2. 西南大学-西藏大学农牧学院药用植物联合研发中心, 西藏大学农牧学院食品科学学院, 西藏 林芝 860000;
3. 西南大学生命科学学院, 重庆, 400715
2. TAAHC-SWU Medicinal Plant R & D Center, Agriculture and Animal Husbandry College, Tibet University, Nyingchi 860000, China;
3. School of Life Sciences, Southwest University, Chongqing 400715, China
波棱瓜子为葫芦科 (Cucurbitaceae) 植物波棱瓜 (Herpetospermum caudigerum Wall) 的干燥成熟种 子[1],主要分布于我国云南、西藏等地,印度和尼泊尔东北部等地也有产。波棱瓜子味苦,性寒,常用于泻肝火,藏医临床上常用于治疗赤巴病、肝胆疾病以及消化不良等。现代药理研究表明: 波棱瓜子提取物具有抗化学性肝损伤[2, 3]、抗氧化[4]、抗疲劳[5]以及抗鸭乙肝病毒[6]等作用。波棱瓜子作为药物治疗肝胆疾病历史悠久,广泛应用于藏药处方中,其化学成分研究也十分广泛。据报道,波棱瓜子主要含有木脂素类、脂肪酸类、香豆素、苯并呋喃类及微量 元素等多种成分[7, 8, 9, 10, 11, 12, 13, 14, 15, 16]。而波棱瓜子乙酸乙酯提取物中则主要含有木脂素类成分,是波棱瓜子治疗肝胆疾病的 有效成分[17, 18, 19]。本文采用多种色谱技术从波棱瓜子提取物分离得到4个化合物,其中化合物1是一个新的联芳基七元内酯类化合物,通过波谱和理化性质鉴定其结构,命名为波棱内酯Ⅲ。化合物2、3为木脂素类化合物,化合物4为黄酮类化合物。
结果与讨论将成熟波棱瓜子的干燥种子经95%乙醇提取后,得到黑色油状浸膏,再经石油醚萃取后,利用正相硅胶柱色谱、羟丙基葡聚糖凝胶LH20及半制备HPLC分离得到一个新的联芳基七元内酯类化合物,命名为波棱内酯Ⅲ (图 1)。
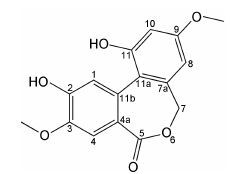
|
Figure 1 Structure of compound 1 |
化合物1为白色无定形粉末。HR-ESI-MS给出准分子离子峰m/z 301.070 4 [M-H]- (计算值为301.071 8),提示化合物的分子式为C16H14O6。化合物1的1H NMR和13C NMR谱 (表 1) 与ulocladol[20]相似,提示其为联芳基七 元内酯类化合物[21, 22, 23]。
化合物1的1H NMR谱显示了2个甲氧基信号 δH 3.77 (3H,s)、δH 3.82 (3H,s); 2个间位偶合的芳烃质子信号δH 6.58 (1H,d,J = 2.2 Hz)、δH 6.61 (1H,d,J = 2.2 Hz); 2个没有偶合的芳烃质子信号δH 7.12 (1H,s)、δH 7.22 (1H,s); 两个酚羟基信号δH 9.62 (1H,s)、δH 9.90 (1H,s) 和一组连氧的亚甲基信号δH 4.78 (1H,d,J = 12.1 Hz)、δH 4.85 (1H,d,J = 12.1 Hz)。13C NMR谱显示1个羰基碳信号(δC 169.6) 及12个芳香碳信号 (δC 100.6~158.0),另外还有两个甲氧基碳信号 (δC 55.5、δC 55.6)、一个连氧的亚甲基信号 (δC 68.8)。HMQC谱上显示了δC 68.8与亚甲基信号δH 4.78 (1H,d,J = 12.1 Hz)、δH 4.85 (1H,d,J = 12.1 Hz) 相关。因此,根据以上的核磁数据和不饱和度Ω = 10,推测化合物1属于联苯[c,e]氧杂䓬-7,5-内酯类化合物,存在两个独立的芳香环,而羰基和亚甲基则可能以内酯的形式存在。结合1H NMR中两个间位偶合的芳烃质 子信号δH 6.58 (1H,d,J = 2.2 Hz)、δH 6.61 (1H,d,J = 2.2 Hz) 和2个没有偶合的芳烃质子信号δH 7.12 (1H,s)、δH 7.22 (1H,s),2个甲氧基信号δH 3.77 (3H,s)、δH 3.82 (3H,s) 以及两个酚羟基信号δH 9.62 (1H,s)、δH 9.90 (1H,s) 推断化合物1中的两个甲氧基和两个酚羟基分别取代于两个苯环上。
|
|
Table 1 1H and 13C NMR data (400 and 100 MHz,respectively,in DMSO-d6) of compound 1 |
HMBC谱显示 (图 2),δH 3.82 (3H,s,OCH3-3) 与δC 149.4 (C-3) 存在关联; δH 3.77 (3H,s ,OCH3-9) 与δC 158.0 (C-9) 存在关联,提示化合物1的C-3和C-9位被甲氧基取代。δH 9.62 (1H,s) 与δC 116.4 (C-1)、δC 145.4 (C-2)、δC 149.4 (C-3) 存在关联,δH 9.90 (1H,s) 与δC 100.6 (C-10)、δC 157.5 (C-11) 存在关联,提示化合物1的C-2和C-11位被羟基取代。另外,δH 4.78 (1H,d,J = 12.1 Hz)、δH 4.85 (1H,d,J = 12.1 Hz) 与δC 169.6 (C-5)、δC 137.6 (C-7a) 存在关联,δH 7.12 (1H,s) 与δC 122.9 (C-11b)、δC 145.4 (C-2) 存在关联,δH 7.22 (1H,s) 与δC 149.4 (C-3)、δC 125.7(C-4a) 存在关联,δH 6.61 (1H,d,J = 2.2 Hz) 与δC 158.0 (C-9)、δC 157.5 (C-11) 存在关联,δH 6.58 (1H,d,J = 2.2 Hz) 与δC 68.8 (C-7)、δC 158.0 (C-9) 存在关联。综合以上信息,最终确定化合物1的结构为2,11-二羟基-3,9-二甲氧基-7H-联苯[c,e]氧杂䓬-5-酮,该化合物为一个新化合物。

|
Figure 2 Key HMBC correlations of 1 |
Avatar 360E.S.P型红外光谱仪 (美国Nicolet公司); UV-260型分光光度仪 (日本岛津公司); Bruker APEXⅢ 7.0 TESLA FTMS型高分辨质谱仪 (德国Bruker公司); Bruker DRX400型核磁共振仪 (德国Bruker公司,TMS为内标); Bruker Avance-300,500型核磁共振光谱仪测定 (德国Bruker公司,TMS为内标); Shimadzu LC-20A半制备型高效液相色谱仪 (日本岛津公司,检测器: UV); RP-C18 (YMC,10 μm,250 mm × 10 mm); Sephadex LH-20凝胶 (美国GE公司);柱色谱硅胶 (青岛海洋化工厂)。
藏药波棱瓜子药材于2010年7月自西藏林芝地区采集,经西藏农牧学院动物科技系植物生物技术教研室兰小中副教授鉴定,凭证标本 (2010-CM-01) 保存于西南大学药学院药物分析教研室。
1 提取与分离干燥的成熟波棱瓜子27 kg,粉碎,95% 乙醇室温下提取3次,合并提取液,减压浓缩,得到总浸膏2.1 kg。将浸膏分散于3 L水中,然后依次用石油醚、乙酸乙酯、正丁醇萃取。萃取液减压回收后,得石油醚萃取物约1 500 g,乙酸乙酯萃取物175 g,正丁醇萃取物120 g。从石油醚萃取物中取出700 g样品,经正相硅胶色谱 (7 000 g) 分离,以石油醚-乙酸乙酯 (100∶0~0∶100) 梯度洗脱,得到28个组分 (Fr.1~28)。Fr.25 (石油醚-乙酸乙酯3∶7) 经硅胶柱色谱,石油醚-乙酸乙酯系统 (9∶1~5∶5),得到8个亚组分 (Fr.25-1~25-8)。Fr.25-7经过羟丙基葡聚糖凝胶LH20分离后得到3个亚组分 (Fr.25-7-1~Fr.25-7-3),Fr.25-7-3再经半制备HPLC分离,流动相为甲醇-水 (60∶40),得到化合物1 (8 mg,tR 12.5 min)。取乙酸乙酯萃取物164 g,用等重的硅胶拌样,烘干后,干法上样。依次用氯仿、氯仿-甲醇 (30∶1~1∶1)、甲醇梯度洗脱,得到10个组分 (Fr.26~Fr.35)。Fr.30 (氯仿-甲醇15∶1) 经反相硅胶柱色谱,用甲醇-水 (25∶75~100∶0) 梯度洗脱,洗脱流分中有晶体析出,重结晶得到化合物2 (20 mg),结晶母液经制备HPLC,以甲醇-水 (55∶45~70∶30) 梯度洗脱,得化合物3 (22 mg)。Fr.33 (氯仿-甲醇6∶1) 经反相硅胶柱色谱,用甲醇-水 (20∶80~100∶0) 梯度洗脱,洗脱流分中有晶体析出,经重结晶得到化合物4 (25 mg)。
2 结构鉴定化合物1 无定形粉末; UV λmax (MeOH)/nm (logε): 303 (2.78),254 (2.96),221 (3.10); IR vmax (KBr)/ cm-1: 3 460,3 186,1 680,1 604,1 458,1 340,1 209,1 170,1 095,1 008,783,545; HR-ESI-MS m/z 301.070 4 [M-H]- (calcd. C16H14O6,301.071 8); 1H NMR (400 MHz,DMSO-d6) 和13C NMR (100 MHz,DMSO-d6) 数据见表 1。
化合物2 白色针状结晶 (甲醇),mp (707.1 ± 60.0) ℃; UV λmax (CH3OH)/nm: 216,278; IR (KBr)/ cm-1: 3 406,1 608,1 517; ESI-MS m/z: 532.73 [M-H]-; 1H NMR (CD3OD,300 MHz): 7.41 (2H,d,J = 8.7 Hz,H- 2',H-6'),7.26 (1H,s,H-6),6.98 (2H,d,J = 10.2 Hz,H-2,H-2''),6.87 (1H,d,J = 8.1 Hz,H-6''),6.79 (1H,d,J = 8.1 Hz,H-5''),6.68 (1H,d,J = 15.6 Hz,H-7),6.35 (1H,d,J = 15.6 Hz,H-8),5.60 (1H,d,J = 6.0 Hz,H-7''),4.80 (2H,s,H-9'),4.24 (2H,d,J = 4.5 Hz,H-9),4.00 (3H,s,3'-OCH3),3.93 (3H,s,3''-OCH3),3.83 (3H,s,3-OCH3),3.61 (2H,m,H-9''),3.31 (1H,s,H-8''); 13C NMR (CD3OD,75 MHz): 155.7 (C-7'),150.4 (C-4'),149.2 (C-3),147.7 (C-4),146.4 (C-3''),145.7 (C-3'),143.9 (C-4''),134.6 (C-1''),134.3 (C-5'),132.9 (C-5''),132.4 (C-7''),130.8 (C-1),128.8 (C-8''),125.1 (C-1'),119.9 (C-6),117.5 (C-6'),116.2 (C-5),115.1 (C-8 '),113.1 (C-2'),111.3 (C-6''),110.7 (C-2),106.0 (C-2''),89.6 (C-7),64.6 (C-9),63.8 (C-9''),56.8 (3'-OCH3),56.5 (3''-OCH3),56.4 (3-OCH3),55.4 (C-9'),55.1 (C-8); 1H NMR、13C NMR数据与文献对照[24],基本一致,鉴定该化合物2为7,8'-didehydroherpetotriol。
化合物3 白色颗粒状结晶 (氯仿-甲醇),mp 175~176 ℃; UV λmax (CH3OH)/nm: 211,273,302; ESI-MS m/z: 536.85 [M+H]+; 1H NMR (CD3OD,500 MHz): 6.90~6.94 (5H,m,H-2,2',6',2'',6''),6.84 (1H,d,J = 8.0 Hz,H-5),6.80 (1H,d,J = 8.0 Hz,H-6),6.54 (1H,d,J = 16.0 Hz,H-7''),6.22 (1H,dt,J = 16.0,6.0 Hz,H-8''),5.60 (1H,d,J = 6.0 Hz,H-7'),5.56 (1H,d,J= 6.5 Hz,H-7),4.23 (2H,d,J = 5.5 Hz,H-9''),3.81~3.92 (4H,m,H-9',H-9),3.91 (3H,s,3'-OCH3),3.86 (3H,s,3''-OCH3),3.84 (3H,s,3-OCH3),3.61 (1H,q,H-8'),3.57 (1H,q,H-8); 13C NMR (CD3OD,125 MHz): 147.7 (C-4'),147.5 (C-3),147.3 (C-4''),145.7 (C-4),143.9 (C-3''),143.8 (C-3'),134.6 (C-1''),132.7 (C-1'),130.9 (C-1),130.5 (C-7''),128.4 (C-5'),128.4 (C-8''),126.1 (C-5''),118.6 (C-6),114.9 (C-6''),114.6 (C-5),114.5 (C-6'),110.1 (C-2''),109.9 (C-2'),109.0 (C-2),88.3 (C-7'),87.9 (C-7),63.6 (C-9'),63.3 (C-9),62.5 (C-9''),55.6 (3'-OCH3),55.5 (3'-OCH3),55.3 (3-OCH3),53.5 (C-8'),53.4 (C-8)。1H NMR、13C NMR数据与文献对照[13],基本一致,鉴定该化合物3为herpetotriol。
化合物4 黄色针状结晶 (甲醇),mp 201~203 ℃; UV λmax (CH3OH)/nm: 264,344; IR (KBr)/cm-1: 3 330,2 976,2 926,2 854,1 656,1 604,1 515,1 491,1 478,1 351,1 260,1 208,1 179,1 137,1 117,1 097,1 060,1 024,994,973; ESI-MS m/z: 577.13 [M-H]-; 1H NMR (CD3OD,500 MHz): 7.79 (2H,d,J = 9.0 Hz,H-6',H-2'),6.95 (2H,d,J = 9.0 Hz,H-3',H-5'),6.72 (1H,d,J = 2.0 Hz,H-6),6.47 (1H,d,J = 2.0 Hz,H-8),5.56 (1H,s,H-Rha1),3.49 (1H,t,H-Rha2),3.86 (1H,m,H-Rha3),4.26 (1H,m,H-Rha4),3.63 (1H,m,H-Rha5),1.28 (3H,d,CH3-Rha6),5.40 (1H,s,H-Rha1'),3.36 (1H,m,H-Rha2'),3.75 (1H,m,H-Rha3'),4.06 (1H,m,H-Rha4'),3.63 (1H,m,H-Rha5'),0.95 (3H,d,CH3-Rha6'); 13C NMR (CD3OD,500 MHz): 179.2 (C-4),163.0 (C-7),162.5 (C-5),161.9 (C-4),158.5 (C-9),157.2 (C-2),136.2 (C-3),131.6 (C-6),131.6 (C-2),121.6 (C-1),116.5 (C-3),116.5(C-5),107.1 (C-10),100.1 (C-6),94.9 (C-8),104.0 (Rha-1),72.0 (Rha-2),72.6 (Rha-3),73.3 (Rha-4),72.1 (Rha-5),18.3 (Rha-CH3),100.4 (Rha-1'),71.8 (Rha-2'),72.4 (Rha-3'),73.6 (Rha-4'),71.4 (Rha-5'),18.6 (Rha- CH3)。1H NMR、13C NMR数据与文献对照[25],基本一致,鉴定该化合物4为kaempferitrin。
| [1] | Ministry of Health of the People's Republic of China. The Drug Standard of Ministry of Health of the People's Republic of China (Tibetan Medicines) (中华人民共和国卫生部药品标准(藏药))[M]. Beijing:Ministry of Health of the People's Republic of China, 1995:641. |
| [2] | Li G, Wang XY, Suo YR, et al. Protective effct of seed oil of Herpetospermum pedunculosum against carbon tetrachlorideinduced liver injury in rats[J]. Saudi Med J, 2014, 35:981-987. |
| [3] | Shen BD, Chen HG, Shen CY, et al. Hepatoprotective effects of lignans extract from Herpetospermum caudigerum against CCl4-induced acute liver injury in mice[J]. J Ethnopharmacol, 2015, 164:46-52. |
| [4] | Fan QM, Zhang H, Gao Y. Anti-oxidant activities of the seed extracts of Herpetospermum pedunculosum against liver injury in rats[J]. West China J Pharm Sci (华西药学杂志), 2008, 23:147-149. |
| [5] | Jin SY, Lv JL, Yuan HL, et al. Studies on anti-fatigue and anoxia-resistant effects of Herpetospermum Caudigerum extracts in mice[J]. Pharm J Chin PLA (解放军药学学报), 2011, 27:417-418. |
| [6] | Han YM, Li ZM, Yuan HL, et al. Curative effect of ganneng dropping pros on ducks in vivo transfected by DHBV[J]. Chin J Exp Tradit Med Form (中国实验方剂学杂志), 2006, 12:43-44. |
| [7] | Kaouadji M, Favre-Bonvin J. Herpepentol, a new lignoid pentamer isolated from Herpetospermum caudigerum Wall[J]. Tetrahedron Lett, 1984, 25:5137-5138. |
| [8] | Kaouadji M, Pieraccini E. Herpetetradione, a new lignoid tetramer isolated from Herpetospermum caudigerum Wall[J]. Tetrahedron Lett, 1984, 25:5135-5136. |
| [9] | Kaouadji M, Favre-Bonvin J. Herpetol, a new dimeric lignoid from Herpetospermum caudigerum Wall[J]. J Biosci, 1984, 39C:307-308. |
| [10] | Kaouadji M, Favre-Bonvin J. Herpetriol and herpetetrol, new lignoids isolated from Herpetospermum caudigerum wall[J]. J Biosci, 1979, 34C:1129-1132. |
| [11] | Kaouadji M, Favre-Bonvin J. Herpetetrone, another tetrameric lignoid from Herpetospermum caudigerum seeds[J]. J Nat Prod, 1987, 50:1089-1094. |
| [12] | Kaouadji M, Favre-Bonvin J. Herpetrione, a trimeric lignoid isolated from Herpetospermum caudigerum[J]. Tetrahedron Lett, 1983, 24:5881-5884. |
| [13] | Kaouadji M, Favre-Bonvin J. Herpetal, a benzofuran isolated from Herpetospermum caudigerum[J]. Phytochemistry, 1978, 17:2134-2135. |
| [14] | Xu B, Liu S, Fan XD, et al. Two new coumarin glycosides from Herpetospermum caudigerum[J]. J Asian Nat Prod Res, 2015, 17:738-743. |
| [15] | Yu JQ, Hang W, Duan WJ, et al. Two new anti-HBV lignans from Herpetospermum caudigerum[J]. Phytochem Lett, 2014, 10:230-234. |
| [16] | Xu B, Yang PP, Wang PL, et al. Study on the chemical constituents of Herpetospermum caudigerum[J]. J Chin Med Mater (中药材), 2012, 35:1080-1082. |
| [17] | Li LY, Deji LM, Wei YF, et al. Literature data investigation in semem of Herpetospermum caudigerum[J]. China J Chin Mater Med (中国中药杂志), 2005, 30:893-894. |
| [18] | Chen L, Zhang M, Li CQ, et al. Protective effect of Herpetospermum ethyl acetate extracts on immunologic liver injury in mice[J]. Pharmacol Clin Chin Mater (中药药理与临床), 2014, 30:111-113. |
| [19] | Shen G, Shen CY, Cheng L, et al. Hepatoprotective effects of total lignans of Herpetospermum caudigerum nanosuspension capsules[J]. Chin Pharm J (中国药学杂志), 2015, 50:1038-1042. |
| [20] | Ulrich H, Gabriele MK, Anthony DW. A new tyrosine kinase inhibitor from a marine isolate of ulocladium botrytis and new metabolites from the marine fungi Asteromyces cruciatus and Varicosporina ramulosa[J]. Eur J Org Chem, 1999, 11:2949-2955. |
| [21] | Ying W, Ming HY, Xiao BW, et al. Bioactive metabolites from the endophytic fungus Alternaria alternate[J]. Fitoterapia, 2014, 99:153-158. |
| [22] | Takao T, Miho K, Akiko K, et al. For phenolics from the cultured lichen mycobiont of Graphis scripta var. pulverulenta[J]. Chem Pharm Bull, 1997, 45:1183-1185. |
| [23] | Kong LY, Min ZD. Studies on chemical constituents of roots of Euphorbia Pekinensis[J]. Acta Pharm Sin (药学学报), 1996, 31:524-529. |
| [24] | Guo R, Luo M, Long CL, et al. Two new lignans from Dipteronia dyeriana[J]. Chin Chem Lett, 2008, 19:1215-1217. |
| [25] | Wu P, Lin FW, Wu TS, et al. Cytotoxic and anti-hiv principles from the rhizomes of Begonia Nantoensis[J]. Chem Pharm Bull, 2004, 52:345-349. |
 2016, Vol. 51
2016, Vol. 51


