2. 上海中药标准化研究中心, 上海 201203
2. Shanghai R & D Center for Standardization of Chinese Medicine, Shanghai 201203, China
野百合为豆科蝶形花亚科 (Papilionoideae) 猪屎豆属 (Crotalaria) 植物野百合 (Crotalaria sessiliflora L.) 的全草,又名野芝麻、狗铃草、农吉利等。产于辽宁、河北、山东、江苏、安徽、浙江等地。有清热解毒、消肿止痛、破血除瘀等效用,可治风湿麻痹、跌打损伤、疮毒、癣疥等症[1]。文献报道野百合中主要有黄酮类化合物[2]。作者曾对野百合中的异黄酮类成分进行了研究[3],后续研究中又从野百合中分离得到12个化合物,包括3个异黄酮: 野百合素B (1)、响铃豆素A (10)、butesuperin B (11); 2个黄酮: 槲 皮素(2) 和山柰酚 (3); 2个三萜类: soyasapogenol B (4)、羊齿烯醇 (5); 2个生物碱: 新海胆灵A (6)、橙黄胡椒酰胺乙酸酯 (12); 其他化合物: 对羟基苯甲酸乙酯 (7)、咖啡酸乙酯 (8)、5,7-二羟基色原酮 (9)。化合物1为新化合物,化合物3~12均是首次从该植物中分离得到。
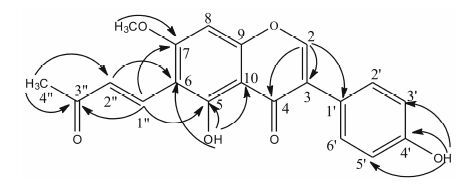
化合物1浅黄色粉末 (甲醇),熔点为235.8~236.7 ℃。ESI-MS给出准分子离子峰为m/z: 353.26 [M+H]+,HR-ESI-MS给出准分子离子峰m/z: 353.103 0 [M+H]+ (计算值353.102 5),提示分子式为C20H16O6,不饱和度为13。IR谱显示该化合物含有羟基 (3 317 cm-1)、苯环 (1 614,1 578,1 519,1 448 cm-1)、羰基 (1 644 cm-1) 等基团。1H NMR (DMSO-d6,600 MHz) (表 1) 谱中可见16个氢信号,其中包括典型的异黄酮2位氢的特征信号δH 8.48 (1H,s,H-2); 一组AA'BB'耦合系统芳香氢信号δH 7.40 (2H,d,J = 8.5 Hz,H-2',6')、6.84 (2H,d,J = 8.5 Hz,H-3',5'); 1个单峰的芳氢信号δH 6.82 (1H,s,H-8); 此外,还有一个甲氧基信号δH 3.99 (3H,s,7-OCH3),2个羟基氢信号δH 14.37 (1H,s,5-OH)、9.64 (1H,s,4'-OH) 以及1对反式双键信号δH 7.77 (1H,d,J = 16.5 Hz,H-1'')、7.15 (1H,d,J = 16.5 Hz,H-2'') 和1个甲基信号δH 2.28 (3H,s,H-4'')。13C NMR (DMSO-d6,150 MHz) (表 1)谱中可见20个碳信号,其中包括1个甲基碳信号δC 27.8、1个甲氧基碳信号δC 56.9、二个羰基信号δC 198.5和180.7、δC 164.0~90.9之间的16个烯碳信号。在化合物1的HMBC谱中 (图 1),δH 2.28 (H-4'') 与δC 198.5 (C-3'')、128.9 (C-2'') 相关; δH 7.15 (H-2'') 与δC 198.5 (C-3'')、27.8 (C-4'')、131.5 (C-1'') 相关; δH 8.48 (H-2) 与δC 120.6 (C-1')、122.9 (C-3)、180.7 (C-4) 相关; δH 3.99 (7-OCH3) 与δC 164.0 (C-7) 相关; 羟基氢信号δH 14.37 (5-OH) 与δC 161.8 (C-5)、106.2 (C-6) 和105.1 (C-10) 相关,羟基氢信号δH 9.64 (4'-OH) 与δC 157.6 (C-4')、115.1 (C-3',5') 相关。另外,HMBC谱 (图 1) 中δH 7.77 (H-1'') 与δC 128.9 (C-2'')、161.8 (C-5)、164.0 (C-7)、198.5 (C-3'') 相关,δH 7.15 (H-2'') 与δC 106.2 (C-6) 相关表明化合物1的C-1''与C-6相连。故化合物1的结构鉴定为5,4'-二羟基-7-甲氧基- 6-(3-丁烯-2-酮-4)-异黄酮。经Scifinder检索,化合物1为新化合物,命名为野百合素B (sessiliflorin B)。
|
|
Table 1 1H NMR (DMSO-d6,600 MHz) and 13C NMR (DMSO- d6,150 MHz) data of compound 1 |
|
Figure 1 Structure and correlations key HMBC of compound 1 |
核磁共振仪 (Bruker AVANCE ⅡI型,400 MHz/ 600 MHz,德国); 熔点仪 (BUCHI,B-540,瑞典); 傅立叶变换红外光谱仪 (Perkin Elmer,FT-IR,Spectrum One,美国); 柱色谱硅胶 (青岛海洋化工厂,100~200目,200~300目); 薄层色谱硅胶板 (烟台江友硅胶开发有限公司); Sephadex LH-20 (GE-Healthcare Bio-Sciences AB,瑞典); ODS (Sepax Technologies Inc.,40~60 μm,美国); MCI GEL CHP20P (Mitsubishi Chemical Corporation,75~150 μm,日本); 石油醚 (沸程60~90 ℃)、二氯甲烷、乙酸乙酯、正丁醇等工业纯有机试剂 (上海润捷化学试剂有限公司); 分析纯化学试剂 (国药集团化学试剂有限公司)。
野百合于2012年9月采自安徽省黄山市黄山区 (原太平县),经上海中医药大学中药研究所徐红研究员鉴定为豆科植物野百合Crotalaria sessiliflora L.。凭证标本 (201209003) 存放于上海中药标准化研究中心。
1 提取与分离野百合全草 (27 kg),干燥、粉碎后经75% 工业乙醇渗漉,减压浓缩渗滤液,得总浸膏2.9 kg。将浸膏加水混悬,依次用石油醚、二氯甲烷、乙酸乙酯、正丁醇萃取,将各部位萃取液减压浓缩,分别得到石油醚部位288 g、二氯甲烷部位238 g、乙酸乙酯部位158 g、正丁醇部位618 g。
乙酸乙酯部位 (158 g),通过硅胶柱色谱,二氯甲烷-甲醇(30∶1→0∶100) 洗脱,得组分A1~A26。组分A11~A13 (8 g),经MCI GEL CHP20P柱色谱,甲醇-水(20%→100%) 洗脱,得到3个组分B1~B3,组分B2,经Sephadex LH-20柱色谱,甲醇洗脱,得化合物2 (7 mg); 组分B3,经ODS-18反相柱色谱,甲醇-水 (10%→100%) 洗脱,得3个组分C1~C3,组分C3经Sephadex LH-20柱色谱,反复纯化,得化合物3 (16 mg)。组分A6 (6 g),经硅胶柱色谱,石油醚-乙酸乙酯 (8∶1→1∶1) 洗脱,得到6个组分D1~D6,组分D2,经Sephadex LH-20柱色谱纯化,得到化合物4(3 mg)。
二氯甲烷部位 (238 g),经硅胶柱色谱,二氯甲烷-乙酸乙酯 (50∶1→0∶100) 为洗脱剂梯度洗脱,得组分E1~E11。组分E1 (12 g) 经硅胶柱色谱,以石油醚-乙酸乙酯 (30∶1→0∶100) 为洗脱剂洗脱 ,得到18个组分F1~F18,其中组分F5经Sephadex LH-20柱色谱,以二氯甲烷-甲醇 (1∶2) 为洗脱剂洗脱,得化合物5 (5 mg); 组分F7经Sephadex LH-20柱色谱,反复纯化,得化合物7 (7 mg)。组分E8 (20 g) 经硅胶柱色谱,石油醚-乙酸乙酯 (3∶1→1∶1) 梯度洗脱,得到10个组分G1~G10,组分G8经Sephadex LH-20柱色谱纯化,得化合物6(4 mg)。组分E2 (14 g) 经MCIGEL CHP20P柱色谱,甲醇-水 (20%→100%) 洗脱,得到16个组分H1~H16,组分H4经Sephadex LH-20柱色谱,二氯甲烷-甲醇 (1∶2) 洗脱,得化合物8(16 mg)、化合物9(5 mg); 组分H8经Sephadex LH-20柱色谱,反复纯化,得化合物10(23 mg)。组分E5 (9 g) 经MCIGEL CHP20P柱色谱,甲醇-水 (60%→100%) 洗脱,得组分I1~I17,组分I15经Sephadex LH-20柱色谱,纯化,得到化合物1(3 mg)。组分E7 (10 g) 经MCIGEL CHP20P柱色谱,甲醇-水 (60%→100%) 洗脱,得12个组分J1~J12,组分J9经重结晶,得化合物11(10 mg); 组分E6 (7 g) 经MCIGEL CHP20P柱色谱,甲醇-水 (60%→100%) 洗脱,得9个组分K1~K9,组分K9经重结晶,得化合物12(2 mg)。
2 结构鉴定 化合物1浅黄色粉末,分子式C20H16O6,熔点235.8~236.7 ℃。IR: 3 317,1 644,1 614,1 578,1 519,1 448 cm-1; ESI-MS m/z: 353.26 [M+H]+; HR-ESI-MS m/z: 353.103 0 [M+H]+(计算值353.102 5); 1H NMR和13C NMR数据见表 1。
化合物2浅黄色粉末,分子式C15H10O7。 1H NMR (CD3OD,400 MHz) δ: 7.76 (1H,s,H-2'),7.65 (1H,d,J = 9.1 Hz,H-6'),6.90 (1H,d,J = 8.5 Hz,H-5'),6.40 (1H,s,H-8),6.20 (1H,s,H-6); 13C NMR (CD3OD,100 MHz) δ: 147.9 (C-2),137.2 (C-3),177.3 (C-4),158.2 (C-5),99.2 (C-6),165.6 (C-7),94.4 (C-8),162.5 (C-9),104.5 (C-10),121.7 (C-1'),116.0 (C-2'),146.2 (C-3'),148.8 (C-4'),116.2 (C-5'),124.2 (C-6')。以上 1H NMR、13C NMR数据与文献[4]一致,故鉴定化合物2为槲皮素。
化合物3黄色粉末,分子式C15H10O6。1H NMR (CD3OD,600 MHz) δ: 8.07 (2H,d,J = 8.6 Hz,H-2',6'),6.90 (2H,d,J = 8.6 Hz,H-3',5'),6.38 (1H,d,J = 2.0 Hz,H-8),6.18 (1H,d,J = 2.0 Hz,H-6); 13C NMR (CD3OD,150 MHz) δ: 148.0 (C-2),137.1 (C-3),177.3 (C-4),158.2 (C-5),99.3 (C-6),165.7 (C-7),94.5 (C-8),162.4 (C-9),104.5 (C-10),123.7 (C-1'),130.6 (C-2'),116.3 (C-3'),160.5 (C-4'),116.3 (C-5'),130.6 (C-6')。以上 1H NMR、13C NMR数据与文献[5]一致,故鉴定化合物3为山柰酚。
化合物4无色针状结晶,分子式C30H50O3。 1H NMR (CDCl3,600 MHz) δ: 5.29 (1H,m,J = 3.5,H-12),4.23 (1H,d,J = 11.2 Hz,H-22α),3.48 (1H,overlap,H-3α),3.46 (1H,overlap,H-24a),3.37 (1H,brd,J = 11.0 Hz,H-24b),2.76 (1H,brs,H-18β),0.89~1.27 (each 3H,s,7×CH3); 13C NMR (CDCl3,150 MHz) δ: 38.4 (C-1),27.7 (C-2),80.9 (C-3),42.8 (C-4),55.9 (C-5),18.4 (C-6),33.1 (C-7),39.7 (C-8),47.7 (C-9),36.7 (C-10),23.8 (C-11),122.3 (C-12),143.9 (C-13),42.1 (C-14),25.9 (C-15),28.2 (C-16),37.4 (C-17),44.7 (C-18),46.2 (C-19),30.5 (C-20),41.5 (C-21),76.6 (C-22),22.4 (C-23),64.5 (C-24),16.2 (C-25),16.9 (C-26),25.4 (C-27),28.2 (C-28),32.8 (C-29),20.0 (C-30)。以上1H NMR、13C NMR数据与文献[6] 一致,故鉴定化合物4为soyasapogenol B。
化合物5无色针状结晶,分子式C30H50O1。 1H NMR (CDCl3,400 MHz) δ: 5.29 (1H,m,H-11),3.20 (1H,dd,J = 10.0,5.8 Hz,H-3),0.89 (3H,d,J = 6.5 Hz,H-29),0.82 (3H,d,J = 6.6 Hz,H-30),1.06,0.96,0.87,0.81,0.75,0.73 (each 3H,s,6×CH3); 13C NMR (CDCl3,100 MHz) δ: 39.4 (C-1),28.4 (C-2),79.3 (C-3),39.5 (C-4),44.4 (C-5),19.3 (C-6),18.1 (C-7),40.1 (C-8),151.2 (C-9),37.9 (C-10),116.3 (C-11),36.9 (C-12),36.3 (C-13),37.8 (C-14),29.4 (C-15),30.9 (C-16),43.1 (C-17),52.1 (C-18),20.3 (C-19),28.3 (C-20),59.8 (C-21),29.9 (C-22),27.6 (C-23),16.0 (C-24),25.4 (C-25),15.5 (C-26),15.2 (C-27),14.1 (C-28),22.3 (C-29),23.2 (C-30)。以上 1H NMR、13C NMR数据与文献[7]一致, 故鉴定化合物5为羊齿烯醇。
化合物6浅黄色粉末,分子式C19H21N3O2。 1H NMR (CD3OD,400 MHz) δ: 7.42 (1H,d,J = 7.6 Hz,H-7),7.25 (1H,d,J = 7.9 Hz,H-4),7.20 (1H,s,H-8),7.12 (1H,m,H-6),7.06 (1H,m,H-5),6.11 (1H,dd,J = 17.3,10.7 Hz,H-17),5.11 (1H,d,J = 10.5 Hz,H-18a),5.09 (1H,d,J = 17.3 Hz,H-18b),4.22 (1H,q,J = 7.0 Hz,H-12),1.55 (3H,s,H-19),1.54 (3H,s,H-20),1.53 (3H,d,J = 7.0 Hz,H-15); 13C NMR (CD3OD,100 MHz) δ: 146.0 (C-2),104.3 (C-3),127.3 (C-3a),119.8 (C-4),121.2 (C-5),122.6(C-6),112.6 (C-7),136.8 (C-7a),114.3 (C-8),124.7 (C-9),162.2 (C-10),52.6 (C-12),168.7 (C-13),20.7 (C-15),40.4 (C-16),146.2 (C-17),112.6 (C-18),28.1 (C-19),28.2 (C-20)。以上1H NMR、13C NMR数据与文献[8]一致,故鉴定化合物6为新海胆灵A。
化合物7无色针状结晶,分子式C9H10O3。 1H NMR (CD3OD,400 MHz) δ: 7.87 (2H,dd,J = 8.8,2.0 Hz,H-3,5),6.86 (2H,dd,J = 8.8,2.0 Hz,H-2,6),4.30 (2H,q,J = 7.1 Hz,-CH2-),1.36 (3H,t,J = 7.1 Hz,-CH3); 13C NMR (CD3OD,100 MHz) δ: 122.5 (C-1),132.7 (C-2,6),116.1 (C-3,5),163.5 (C-4),168.3 (-C=O),61.7 (-OCH2-),14.7 (-CH3)。以上1H NMR、13C NMR数据与文献[9]一致,故鉴定化合物7为对羟基苯甲酸乙酯。
化合物8浅棕色结晶,分子式C11H12O4。 1H NMR (CD3OD,600 MHz) δ: 7.54 (1H ,d ,J = 15.8 Hz,H-α),7.03 (1H ,d ,J = 2.0 Hz,H-2),6.94 (1H ,dd,J = 8.2,2.0 Hz,H-6),6.78 (1H ,d ,J = 8.2 Hz,H-5),6.25 (1H,d,J = 15.8 Hz,H-β),4.21 (2H,q,J = 7.1 Hz,-OCH2),1.31 (3H,t,J = 7.1 Hz,-CH3); 13C NMR (CD3OD,150 MHz) δ: 127.7 (C-1),115.3 (C-2),146.8 (C-3),146.7 (C-4),116.5 (C-5),122.9 (C-6),149.5 (C-α),115.1 (C-β),169.3 (C=O),61.4 (-OCH2-),14.6 (-CH3)。以上1H NMR、13C NMR数据与文献[10]一致,故鉴定化合物8为咖啡酸乙酯。
化合物9浅棕色针状结晶,分子式C9H6O4。 1H NMR (CD3OD,400 MHz) δ: 7.97 (1H,d,J = 5.9 Hz,H-2),6.33 (1H,d,J = 2.1 Hz,H-8),6.20 (1H,d,J = 2.2 Hz,H-6),6.19 (1H,d,J = 6.0 Hz,H-3); 13C NMR (CD3OD,100 MHz) δ: 158.1 (C-2),111.6 (C-3),183.4 (C-4),163.4 (C-5),100.3 (C-6),166.2 (C-7),95.1 (C-8),159.9 (C-9),106.6 (C-10)。以上1H NMR、13C NMR 数据与文献[11]一致,故鉴定化合物9为5,7-二羟基色原酮。
化合物10黄色针状结晶,分子式C20H16O5。 1H NMR (DMSO-d6,400 MHz) δ: 8.18 (1H,s,H-2),7.95 (1H,d,J = 8.8 Hz,H-5),7.95 (1H,d,J = 2.2 Hz,H-8),6.94 (1H,dd,J = 8.8,2.2 Hz,H-6),6.92 (1H,d,J = 8.1 Hz,H-6'),6.35 (1H,d,J = 8.1 Hz,H-5'),5.24 (1H,t,J = 8.6 Hz,H-2''),5.06 (1H,s,Hα-4''),4.90 (1H,s,Hβ-4''),3.31 (1H,dd,J = 15.7,7.8 Hz,Hα-1''),2.89 (1H,dd,J = 15.7,7.8 Hz,Hβ-1''),1.73 (3H,s,H-5''); 13C NMR (DMSO-d6,100 MHz) δ: 154.5 (C-2),122.1 (C-3),175.6 (C-4),127.2 (C-5),115.2 (C-6),162.6 (C-7),102.1 (C-8),157.6 (C-9),116.4 (C-10),112.6 (C-1'),152.3 (C-2'),112.9 (C-3'),160.8 (C-4'),100.4 (C-5'),131.1 (C-6'),32.0 (C-1''),85.4 (C-2''),144.0 (C-3''),111.6 (C-4''),17.1 (C-5'')。以上1H NMR、13C NMR数据与文献[12]一致,故鉴定化合物10为响铃豆素A。
化合物11浅黄色颗粒,分子式C27H24O9。 1H NMR (DMSO-d6,400 MHz) δ: 8.60 (1H,s,7''-OH),8.50 (1H,s,H-2),7.62 (1H,d,J = 8.9 Hz,H-5),7.54 (2H,d,J = 8.8 Hz,H-2',6'),7.11 (1H,d,J = 8.9 Hz,H-6),6.78 (2H,s,H-5'',9''),7.00 (2H,d,J = 8.8 Hz,H-3',5'),5.08 (1H,d,J = 8.0 Hz,H-1''),4.39 (1H,m,H-2''),3.79 (3H,s,-OCH3),3.78 (6H,s,2×OCH3),3.68 (1H,s,Ha-3''),3.42 (1H,m,Hb-3''); 13C NMR (DMSO- d6,100 MHz) δ: 153.2 (C-2),123.3 (C-3),174.7 (C-4),116.6 (C-5),115.0 (C-6),147.4 (C-7),132.0 (C-8),145.9 (C-9),118.4 (C-10 ),124.0 (C-1'),130.2 (C-2'),113.6 (C-3'),159.1 (C-4'),113.6 (C-5'),130.2 (C-6'),76.4 (C-1''),78.2 (C-2''),59.8 (C-3''),125.7 (C-4''),105.4 (C-5''),148.0 (C-6''),136.2 (C-7''),148.0 (C-8''),105.4 (C-9''),56.1 (2×OCH3),55.2 (4'-OCH3)。以上 1H NMR、13C NMR数据与文献[13]一致,故鉴定化合物11为butesuperin B。
化合物12白色粉末,分子式为C27H28N2O4。 1H NMR (DMSO-d6,400 MHz) δ: 8.50 (1H,d,J = 8.4 Hz,H-1),8.13 (1H,d,J = 8.4 Hz,H-4),7.78 (2H,d,J = 7.1 Hz,H-3',7'),7.51 (1H,m,H-5'),7.44 (2H,m,H-8,12),7.31 (2H,d,J = 7.1 Hz,H-4',6'),7.26~7.19 (5H,m,H-15~H-19),7.15 (3H,m,H-9,10,11),4.67 (1H,m,H-2),4.18 (1H,m,H-5),4.01 (1H,dd,J = 11.0,4.8 Hz,H-20a),3.85 (1H,dd,J = 11.0,6.7 Hz,H-20b),2.98 (2H,m,H-13),2.78 (2H,m,H-6),1.98 (3H,s,H-2''); 13C NMR (DMSO-d6,100 MHz) δ: 54.8 (C-2),171.2 (C-3),49.1 (C-5),37.2 (C-6),138.0 (C-7),128.2 (C-8,12),129.1 (C-9,11),126.2 (C-10),37.8 (C-13),138.3 (C-14),128.0 (C-15,19),129.1 (C-16,18),127.4 (C-17),64.6 (C-20),166.1 (C-1'),134.0 (C-2'),127.1 (C-3',7'),128.1 (C-4',6'),131.3 (C-5'),170.3 (C-1''),20.6 (C-2'')。以上1H NMR、13C NMR数据与文献[14]一致,故鉴定化合物12为橙黄胡椒酰胺乙酸酯。
| [1] | Editorial Committee of Flora of China of Chinese Academy of Sciences. Flora of China (中国植物志)[M]. Vol 42. Beijing:Science Press, 1998:366. |
| [2] | Zeng JY, Li SH, Wu XJ, et al. Flavonoid compounds of whole plant of Crotalaria sessiliflora L.[J]. Chin Pharm J (中国药学杂志), 2014, 49:1190-1193. |
| [3] | Fan CM, Chou GX, Zhu EY, et al. Isoflavones from Crotalaria sessiliflora L.[J]. Chin Tradit Herb Drugs (中草药), 2015, 46:3297-3303. |
| [4] | Guo N, Chen XQ, Zhao QS. A new polyisoprenylated benzoylphloroglucinol derivative from Hypericum henryi subsp. uraloides (Guttiferae)[J]. Acta Bot Yunnan (云南植物研究), 2008, 30:515-518. |
| [5] | Fang W, Ruan JL, Wang Z, et al. Studies on chemical constituents of Arachniodes rhomboidea[J]. China J Chin Mater Med (中国中药杂志), 2008, 33:649-650. |
| [6] | Nartowska J, Wawer I, Strzelecka H. Triterpenoid sapogenin from Anthyllis vulneraria L.[J]. Acta Pol Pharm, 2001, 58:289-291. |
| [7] | Li JJ, Wang AL, Yuan ZZ, et al. Triterpenoids from Ainsliaea yunnanensis[J]. China J Chin Mater Med (中国中药杂志), 2013, 38:3918-3922. |
| [8] | Zhang F, Qin JJ, Cheng XR, et al. Chemical constituents from Inula hepehensis[J]. Nat Prod Res Dev (天然产物研究与开发), 2012, 24:427-431. |
| [9] | Yang LM, Hu R, Qi W, et al. Chemical constituents of Rhodiola kirilowii Maxim.[J]. J Chin Pharm Sci, 2011, 20:154-158. |
| [10] | Chen YL, Tan JJ, Lu LL, et al. Water-soluble constituents of Clerodendranthus spicatus[J]. Chin Tradit Herb Drugs (中草药), 2009, 40:689-693. |
| [11] | Wang YL, Duan SL, Zhang QY, et al. Chemical constituents from stems of Ficus tsiangii[J]. Chin Tradit Herb Drugs (中草药), 2014, 45:333-336. |
| [12] | Sun QH, Chou GX. Isoflavonoids from Crotalaria albida inhibit adipocyte differentiation and lipid accumulation in 3T3-L1 cells via suppression of PPAR-γ pathway[J]. PLoS One, 2015, 10:e0135893. |
| [13] | Kai M, Tsutomu I, Horiko S, et al. Isolation of new isoflavonolignans, butesuperins A and B, from a Thai Miracle Herb, Butrasuperba[J]. Heterocycles, 2005, 65:893-900. |
| [14] | Tang J, Supinya T, Wang ZT, et al. Aurantiamide acetate from stems of Zanthoxylum dissitum Hemsley[J]. J Chin Pharm Sci, 2003, 12:231-233. |
 2016, Vol. 51
2016, Vol. 51


