites tricholobus and their neuroprotective effects related to antioxidant activities[J]. Planta Med , 2009, 75 :230–235. DOI:10.1055/s-0028-1088377 |
| [16] | Zhang N, Guo ML, Zhang G, et al. A new neuroprotective bakkenolide from the rhizome of Petasites tricholobus[J]. Chin Chem Lett , 2008, 19 :841–844. DOI:10.1016/j.cclet.2008.04.036 |
| [17] | Li YX, Wang Y, Guo ML. Study on chemical constituents of Petasites japonicus[J]. Acad J Second Mil Med Univ (第二军医大学学报) , 2010, 31 :779–781. |
 2016, Vol. 51
2016, Vol. 51


