耐甲氧西林金葡菌 (methicillin-resistant Staphylococcus aureus,MRSA) 被称作“超级病菌”,自1961年在英国被首次发现以来,即以惊人的速度在世界范围内蔓延,目前MRSA感染已超过艾滋病、结核和病毒性肝炎成为患者首位致死原因,严重威胁公共卫生安全。虽然利奈唑胺[1]、达托霉素[2]、头孢洛林[3]、奥利万星[4]、达巴万星[5]、磷酸泰地唑胺[6]等新药陆续获得FDA批准用于MRSA的治疗,但已在临床使用多年的万古霉素仍然是治疗MRSA感染的一线药物[7]。因此,寻找结构新颖、高效低毒的抗MRSA先导化合物成为近年来抗生素研究领域的重要方向。本文对2010年至今发现的具有显著抗MRSA活性的天然产物及其药物化学研究进行综述。
1 抗MRSA活性的天然产物 1.1 木脂素和醌Hao等[8]发现,来源于Manglietiastrum sinicum的木脂素化合物manglisins A~D (1~4) 具有显著的抗MRSA活性,其MIC为0.006 4~0.052 μg·mL-1。Tomoda等[9]从Penicillium radicum FKI- 3765-2中分离得到结构新颖的蒽醌二聚物rugulosins A~C (5~7),其中仅rugulosin A (5) 抗MRSA活性显著 (MIC = 0.125 μg·mL-1),表明C15或C15'的羟基将导致活性降低。Müller等[10]发现Hyalangium minutum NOCB-2T DSM 14724T的提取物hyaladione (8) 表现出显著的抗MRSA活性 (MIC = 0.83 μg·mL-1)。此外,传统中药虎杖的主要活性成分大黄素9[11]对MRSA也具有良好的抑制活性,其MIC为2~4 μg·mL-1。
1.2 黄酮Qian等[12]从Streptomyces caelestis中 分离的七环化合物citreamicins θ A和θ B (10、11) 抗MRSA活性显著,MIC均为0.25 μg·mL-1。结构较为简单的macluraxanthones (12、13) 由Laphookhieo等[13]从Garcinia propinqua枝中分离得到,MIC均为 4 μg·mL-1。Cichewicz等[14]从夏威夷土壤Alternaria sp.中获得了通过硫键相连的二聚体polluxochrin (14) 和dioschrin (15)。在水存在下,14可经分子内环合得到15和castochrin (16),14~16均表现出抗MRSA 活性,MIC范围为2~3.2 μg·mL-1。
1.3 萜Lin等[15]从中国南海海绵Dysidea sp.中分离的dysidinoid A (17) 是首个来源于自然界的具有9,4- friedodrime骨架和2,5-二酮吡咯片段的杂萜,具有 中等抗MRSA活性,MIC为8 μg·mL-1。Zhang等[16]从苏木中分离的二萜caesanines A和B (18、19) 在C19/C20之间具有独特的氮桥结构,对MRSA表现出良好的活性,MIC范围为3.125~12.5 μg·mL-1。Roussis等[17]从褐藻Dilophus spiralis中提取了17个具有dolabellane骨架的二萜化合物,其中化合物20抗MRSA活性显著,MIC为2 μg·mL-1,其C-14异构 体21和环氧化合物22活性较弱,MIC分别为4和 8 μg·mL-1,可见C14位羟基对增强活性有一定作用。
1.4 生物碱Gibbons等[18]从南美植物Pterogyne nitens中发现了抗MRSA活性显著的异戊烯基胍类生物碱galegine (23) 和pterogynidine (24),其MIC均为4 μg·mL-1,构效关系研究表明,异戊烯基是重要的活性基团,如将其替代为香叶基或将亚胺氮原子变为硫或氧 (25) 均将导致活性降低。Hertweck等[19]发现,来源于Kandelia candel Streptomyces sp.发酵液的吲哚倍半萜xiamycin B (26)、indosespene (27) 和sespenine (28) 表现出中等至显著的抗MRSA活性。Hao等[20]从Myrioneuron faberi中分得的myrifabine (29) 对MRSA的MIC为6.32~12.54 μg·mL-1,该化合物结构新颖,其十环骨架上具有12个手性中心,是首个发现的Myrioneuron型二聚体生物碱。Nanayakkara等[21]从Septoria pistaciarum分得吡啶酮生物碱30和31,其中化合物31对MRSA的MIC为5 μg·mL-1,但化合物30无活性,说明N-羟基化会导致活性消失。Laphookhieo等[22]从Clausena wallichii根中发现的咔唑生物碱clausenawalline E (32) 和heptaphylline (33) 具有中等抗MRSA活性,MIC分别为8和4 μg·mL-1。
1.5 大环化合物Fenical等[23]从加利福尼亚近岸沉积泥的海洋微生物Streptomyces sp.中发现了具有独特环系结构的大环内酯anthracimycin (34),该分子由含14个碳原子的大环与两个环己烷稠合而成,在体内和体外实验中对包括MRSA在内的多个耐药性金葡菌活性显著,MIC≤0.25 μg·mL-1。Müller等[24]从 粘杆菌Pyxidicoccus fallax AndGT8的发酵液中分离得到大环内酯苷disciformycins A和B (35、36),其中35活性优于万古霉素,且无交叉耐药性,其抗MRSA和MRSA/VRSA (耐万古霉素金葡菌) 的MIC分别为0.6~1.2 μg·mL-1和0.6 μg·mL-1,其双键位置异 构体36活性稍弱,对上述菌株的MIC范围为2~8 μg·mL-1。Lacret等[25]从Streptomyces zhaozhouensis CA-185989中分离得到的异斑鸠霉素 (37) 和28-N-甲基斑鸠霉素 (38) 均表现出较好的抗MRSA活性,其MIC分别为2~4 μg·mL-1和1~2 μg·mL-1。Li等[26]从Bacillus amyloliquefaciens AP183中也得到活性优于万古霉素的大环多烯bacillusin A (39),其对MRSA和VRE (耐万古霉素肠球菌) 的MIC分别为1.2和0.6 μg·mL-1。
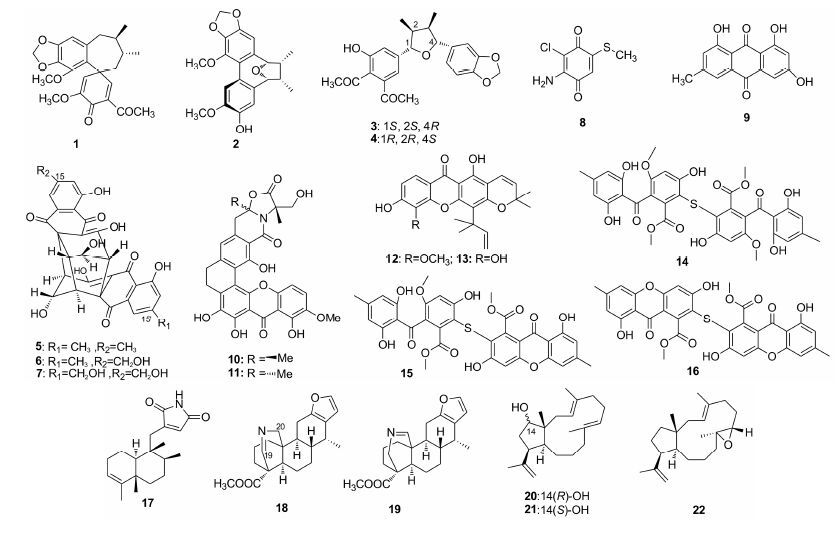

Reyes等[27]发现,来源于Kocuria palustris的噻唑多肽化合物kocurin (40) 抗MRSA活性显著,MIC为0.25 μg·mL-1。Fenical等[28]从Streptomycesstrain中分离的缩肽化合物fijimycins A~C (41~43) 表现出中等至显著的抗MRSA活性 (MIC = 4~32 μg·mL-1),其中fijimycin B (42) 活性最差,说明苯基片段是保持活性的关键,但空间构型对活性无明显影响。与之类似,Inoue等[29]发现,分离自巴布亚新几内亚海洋Brevibacillus laterosporus培养液的bogorol A (44) 也与其Z式异构体 (45) 具有一致的抗MRSA活性,MIC均为4 μg·mL-1。此外,Kazuhisa等[30]发现,分离自Lysobactersp. RH2180-5发酵液的环十二肽lysocin E (46) 以MRSA的原生质体膜中的甲基萘醌为靶点,MIC达到4 μg·mL-1,且对小鼠的ED50 (0.5 mg·kg-1) 明显优于万古霉素 (5.8 mg·kg-1),是具有临床应用价值的化合物。Inoue等[31]进一步发现,异构体47与天然产物46抗菌活性相同,说明lysocin E的活性不受空间构型影响,但氮去甲基化 (48) 会导致活性降低。
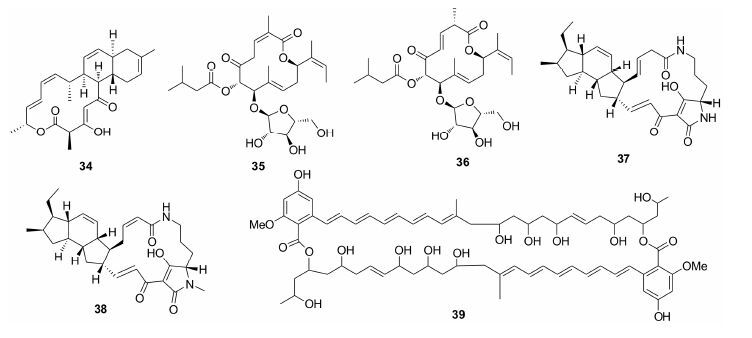
1.7 糖苷类Andersen等[32]发现采集于加拿大海洋沉积物链霉菌的novobiocin (49) 抗MRSA活性显著,MIC为0.25 μg·mL-1,其3''和4''上的氨基甲酰基和甲氧基以及5位氢均是重要活性基团。具有中等抗MRSA活性的甾醇糖苷50和51由Kubanek等[33]分离自Peyssonnelia sp.,MIC分别为6.3和3.1 μg·mL-1。Xiao等[34]从Streptomycessp.中分离的糖苷52及其羟基类似物 (53~55) 均表现出显著的抗MRSA活性,MIC范围为1~4 μg·mL-1,C11/C11'的半缩酮结构及C14/C14' 的烷基化有助于增加该类化合物的活性。Oberlies等[35]从Bionectria sp. MSX 47401中获得化合物56~58,其中58活性最优,对MRSA的MIC范围为2.8~5.5 μg·mL-1,化合物56和57则表现出中等的抗菌活性,MIC为7.8~28 μg·mL-1。
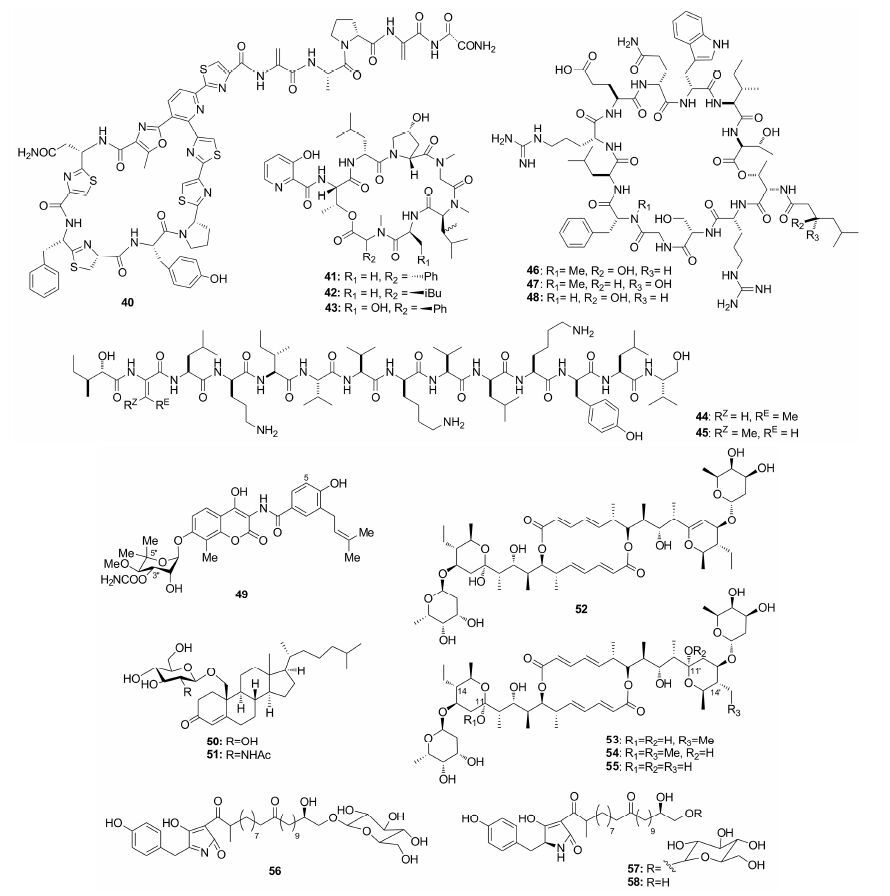
Hemscheidt等[36]从Pseudoalteromonas sp. 发酵液中提取的溴代物59和60抗MRSA活性显著,其IC50分别为0.9和0.92 μg·mL-1。Gerwick等[37]从蓝藻 (Leptolyngbya crossbyana) 中得到多溴代苯酚醚crossbyanols A~D,其中crossbyanol B (61) 抗MRSA活性较好,MIC范围为2.0~3.9 μg·mL-1。Lin等[38]从深海Spiromastix sp. 真菌发酵液中得到多个在A环和C环上具有丙基取代的多卤代化合物,其活性与卤代数量成正比 (四氯代65: IC50 = 0.96 μg·mL-1,三氯代62~64: IC50 = 1.7 μg·mL-1)。Pestalone (66) 来源于Pestalotia和单细胞海洋细菌CNJ-328的共培养物,2001年首次发现时被认为抗MRSA活性极为显著 (MIC =37 ng·mL-1)。Schmalz等[39]在全合成的基础上发现其活性低于此前报道,MIC为6.25 μg·mL-1,且其合成衍生物67~69也表现出较好的抗MRSA活性 (MIC = 3~10 μg·mL-1)。Fenical等[40]从Streptomyces sp. CNQ-329发酵液中得到C7和C10a由线性单萜桥相连的混源萜类napyradiomycin A和B (70、71),虽然结构新颖,但其抗MRSA活性远远低于napyradiomycins B3 (72) (70、71: MIC = 16~64 μg·mL-1,72: MIC = 2 μg·mL-1),研究发现,C-3位和C-4a位上的氯原子是活性关键所在。Hensler等[41]从Streptomyces sp. CNQ-525中分得的napyradiomycin衍生物A80915A (73) 及其重氮酮异构体A80915B (74) 对MRSA均显示出理想的活性,MIC范围为1~3 μg·mL-1,且74对VRSA也具有抑制作用 (MIC =0.5~4 μg·mL-1)。Fenical等[42]从Streptomycessp. CNH-189分得的杂萜merochlorins A和B (75、76) 活性相似,抗MRSA的MIC范围为2~4 μg·mL-1。Bewley等[43]从海洋金藻Chrysophaeum taylori中分得的chrysophaentin A (77) 抗MRSA活性显著,MIC50为 (1.5 ±0.7) μg·mL-1。研究表明其芳环上的羟基及氯原子取代有助于提高活性。此外,保持其基本的大环骨架对活性也具有重要意义。

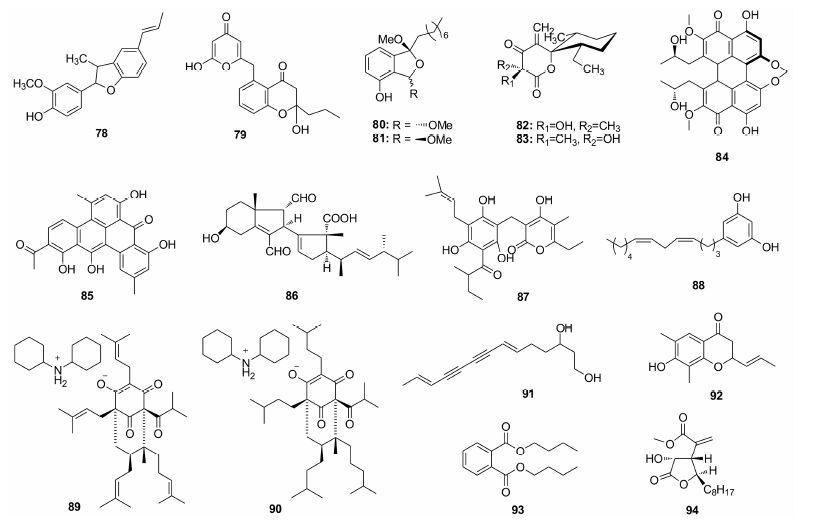
Filho等[44]从巴西传统草药Piper regnellii的叶子中提取了对MRSA和MSSA (甲氧西林敏感 金葡菌) 均表现出良好活性的酚eupomatenoid-5 (78),MIC均为4 μg·mL-1。Mancini等[45]发现,来源于Streptomyces sundarbansensis的聚酮化合物chromanone (79) 能选择性抑制MRSA,MIC为2 μg·mL-1。Jung等[46]从Paecilomyces variotii提取的化合物80抗MRSA活性明显高于81 (80: MIC =5~20 μg·mL-1; 81: MIC =20~40 μg·mL-1),说明构型对该类化合物的活性具有重要影响。Shiomi等[47]从Simplicillium sp. FKI-5985培养液中分得骨架新颖的aogacillins A和B (82、83),该类化合物的内酯环与环已烷通过螺原子相连,可使阿贝卡星对耐阿贝卡星的MRSA的MIC由256 降至8 μg·mL-1,具有良好的应用前景。Nanayakkara等[48]从Septoria pistaciarum中提取的 (+)-cercosporin (84) 对MRSA的MIC为5.0 μg·mL-1。 Hertweck等[49]从Clostridium beijerinckii HKI0724中得到的clostrubin (85) 是首个在严格厌氧菌环境中发现的多酚化合 物,对MRSA的MIC为0.05 μg·mL-1,其独特的五 环骨架是首次在天然产物中被发现。来源于斯里兰卡Rhizoctonia solani的solanioic acid (86) 对包括MRSA在内的多种革兰阳性菌表现出良好的抑制活性 (MIC = 1 μg·mL-1),其多取代碳骨架由类固醇重排而得[50]。Carpinella等[51]从阿根廷本土植物Achyrocline satureioides和Lithrea molleoides中分离的87和88对MRSA的MIC均为2 μg·mL-1,且化合物87与 红霉素或庆大霉素联用时,可分别降低其使用浓度至1/300和1/260,具有临床联合用药的潜力。Verotta等[52]发现来源于Hypericum perforatum的不稳定多 异戊烯基取代化合物hyperforin (89) 及其氢化类 似物90的MIC分别为1和4 μg·mL-1。Kim等[53]从Atractylodes japonica根中提取的不饱和碳链化合物91表现出中等的抗MRSA活性,其MIC范围为4~32 μg·mL-1。Ye等[54]从Penicillium sp. XGH2321发酵液中分离的烯92和酯93对MRSA具有与万古霉素相当的抑菌活性。Wu等[55]以控制肽聚糖合成的MurA酶为靶点筛选到产自Neosartorya fischeri的化合物avenaciolide (94),94对野生型MRSA MurA和MurAC119D (耐磷霉素突变体) 都具有显著抑制活性,IC50为2~2.4 μg·mL-1,其结构中的α,β-不饱和羰基是重要活性基团。
值得注意的是,一些天然产物虽然自身抗MRSA活性较弱,但与现有抗生素联用表现出显著的协同作用,有望开发成治疗抗MRSA感染的辅助药物。如芹菜素[56]和香芹酮[57]可明显增加喹诺酮类和 (或) 氨基糖苷类抗生素的抗菌作用; 绿茶的主要成分表儿茶素没食子酸酯 (ECg) 和表没食子儿茶素没食子酸酯 (EGCg)[58]、山楂提取物[59]、桑色素[60]和鼠尾草酸[61]对β-内酰胺类抗生素均显示出良好的协同作用。
DNA旋转酶和拓扑异构酶Ⅳ是抗MRSA药物的重要靶标之一。活性显著的山柰酚鼠李糖苷95[62]和吲哚并喹啉化合物97[63]均以此为靶点,其MIC分别为1~2 μg·mL-1和0.063 μg·mL-1。Hossion等[64]以95为先导化合物合成了系列槲皮素酰基糖苷衍生物,发现半乳糖苷96对MRSA、VRE、VISA (万古霉素中介金葡菌) 和MSSA均表现出良好的抗菌活性,其MIC范围为0.13~0.5 μg·mL-1,为万古霉素和诺氟沙星的2倍以上,体内实验表明该化合物具有良好的吸收性和低毒性。 构效关系研究表明,其糖片段上合适大小的亲脂取代对活性具有重要影响。Lin等[63]发现卤代酯基对先导化合物97的活性具有积极作用,并得到了活性优于万古霉素的三氟乙酯98,其MIC为0.031 μg·mL-1。
PBP2是另一个常见的抗MRSA药物靶点。Talele等[65, 66]以具有PBP2抑制作用的罗丹宁衍生物99为先导化合物 (MIC = 62.5 μg·mL-1) ,发现罗丹宁骨架的N3亲脂侧链及C5苄亚甲基有助于增加活性,其中N3以苯丙氨酸取代活性最佳。进一步研究发现,苄亚甲基3位上具有与其保持一定空间距离的脂溶性芳香基团或在其芳环上引入卤代基团均可提高其活性。结构修饰后的衍生物100 ~102活性显著提高,MIC范围为0.98~1.95 μg·mL-1,是具有开发潜力的抗MRSA候选物。
多项研究表明,增加天然产物的脂溶性是获得具有更优抗MRSA活性化合物的重要手段。来源于地钱(Reboulia hemisphaerica) 的大环双联苄天然产物riccardin C (103) 具有中等抗MRSA活性(MIC = 3.2 μg·mL-1)[67],Miyachi等[68]发现B环无取代衍生物104的抗MRSA活性显著提高 (MIC =1 μg·mL-1),进一步改变骨架结构,将AB环通过碳键直接相连,发现衍生物105活性进一步提升,MIC为0.5 μg·mL-1,而化合物106则活性丧失,说明C/D环上的羟基及大环的刚性结构对活性保持具有重要影响[69]。Goker等[70]经过结构修饰得到的苯并咪唑类化合物10 7具有与万古霉素相似的抗MRSA活性 (MIC = 0.78 μg·mL-1),其胍侧链上的苄基变为烷基后活性将显著降低。
α-Mangostin (108) 抗MRSA活性显著,MIC为1.56 μg·mL-1。Beuerman等[71, 72]发现其刚性芳香并环骨架及疏水侧链是活性所必需的。同时,具有两亲性特点的阳离子氨基酸残基如精氨酸对膜靶向也具有重要影响。所合成衍生物对包括MRSA在内的多种革兰阳性菌具有良好的抑制活性,其中109对MRSA的MIC达到0.095~1.56 μg·mL-1。精氨酸衍生物110和111也显示出良好的抗菌活性 (MIC = 2~4 μg·mL-1)。
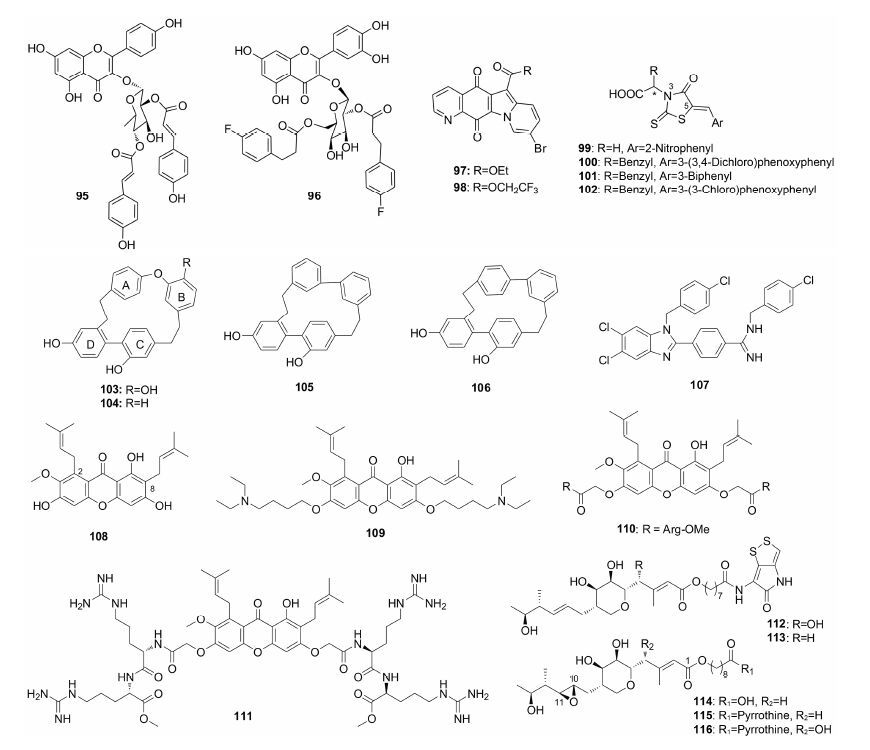
将基因工程应用于抗MRSA活性化合物的构效关系研究是近年来的新方向。Simpson等[73]发现天然产物thiomarinols A、C (112、113) 和pseudomonic acid A (114) 具有较好的抗MRSA活性,并通过生物合成获得了抗MRSA化合物115和116,所合成衍生物与天然产物活性相当。
如前所述,众多含卤化合物均具有显著的抗MRSA活性。因此,在天然产物中引入卤原子往往能获得理想的效果。Zadrazilova等[74]合成了多个具有显著抗MRSA活性的N-烷基氨基甲酸酯取代的水杨酰苯胺衍生物 (MIC < 0.008~4 μg·mL-1),其中化合物117和118的抗MRSA活性是万古霉素的250倍 (MIC < 0.008 μg·mL-1),且抗MSSA活性显著,MIC分别为 < 0.008 μg·mL-1和0.016 μg·mL-1。构效关系研究表明,其抗菌活性与酰胺烷基碳链的长度成反比; 同时,苯环上较多的卤代有助于活性的增加,且4位卤代产物活性优于3位卤代产物。
海洋生物碱cis-3,4-dihydrohamacanthin B (119) 以丙酮酸激酶为靶点,抗MRSA的MIC为12.5 μg·mL-1。Young等[75]发现保持该类化合物的双吲哚线性结构尤其是游离吲哚氮氢及溴原子取代对活性具有重要意义,但内酰胺片段并非活性必需基团。在吲哚环 的5位、6位、5' 位和6' 位分别引入两个或三个卤代基团 (化合物120),将极大提高其抗MRSA活性,MIC范围为0.3~1 μg·mL-1,较万古霉素更优 (MIC = 2 μg·mL-1)。此外,Veale等[76]对结构和机制类似的bromodeoxytopsentin (121) 进行研究时也发现,在苯环上引入卤代基团有助于提高其活性,二卤代衍生物122的活性明显优于先导化合物121 (121: IC50 = 24 ng·mL-1; 122: IC50 = 0.59~1.90 ng·mL-1)。
高卤代双吡咯化合物marinopyrroles分离自海洋Streptomyces sp.,其中marinopyrroles A和C (123、124) 表现出与万古霉素一致的抗MRSA活性,其MIC分别为0.16和0.088 μg·mL-1 (万古霉素: 0.2~0.4 μg·mL-1)[77]。Qin 等[78]发现吡咯环上的氯原子和苯环上邻位羟基取代基的存在是保持该类化合物活性的关键所在,同时,苯环上对位和间位吸电子保护基的存在也有利于其活性,所得苯环上4,4'-位具有CF3取代的衍生物125对MRSA的活性为万古霉素的4倍,并对MRSE (耐甲氧西林表皮葡球菌)、MSSA和ATCC (粪肠球菌) 均表现出优于万古霉素的抑制活性,是极具临床开发价值的抗生素候选药物。

Salinomycin (126) 来源于Streptomyces albus,抗MRSA的MIC范围为8~16 μg·mL-1。Huczynski等[79]发现其三氟乙基衍生物127的活性明显优于天然产物,MIC为0.5~1 μg·mL-1。分离自Magnoliae officinalis树皮的magnolol (128) 是结构独特的双苯酚化合物,以对位具有烷基侧链取代为特征。Kumar等[80]发现,芳环上的烷基取代和卤代尤其是溴代会增加其活性,且通常双卤代衍生物活性优于单卤代衍生物,衍生物129的抗MRSA的MIC为1 μg·mL-1。
某些活性天然产物虽然对MRSA无效,但通过结构改造也可以成为具有良好抗MRSA活性的药物候选物。肽130对金葡菌 (SA) 和VRE的MIC分别为2和31 μg·mL-1,Bremner等[81]对其萘骨架上的醚基团和C-端进行了修饰,发现衍生物131对MRSA和VRSA的MIC均为2~4 μg·mL-1。从Hypericum olympicum中提取的天然产物olympicin A (132) 对SA具有良好的抗菌活性,MIC为0.5~1 μg·mL-1,以其为先导化合物,Sun等[82]合成了多个黄酮衍生物,其中查尔酮133具有良好抗MRSA活性,MIC为0.39 μg·mL-1。构效关系研究表明,对4-二氢色原酮骨架而言,其2位上的亲脂取代和4位上的氢键供体或受体,及5位和7位上的羟基有利于活性; 对查尔酮骨架而言,2'和4'位上的双羟基及亲脂性侧链是活性的必要条件,且查尔酮衍生物的活性总体高于4-二氢色原酮。Mobashery等[83]以具有抗SA活性的喹唑啉酮134 (MIC = 2 μg·mL-1) 为先导化合物合成了与利奈唑胺和万古霉素活性相似的135,135以PBP1与PBP2a为靶点,对某些MRSA菌株的MIC低至2 μg·mL-1。Pigeonpea树叶提取物cajaninstilbene acid (136) 对SA的MIC为13 μg·mL-1,Chen等[84]发现苯环上具有氟原子取代的衍生物137表现出理想的抗MRSA活性,MIC范围为0.5~2 μg·mL-1。Pseudopteroxazole (138) 对结核杆菌活性显著,MIC为2 μg·mL-1,但对MRSA 无活性,Kerr等[85]发现C-21上的极性取代基有助于提高其抗MRSA活性,衍生物139~141对MRSA的IC50分别为3、7和12 μg·mL-1,优于万古霉素 (12.25 μg·mL-1)。

综上所述,自1994年报道抗MRSA天然产物andrimid和moiramide B以来[86],从天然产物中发现具有临床应用前景的抗MRSA活性先导化合物成为研究的热点。近年来所报道的抗MRSA天然产物结构类型多样,其中大环类化合物和多肽类化合物较多,且含卤化合物占据了相当比例; 从生物来源看,海洋微生物仍然是抗MRSA活性天然产物的主要来源。此外,以活性天然产物为先导化合物开展药物化学研究,已成为发现抗MRSA候选药物的重要手段,增加先导化合物的脂溶性和引入卤代基团往往能取得良好的效果。总之,对来源于自然界的抗MRSA活性成分开展广泛而深入的研究将积极推动新型抗MRSA抗生素的研发进程。
| [1] | Watkins RR, Lemonovich TL, File TM. An evidence-based review of linezolid for the treatment of methicillin-resistant Staphylococcus aureus (MRSA):place in therapy[J]. Core Evid, 2012, 7:131-143. |
| [2] | Gould IM, Miro JM, Ryba MJ. Daptomycin:the role of highdose and combination therapy for Gram-positive infections[J]. Int J Antimicrob Agents, 2013, 42:202-210. |
| [3] | Arshad S, Hartman P, Zervos MJ. A novel treatment option for MRSA pneumonia:ceftaroline fosamil-yielding new hope in the fight against a persistent infection[J]. Exp Rev Antiinfect Ther, 2014, 12:727-729. |
| [4] | Karaoui LR, El-Lababidi R, Chahine EB. Oritavancin:an investigational lipoglycopeptide antibiotic[J]. Am J Health Syst Pharm, 2013, 70:23-33. |
| [5] | Scott LJ. Dalbavancin:a review in acute bacterial skin and skin structure infections[J]. Drugs, 2015, 75:1281-1291. |
| [6] | Zhanel GG, Love R, Adam H, et al. Tedizolid:a novel oxazolidinone with potent activity against multidrug-resistant Gram-positive pathogens[J]. Drugs, 2015, 75:253-270. |
| [7] | Daniel A. Anti-infective drug development for MRSA[J]. Methods Mol Biol, 2014, 1085:311-331. |
| [8] | Ding JY, Li SL, Hao XJ, et al. Antimicrobial constituents of the mature carpels of Manglietiastrum sinicum[J]. J Nat Prod, 2014, 77:1800-1805. |
| [9] | Yamazaki H, Koyama N, Tomoda H, et al. Anti-MRSA antibiotics, produced by Penicillium radicum FKI-3765-2[J]. Org Lett, 2010, 12:1572-1575. |
| [10] | Okanya PW, Mohr KI, Müller R, et al. Hyaladione, an S-methyl cyclohexadiene-dione from Hyalangium minutum[J]. J Nat Prod, 2012, 75:768-770. |
| [11] | Cao F, Peng W, Zhou H, et al. Emodin is identified as the active component of ether extracts from Rhizoma Polygoni Cuspidati, for anti-MRSA activity[J]. Can J Physiol Pharmacol, 2015, 93:485-493. |
| [12] | Liu LL, Xu Y, Qian PY, et al. Four new antibacterial xanthones from the marine-derived actinomycetes Streptomyces caelestis[J]. Mar Drugs, 2012, 10:2571-2583. |
| [13] | Tantapakul C, Phakhodee W, Laphookhieo S, et al. Rearranged benzophenones and prenylated xanthones from Garcinia propinqua twigs[J]. J Nat Prod, 2012, 75:1660-1664. |
| [14] | Cai S, King JB, Cichewicz RH, et al. Bioactive sulfurcontaining sulochrin dimers and other metabolites from an Alternaria sp. isolate from a Hawaiian soil sample[J]. J Nat Prod, 2014, 77:2280-2287. |
| [15] | Jiao WH, Li J, Lin HW, et al. Dysidinoid A, an unusual meroterpenoid with anti-MRSA activity from the South China Sea sponge Dysidea sp.[J]. Molecules, 2014, 19:18025-18032. |
| [16] | Liu X, Liang J, Zhang L, et al. Caesanines A-D, new cassane diterpenes with unprecedented N bridge from Caesalpinia sappan[J]. Org Lett, 2013, 15:4726-4729. |
| [17] | Ioannou E, Quesada A, Roussis V. Dolabellanes with antibacterial activity from the brown alga Dilophus spiralis[J]. J Nat Prod, 2011, 74:213-222. |
| [18] | Coqueiro A, Regasini LO, Gibbons S, et al. In vitro antibacterial activity of prenylated guanidine alkaloids from Pterogyne nitens and synthetic analogues[J]. J Nat Prod, 2014, 77:1972-1975. |
| [19] | Ding L, Maier A, Hertweck C. A family of multicyclic indolosesquiterpenes from a bacterial endophyte[J]. Org Biomol Chem, 2011, 9, 4029-4031. |
| [20] | Cao MM, Zhang Y, Hao XJ, et al. Myrifabine, the first dimeric Myrioneuron alkaloid from Myrioneuron faberi[J]. Org Lett, 2014, 16:528-531. |
| [21] | Kumarihamy M, Fronczek FR, Nanayakkara NPD, et al. Bioactive 1,4-dihydroxy-5-phenyl-2-pyridinone alkaloids from Septoria pistaciarum[J]. J Nat Prod, 2010, 73:1250-1253. |
| [22] | Maneerat W, Ritthiwigrom T, Laphookhieo S, et al. Bioactive carbazole alkaloids from Clausena wallichii roots[J]. J Nat Prod, 2012, 75:741-746. |
| [23] | Jang KH, Nam SJ, Fenical W, et al. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete[J]. Angew Chem Int Ed, 2013, 52:7822-7824. |
| [24] | Surup F, Viehrig K, Müller R, et al. Disciformycins A and B:12-membered macrolide glycoside antibiotics from the myxobacterium Pyxidicoccus fallax active against multiresistant Staphylococci[J]. Angew Chem Int Ed, 2014, 53:13588-13591. |
| [25] | Lacret R, Oves-Costales D, Reyes F, et al. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis[J]. Mar Drugs, 2015, 13:128-140. |
| [26] | Ravu RR, Jacob MR, Li XC, et al. Bacillusin A, an antibacterial macrodiolide from Bacillus amyloliquefaciens AP183[J]. J Nat Prod, 2015, 78:924-928. |
| [27] | Martín J, Sousa TS, Reyes F, et al. Kocurin, the true structure of PM181104, an anti-methicillin-resistant Staphylococcus aureus (MRSA) thiazolyl peptide from the marine-derived bacterium Kocuria palustris[J]. Mar Drugs, 2013, 11:387-398. |
| [28] | Sun P, Maloney KN, Fenical W, et al. Fijimycins A-C, three antibacterial etamycin-class depsipeptides from a marinederived Streptomyces sp.[J]. Bioorg Med Chem, 2011, 19:6557-6562. |
| [29] | Yamashita T, Kuranaga T, Inoue M. Solid-phase total synthesis of bogorol A:stereocontrolled construction of thermodynamically unfavored (E)-2-amino-2-butenamide[J]. Org Lett, 2015, 17:2170-2173. |
| [30] | Hamamoto H, Urai M, Kazuhisa S, et al. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane[J]. Nat Chem Biol, 2015, 11:127-133. |
| [31] | Murai M, Kaji T, Inoue M, et al. Total synthesis and biological evaluation of the antibiotic lysocin E and its enantiomeric, epimeric, and N-demethylated analogues[J]. Angew Chem Int Ed, 2015, 54:1556-1560. |
| [32] | Dalisay DS, Williams DE, Andersen RJ, et al. Marine sediment-derived Streptomyces bacteria from British Columbia, Canada are a promising microbiota resource for the discovery of antimicrobial natural products[J]. PLoS One, 2013, 8:e77078. |
| [33] | Lin AS, Engel S, Kubanek J, et al. Structure and biological evaluation of novel cytotoxic sterol glycosides from the marine red alga Peyssonnelia sp.[J]. Bioorg Med Chem, 2010, 18:8264-8269. |
| [34] | Wu C, Tan Y, Xiao C, et al. Identification of elaiophylin derivatives from the marine-derived actinomycete Streptomyces sp. 7-145 using PCR-based screening[J]. J Nat Prod, 2013, 76:2153-2157. |
| [35] | Figueroa M, Raja H, Oberlies NH, et al. Peptaibols, tetramic acid derivatives, isocoumarins, and sesquiterpenes from a Bionectria sp. (MSX 47401)[J]. J Nat Prod, 2013, 76:1007-1015. |
| [36] | Feher D, Barlow R, Hemscheidt TK, et al. Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp.[J]. J Nat Prod, 2010, 73:1963-1966. |
| [37] | Choi H, Engene N, Gerwick WH, et al. Crossbyanols A-D, toxic brominated polyphenyl ethers from the Hawai'ian bloomforming cyanobacterium Leptolyngbya crossbyana[J]. J Nat Prod, 2010, 73:517-522. |
| [38] | Niu S, Shao Z, Lin W, et al. Spiromastixones A-O, antibacterial chlorodepsidones from a deep-sea-derived Spiromastix sp. fungus[J]. J Nat Prod, 2014, 77:1021-1030. |
| [39] | Augner D, Krut O, Schmalz HG, et al. On the antibiotic and antifungal activity of pestalone, pestalachloride A, and structurally related compounds[J]. J Nat Prod, 2013, 76:1519-1522. |
| [40] | Cheng YB, Jensen PR, Fenical W. Cytotoxic and antimicrobial napyradiomycins from two marine-derived Streptomyces strains[J]. Eur J Org Chem, 2013, 18:3751-3757. |
| [41] | Haste NM, Farnaes L, Hensler ME, et al. Bactericidal kinetics of marine-derived napyradiomycins against contemporary methicillin-resistant Staphylococcus aureus[J]. Mar Drugs, 2011, 9:680-689. |
| [42] | Kaysser L, Fenical W, Moore BS, et al. Merochlorins A-D, cyclic meroterpenoid antibiotics biosynthesized in divergent pathways with vanadium-dependent chloroperoxidases[J]. J Am Chem Soc, 2012, 134:11988-11991. |
| [43] | Plaza A, Keffer JL, Bewley CA, et al. Chrysophaentins A-H, antibacterial bisdiarylbutene macrocycles that inhibit the bacterial cell division protein FtsZ[J]. J Am Chem Soc, 2010, 132:9069-9077. |
| [44] | Marçal FJB, Cortez DAG, Filho BPD, et al. Activity of the extracts and neolignans from Piper regnellii against methicillin-resistant Staphylococcus aureus (MRSA)[J]. Molecules, 2010, 15:2060-2069. |
| [45] | Djinni I, Defant A, Mancini I. Antibacterial polyketides from the marine alga-derived endophitic Streptomyces sundarbansensis:a study on hydroxypyrone tautomerism[J]. Mar Drugs, 2013, 11:124-135. |
| [46] | Liu J, Li F, Jung JH, et al. Antibacterial polyketides from the jellyfish-derived fungus Paecilomyces variotii[J]. J Nat Prod, 2011, 74:1826-1829. |
| [47] | Takata K, Omura S, Shiomi K, et al. Aogacillins A and B produced by Simplicillium sp. FKI-5985:new circumventors of arbekacin resistance in MRSA[J]. Org Lett, 2013, 15:4678-4681. |
| [48] | Kumarihamy M, Khan SI, Nanayakkara NPD, et al. Antiprotozoal and antimicrobial compounds from the plant pathogen Septoria pistaciarum[J]. J Nat Prod, 2012, 75:883-889. |
| [49] | Pidot S, Ishida K, Hertweck C, et al. Discovery of clostrubin, an exceptional polyphenolic polyketide antibiotic from a strictly anaerobic bacterium[J]. Angew Chem Int Ed, 2014, 53:7856-7859. |
| [50] | Ratnaweera PB, Silva ED, Andersen RJ, et al. Solanioic acid, an antibacterial degraded steroid produced in culture by the fungus Rhizoctonia solani isolated from tubers of the medicinal plant Cyperus rotundus[J]. Org Lett, 2015, 17:2074-2077. |
| [51] | Joray MB, Gonzalez ML, Carpinella MC, et al. Antibacterial activity of the plant-derived compounds 23-methyl-6-Odesmethylauricepyrone and (Z,Z)-5-(trideca-4,7-dienyl)resorcinol and their synergy with antibiotics against methicillinsusceptible and -resistant Staphylococcus aureus[J]. J Agric Food Chem, 2011, 59:11534-11542. |
| [52] | Schiavone BIP, Rosato A, Verotta L, et al. Biological evaluation of hyperforin and its hydrogenated analogue on bacterial growth and biofilm production[J]. J Nat Prod, 2013, 76:1819-1823. |
| [53] | Jeong SI, Kim SY, Kim KJ, et al. Antibacterial activity of phytochemicals isolated from Atractylodes japonica against methicillin-resistant Staphylococcus aureus[J]. Molecules, 2010, 15:7395-7402. |
| [54] | Zhao R, Li JJ, Ye BP, et al. Isolation and structure elucidation of the antibacterial metabolites from marinederived Penicillium strain XGH2321[J]. J Chin Antibiot (中国抗生素杂志), 2012, 37:261-264. |
| [55] | Chang CM, Chern J, Wu SH, et al. Avenaciolides:potential MurA-targeted inhibitors against peptidoglycan biosynthesis in methicillin-resistant Staphylococcus aureus (MRSA)[J]. J Am Chem Soc, 2015, 137:267-275. |
| [56] | Zhang XJ, Zuo GY, Zhang YL, et al. Research on joint anti-MRSA activity of apigenin in vitro[J]. Chin Hosp Pharm J (中国医院药学杂志), 2012, 32:755-758. |
| [57] | Mun SH, Kang OH, Kwon DY, et al. In vitro anti-MRSA activity of carvone with gentamicin[J]. Exp Ther Med, 2014, 7:891-896. |
| [58] | Hua DX, Peng Q, Qian YS, et al. Study of antibacterial activity of green tea and its extracts against MRSA[J]. J Chin Antibiot (中国抗生素杂志), 2010, 35:228-233. |
| [59] | Qin RX, Zheng J, Zhou H, et al. The combination of catechin and epicatechin gallate from fructus crataegi potentiates β-lactam antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) in vitro and in vivo[J]. Int J Mol Sci, 2013, 14:1802-1821. |
| [60] | Mun SH, Lee YS, Han SH, et al. In vitro potential effect of morin in the combination with β-lactam antibiotics against methicillin-resistant Staphylococcus aureus[J]. Foodborne Pathogens Dis, 2015, 12:545-550. |
| [61] | Yuan GJ, Li PB, Yang H. Anti-MRSA activity of carnosic acid in rosemary[J]. Chin J Mod Appl Pharm (中国现代应用药学), 2012, 29:571-574. |
| [62] | Otsuka N, Liu MH, Tsuchiya T, et al. Anti-methicillin resistant Staphylococcus aureus (MRSA) compounds isolated from Laurus nobilis[J]. Biol Pharm Bull, 2008, 31:1794-1797. |
| [63] | Wu XW, Wu ZP, An LK, et al. Synthesis, antimicrobial activity and possible mechanism of action of 9-bromosubstituted indolizinoquinoline-5,12-dione derivatives[J]. Eur J Med Chem, 2011, 46:4625-4633. |
| [64] | Hossion AML, Zamami Y, Sasaki K, et al. Quercetin diacylglycoside analogues showing dual inhibition of DNA gyrase and topoisomerase Ⅳ as novel antibacterial agents[J]. J Med Chem, 2011, 54:3686-3703. |
| [65] | Patel BA, Hardej D, Talele TT, et al. The synthesis and SAR study of phenylalanine-derived (Z)-5-arylmethylidene rhodanines as anti-methicillin-resistant Staphylococcus aureus (MRSA) compounds[J]. Bioorg Med Chem Lett, 2013, 23:5523-5527. |
| [66] | Hardej D, Ashby JCR, Talele TT, et al. The synthesis of phenylalanine-derived C5 substituted rhodanines and their activity against selected methicillin-resistant Staphylococcus aureus (MRSA) strains[J]. Eur J Med Chem, 2010, 45:5827-5832. |
| [67] | Sawada H, Okazaki M, Miyachi H, et al. Riccardin C derivatives as anti-MRSA agents:structure-activity relationship of a series of hydroxylated bis(bibenzyl)s[J]. Bioorg Med Chem Lett, 2012, 22:7444-7447. |
| [68] | Sawada H, Onoda K, Miyachi H, et al. Structure-anti-MRSA activity relationship of macrocyclic bis(bibenzyl) derivatives[J]. Bioorg Med Chem Lett, 2013, 23:6563-6568. |
| [69] | Onoda K, Sawada H, Miyachi H, et al. Anti-MRSA activity of isoplagiochin-type macrocyclic bis(bibenzyl)s is mediated through cell membrane damage[J]. Bioorg Med Chem, 2015, 23:3309-3316. |
| [70] | Karatas H, Alp M, Goker H, et al. Synthesis and potent in vitro activity of novel 1H-benzimidazoles as anti-MRSA agents[J]. Chem Biol Drug Des, 2012, 80:237-244. |
| [71] | Cao D, Liu S, Beuerman RW, et al. Amino acid modified xanthone derivatives:novel, highly promising membraneactive antimicrobials for multidrug-resistant Gram-positive bacterial infections[J]. J Med Chem, 2015, 58:739-752. |
| [72] | Cao D, Liu S, Beuerman RW, et al. Design and synthesis of amphiphilic xanthone-based, membrane-targeting antimicrobials with improved membrane selectivity[J]. J Med Chem, 2013, 56:2359-2373. |
| [73] | Murphy AC, Fukuda D, Simpson TJ, et al. Engineered thiomarinol antibiotics active against MRSA are generated by mutagenesis and mutasynthesis of Pseudoalteromonas SANK73390[J]. Angew Chem Int Ed, 2011, 50:3271-3274. |
| [74] | Zadrazilova I, Pospisilova S, Jampilek J, et al. Salicylanilide carbamates:promising antibacterial agents with high in vitro activity against methicillin-resistant Staphylococcus aureus (MRSA)[J]. Eur J Pharm Sci, 2015, 77:197-207. |
| [75] | Kumar NS, Dullaghan EM, Young RN, et al. Discovery and optimization of a new class of pyruvate kinase inhibitors as potential therapeutics for the treatment of methicillin-resistant Staphylococcus aureus infections[J]. Bioorg Med Chem, 2014, 22:1708-1725. |
| [76] | Veale CGL, Zoraghi R, Young RM, et al. Synthetic analogues of the marine bisindole deoxytopsentin:potent selective inhibitors of MRSA pyruvate kinase[J]. J Nat Prod, 2015, 78:355-362. |
| [77] | Clive DLJ, Cheng P. The marinopyrroles[J]. Tetrahedron, 2013, 69:5067-5078. |
| [78] | Cheng C, Qin Y, Li R, et al. Marinopyrrole derivatives as potential antibiotic agents against methicillin-resistant Staphylococcus aureus (Ⅱ)[J]. Mar Drugs, 2013, 11:2927-2948. |
| [79] | Antoszczak M, Popiel K, Huczynski A, et al. Synthesis, cytotoxicity and antibacterial activity of new esters of polyether antibiotic-salinomycin[J]. Eur J Med Chem, 2014, 76:435-444. |
| [80] | Jada S, Doma MR, Kumar HMS, et al. Design and synthesis of novel magnolol derivatives as potential antimicrobial and antiproliferative compounds[J]. Eur J Med Chem, 2012, 51:35-41. |
| [81] | Bremner JB, Keller PA, Pyne SG, et al. Binaphthyl-based dicationic peptoids with therapeutic potential[J]. Angew Chem Int Ed, 2010, 49:537-540. |
| [82] | Feng L, Maddox MM, Sun D, et al. Synthesis, structureactivity relationship studies, and antibacterial evaluation of 4-chromanones and chalcones, as well as olympicin A and derivatives[J]. J Med Chem, 2014, 57:8398-8420. |
| [83] | Bouley R, Chang M, Mobashery S, et al. Discovery of antibiotic (E)-3-(3-carboxyphenyl)-2-(4-cyanostyryl)quinazolin-4(3H)-one[J]. J Am Chem Soc, 2015, 137:1738-1741. |
| [84] | Geng ZZ, Lin J, Chen WM, et al. Novel cajaninstilbene acid derivatives as antibacterial agents[J]. Eur J Med Chem, 2015, 100:235-245. |
| [85] | McCulloch MWB, Berrue F, Kerr RG, et al. One-pot syntheses of pseudopteroxazoles from pseudopterosins:a rapid route to non-natural congeners with improved antimicrobial activity[J]. J Nat Prod, 2011, 74:2250-2256. |
| [86] | Needham J, Kelly MT, Ishige M, et al. Andrimid and moiramides A-C, metabolites produced in culture by a marine isolate of the bacterium Pseudomonas fluorescens:structure elucidation and biosynthesis[J]. J Org Chem, 1994, 59:2058-2063. |
 2016, Vol. 51
2016, Vol. 51


