2. 中南大学湘雅医院药学部, 湖南长沙 410008
2. Department of Pharmacy, Xiangya Hospital, Central South University, Changsha 410008, China
党参为常用传统中药,具有补中益气、健脾养胃益肺、养血生津等功效[1]。现行《药典》规定党参药材为桔梗科 (Campanulaceae) 植物党参 (Codonopsis pilosula (Franch.) Nannf.)、素花党参 (C. pilosula Nannf. var. modesta (Nannf.) L. T. Shen) 或川党参 (C. tangshen Oliv.) 的干燥根; 其中,党参 (C. pilosula) 在甘肃、山西和陕西等地长期大量种植,不但是目前党参药材的主要来源品种,也成为定西等部分干旱地区的特色经济作物和当地药农的重要经济来源。现代药 理学研究表明党参具有保护胃黏膜、免疫调节、改善学习记忆能力、抗衰老、神经细胞保护和抗肿瘤等多种药效[2, 3, 4, 5, 6]。在化学成分研究方面,从党参中已分离鉴定了包括甾体、萜类、生物碱类、多烯炔类等类型的30余个化合物。除多糖外[7],早期的研究主要是 以乙醇或甲醇提取为主[8, 9, 10, 11],这与党参及其复方通常以水煎应用的情况不完全一致。因此,作为对常用中药化学成分及其药理活性多样性系统研究的内容之一[12, 13, 14, 15, 16, 17, 18, 19, 20, 21],作者对党参水提物的化学成分进行了研究。
前文已报道了24个新化合物的分离和结构鉴定及其初步药理活性筛选的研究结果,其中包括15个C14-烯炔苷、3个结构类型不同的倍半萜苷、2个C14-烯炔醇、2个C8-烯炔酸、1个w -羟基不饱和C14-脂肪酸和1个具有新颖骨架的木脂素[22, 23, 24, 25, 26]。本文继续报道2个新的 (1和2) (图 1) 和14个首次从党参中得到的已知木脂素类成分的分离和结构鉴定及其初步活性筛选结果。
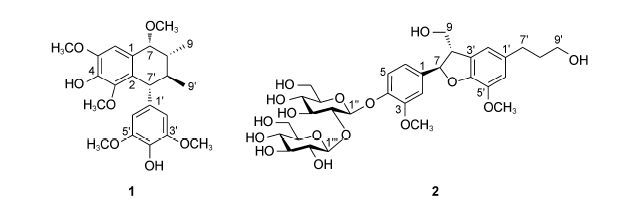
|
Figure 1 Structures of compounds 1 and 2 |
化合物1 淡黄色无定形粉末,易溶于甲醇; [α] -13.5 (c 0.06,MeOH); UV (MeOH) λmax (logε) 203 (4.74),279 (3.37) nm; CD (MeOH) 226 (Δε +0.52),271 (Δε -0.05),294 (Δε +0.06) nm; IR νmax 3 378,2 931,2 856,1 685,1 514,1 453,1 377,1 285,1 208,1 143,1 050,979,844,801,725 cm-1; 1H NMR (CD3OD,600 MHz)、13C NMR (CD3OD,150 MHz) 数据见表 1; (-)- ESI-MS m/z 453 [M+Cl]-; HR-ESI-MS m/z 441.189 0 [M+Na]+ (C23H30O7Na计算值441.188 4)。
|
|
Table 1 NMR spectroscopic data for compound 1. NMR data (δ) were measured in CD3OD at 600 MHz for 1H and 150 MHz for 13C. Proton coupling constants (J) in Hz are given in parentheses. The assignments were based on 1H-1H COSY,HSQC,and HMBC experiments |
化合物1的红外光谱显示存在羟基 (3 378 cm-1) 和苯环 (1 514 cm-1) 的特征吸收峰,(+)-HR-ESI-MS和NMR谱数据提示其分子式为C23H30O7。在1H NMR谱的芳香区显示积分比为1∶2的两个芳香氢信号 [δH 6.63 (s,H-6) 和6.49 (s,H-2',H-6')]; 脂肪族区有4个次甲基的氢信号 [δH 3.95 (d,J = 1.8 Hz,H-7)、3.45 (d,J = 7.8 Hz,H-7')、1.93 (ddq,J = 8.4,7.8,6.6 Hz,H-8')和1.50 (ddq,J = 1.8,8.4,6.6 Hz,H-8)],两个甲基的氢信号 [δH 1.12 (d,J = 6.6 Hz,H3-9) 和1.02 (d,J = 6.6 Hz H3-9')],以及5个甲氧基的氢信号 [3.87 (s,MeO-5),3.76 (s,MeO-3',MeO-5'),3.43 (s,MeO-7),3.11 (s,MeO-3)]。化合物1的13C NMR和DEPT谱数据 (表 1) 除显示与以上基团相对应的碳共振信号外,还给出可归属于两个苯环的9个季碳的共振信号; 其中,一个苯环的两个次甲基碳信号相互重叠,两个连氧的季碳信号相互重叠,表明该化合物的结构中存在一个对称取代的苯环。由以上波谱数据,初步推断化合物1为含有5个甲氧基的木脂素类化合物,并得到其2D NMR图谱解析的确证。
通过化合物1的HSQC图谱解析,对其NMR中氢和与其相连结的碳的共振信号进行了准确归属。化合物1的1H-1H COSY谱显示H-7、H-8、H-8'、H-7'依次偶合相关,H-8和H3-9偶合相关,H-8'和H3-9'偶合相关 (图 2)。由此确证结构中存在7,7,7',7'-四取代的8,8'-二甲基丁烷单元。化合物1的HMBC图谱除显示进一步确证该结构单元的异核远程相关信号外 (图 2),还显示H-6与C-1、C-2、C-4、C-5和C-7相关,H-7与C-1、C-2、C-6、C-8、C-8'和C-9相关,MeO-3与C-3相关,MeO-5与C-5相关,MeO-7与C-7相关; 结合这些氢和碳的化学位移,推断7,7,7',7'-四取代的8,8'-二甲基丁烷单元的7,7-位分别被一个甲氧基和一个2-取代的4-羟基-3,5-二甲氧基苯基取代。同时,HMBC图谱显示H-2' (H-6') 与C-3' (C-5')、C-4'和C-7'相关,H-7'与C-1'、C-2' (C-6')、C-8、C-8'和C-9'相关,MeO-3' (MeO-5') 与C-3' (C-5') 相关,结合这些氢和碳的化学位移,推定7,7,7',7'-四取代的8,8'-二甲基丁烷单元的7'-位被一个对称的4'-羟基-3',5'-二甲氧基苯基取代。另外,HMBC图谱还显示H-7'与C-1、C-2和C-3相关,表明8,8'-二甲基丁烷单元的7'-位与2-取代4-羟基-3,5-二甲氧基苯基的2-位相互连接。因此,化合物1的平面结构确定为4,4'-二羟基-3,3',5,5',7-五甲氧基-2,7'-环木脂烷。化合物1的1H NMR显示H-7与H-8之间、H-7'与H-8'之间以及H-8'与H-8之间的的偶合常数 (J) 分别为1.8、7.8和8.4 Hz。由此推断H-7'、H-8'和H-8均处于结构中四氢萘环的准a键上,相邻的氢之间为反式取向; 而H-7处于准e键上,与H-8之间为顺式取向。化合物1的CD谱中,271 nm处的负Cotton效应和294 nm处的正Cotton效应,与其UV图谱中280 nm附近苯环的吸收峰对应,呈现出比较特征的激子偶合Cotton效应峰。运用激子手征性规则[27],以上特征的激子偶合Cotton效应峰,表明两个苯环发色团的偶极之间为顺时针排列,据此推断化合物1具有7'R构型,并通过与类似物的CD数据[28]比较得到确证。结合前述通过偶合常数分析确定的相对构型,推定化合物1的绝对构型为7R,7'R,8R,8'S。因此,化合物1的结构确定为 (-)-(7R,7'R,8R, 8'S)-4,4'-二羟基-3,3',5,5',7-五甲氧基-2,7'-环木脂烷。文献[29]报道通过 (E)-2,6-甲氧基-4-(1-丙烯) 苯酚在甲醇中的阳极氧化反应,制备得到了具有与化合物1相同平面结构和相对构型的化合物及其7-位差向异构体,但文献中仅报道了其在CDCl3中1H NMR数据,且与化合物1的数据之间存在明显差异。由于文献报道的反应涉及形成4个手性中心,相关产物的具体结构和构型尚需通过其他实验数据进一步进行确证。
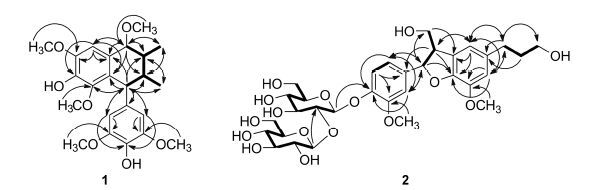
|
Figure 2 Key HMBC (arrows,from H to C) and COSY (thick lines) correlations of compounds 1 and 2 |
化合物2 白色无定形粉末; [α] -29.2 (c 0.04,MeOH); UV (MeOH) λmax (logε) 205 (3.86),231 (3.25),282 (2.71) nm; CD (MeOH) 221 (Δε+1.71),243 (Δε -1.02),279 (Δε -0.59) nm; IR νmax 3 394,3 190,3 011,2 921,2 850,1 647,1 600 (sh),1 515,1 504,1 469,1 419,1 369,1 343,1 300,1 269,1 214,1 163,1 139,1 119,1 082,1 057,1 033,1 019,981,951,897,859,810,764,739,722,652 cm-1; 1H NMR (CD3OD,600 MHz)、13C NMR (CD3OD,150 MHz) 数据见表 2; (+)-ESI- MS m/z 707 [M+Na]+,723 [M+K]+; (-)-ESI-MS m/z 683 [M-H],719 [M+Cl]-; HR-ESI-MS m/z 707.252 5 [M+Na]+ (C32H44O16Na计算值 707.252 2)。
|
|
Table 2 NMR spectroscopic data for compound 2. NMR data (δ) were measured in CD3OD at 600 MHz for 1H and 150 MHz for 13C. Proton coupling constants (J) in Hz are given in parentheses. The assignments were based on 1H-1H COSY,HSQC,and HMBC experiments |
化合物2的红外光谱显示羟基 (3 394 cm-1)、共轭双键 (1 647 cm-1) 和苯环 (1 600、1 515和1 504 cmͨ 2;1) 的特征吸收峰; (+)-HR-ESI-MS结合NMR数据 (表 2) 提示其分子式为C32H44O16。在化合物2的NMR图谱中,除可归属于2个β-吡喃葡萄糖基单元的特征共振信号外,还观察到与同时分离得到的已知化合物3相似的信号。由此初步推断化合物2为3的双β-葡萄糖苷,并得到2D NMR图谱测定和解析的确证。通过化合物2的HSQC图谱解析准确归属了NMR谱中氢及其相连碳的共振信号 (表 2)。在1H-1H COSY谱中,除显示两个葡糖糖基单元邻位氢依次偶合的交叉峰信号外 (图 2),H-8/H-7/H2-9和H2-7'/H2-8'/H2-9'的偶合交叉峰信号,结合它们的化学位移,确证苷元部分含有7,8-二取代丙醇和7'-取代丙醇单元。根据1H-1H COSY谱中H-5/H-6的交叉峰信号,以及HMBC谱中H-2与C-4 、C-6和C-7,H-5与C-1和C-3,H-6与C-2、C-4和C-7,H-7与C-2、C-6、C-8和C-9,MeO-3与C-3的远程异核相关信号,并结合这些氢和碳共振信号的化学位移,推断7,8-二取代丙醇单元的7-位与一个3-甲氧基-4-羟基苯基相连。同时,根据HMBC谱中H-2'和H-6'与C-4'和C-7',H-2'与C-3'和C-6',MeO-5'和H-6'与C-5',以及H2-7'与C-1'、C-2'、C-6'、C-8'和C-9'的相关信号,结合它们的化学位移,推定7'-取代丙醇单元的7'-位连接在一个3',4'-二取代的5'-甲氧基苯环上。另外,由H-2'与C-8,H-7与C-3'和C-4',H-8与C-2'和C-4',以及H2-9与C-3'的HMBC相关信号,确定7,8-二取代3-甲氧基-4-羟基苯丙醇单元的7-和8-位分别与3',4'-二取代5'-甲氧基苯丙醇单元的4'-和3'-位相连,并证明了化合物2的苷元结构为4-羟基-3,5'-二甲氧基-4',7-环氧-8,3'-新木脂烷- 9,9'-三醇 (dihydrodehydrodiconiferyl alcohol,二氢脱氢双松柏基醇)。根据HMBC谱中,H-1''与C-4以及H-1'''与C-2''的相关信号,推定2个β-吡喃葡萄糖基 单元之间通过1'''→2''相互连接,且内侧糖基与苷元的C-4以苷键相连,得到化合物2的平面结构。在 化合物2的1H NMR谱中,H-7与H-8的偶合常数 (J7,8 = 6.0 Hz) 与化合物3的完全相同,表明它们的C-7和C-8具有相同的相对构型。在化合物2的CD谱中,221 nm处呈现正Cotton效应,243和279 nm 处为负Cotton效应,通过与文献中类似化合物的CD数据比较[12, 30],确定其具有7R,8S构型。进一步通过酶水解后,从2的水解产物中分离得到苷元和糖,并经1H NMR谱 (丙酮-d6) 及在甲醇中测定比旋光值和CD谱分析,确证苷元为3,糖为D-葡萄糖。因此,化合物2的结构确定为 (-)-(7R,8S)-二氢脱氢双松柏基醇4-O-β-D- 吡喃葡萄糖基-(1'''→2'')-β-D-吡喃葡萄糖苷。根据IUPAC关于木脂素命名的建议[31],该化合物命名为 (-)-(7R,8S)-4-羟基-3,5'-二甲氧基-4',7-环 氧-8,3'-新木脂烷-9,9'-二醇4-O-β-D-吡喃葡萄糖基- (1'''→2'')-β-D-吡喃葡萄糖苷。
化合物3 白色无定形粉末; [α] -2.0 (c 0.69, CH3OH); UV (MeOH) λmax (logε) 216 (5.13),234 (5.03),284 (4.62) nm; CD (MeOH) 228 (Δε+1.12),246 (Δε -1.20),292 (Δε -0.58) nm; ESI-MS m/z 383 [M+Na]+,399 [M+K]+,359 [M-H]-,395 [M+Cl]-; 1H NMR (DMSO-d6,500 MHz) δ: 6.91 (1H,s,H-2),6.76 (2H,s,H-5,H-6),6.69 (1H,brs,H-2'),6.68 (1H,brs,H-6'),5.41 (1H,d,J = 7.0 Hz,H-7),3.76 (3H,s,MeO-5 '),3.74 (3H,s,MeO-3),3.69 (1H,dd,J = 10.5,5.5 Hz,H-9a),3.61 (1H,dd,J = 10.5,7.0 Hz,H-9b),3.43 (1H,m,H-8),3.42 (2H,t,J = 7.0 Hz,H2-9),2.53 (2H,t,J = 7.0 Hz,H2-7'),1.69 (2H,quin,J = 6.5 Hz,H2-8'); 13C NMR (DMSO-d6,125 MHz) δ: 147.7 (C-4),146.5 (C-3),145.6 (C-4'),143.4 (C-5'),135.1 (C-1),132.6 (C-1'),129.2 (C-3'),118.6 (C-6),116.6 (C-2'),115.4 (C-5),112.5 (C-6') ,110.4 (C-2),87.0 (C-7),63.1 (C-9),60.3 (C-9'),55.8 (MeO-5'),55.7 (MeO-3),53.4 (C-8),34.8 (C-8'),31.7 (C-7')。以上NMR数据与文献[30]报道 (-)-(7R,8S)-dihydrodehydrodiconiferyl alcohol (二氢脱氢双松柏基醇) 在丙酮-d6中的数据一致,差别可归属于溶剂效应所致。
化合物4 淡黄色无定形粉末; [α] +10.5 (c 0 .06,MeOH); UV (MeOH) λmax (logε) 205 (5.05),279 (4.81) nm; CD (MeOH) 221 (Δε +3.78),245 (Δε -0.30),268 (Δε +0.45),294 (Δε +0.13) nm; ESI-MS m/z 381 [M+Na]+,397 [M+K]+,393 [M+Cl]-; 1H NMR (CD3OD,600 MHz) δ: 6.91 (1H,s,H-2'),6.89 (1H,d,J = 1.8 Hz,H-2),6.88 (1H,s,H-6'),6.77 (1H,dd,J = 8.4,1.8 Hz,H-6),6.71 (1H,d,J = 8.4 Hz,H-5),6.48 (1H,d,J = 16.2 Hz,H-7'),6.17 (1H,dt,J = 6.0,16.2 Hz,H-8'),5.46 (1H,d,J = 6.0 Hz,H-7),4.14 (2H,dd,J = 6.0,1.2 Hz,H2-9'),3.82 (3H,s,MeO-5'),3.78 (1H,dd,J = 11.6,5.4 Hz,H-9a),3.76 (3H,s,MeO-3),3.72 (1H,dd,J = 11.6,6.6 Hz,H-9b),3.43 (1H,q,J = 6.0 Hz,H-8); 13C NMR (CD3OD,150 MHz) δ: 149.6 (C-4),149.4 (C-3),147.9 (C-4'),145.8 (C-5'),134.8 (C-1),132.9 (C-1'),132.3 (C-7'),130.6 (C-3'),127.8 (C-8'),120.0 (C-6),116.8 (C-2'),116.5 (C-5),112.4 (C-6'),110.8 (C-2),89.6 (C-7),65.2 (C-9),64.2 (C-9'),57.0 (MeO-3),56.7 (MeO-3'),55.4 (C-8)。以上NMR数据与文献[32]报道(+)-(7S,8R)-dehydrodiconiferyl alcohol (脱氢双松柏基醇) 在丙酮-d6中的数据一致,差别可归属于溶剂效应所致。
化合物5 黄色无定形粉末; [α] +31.2 (c 0.12, MeOH); UV (MeOH) λmax (logε) 210 (5.12),232 (5.03),345 (5.08) nm; CD (MeOH) 206 (Δε -2.01),225 (Δε +3.22),246 (Δε -0.40),261 (Δε +0.30),312 (Δε +0.52),336 (Δε +0.62),357 (Δε +0.32) nm; ESI-MS m/z 379 [M+Na]+,395 [M+K]+,391 [M+Cl]-; 1H NMR (CD3OD,500 MHz) δ: 9.53 (1H,d,J = 7.5 Hz,H-9'),7.57 (1H,d,J = 15.5 Hz,H-7'),7.24 (1H,s,H-2'),7.18 (1H,s,H-6'),6.89 (1H,d,J = 1.5 Hz,H-2),6.77 (1H,dd,J = 8.0,1.5 Hz,H-6),6.72 (1H,d,J = 8.0 Hz,H-5),6.63 (1H,dd,J = 15.5,7.5 Hz,H-8'),5.55 (1H d,J = 6.0 Hz,H-7),3.86 (3H,s,MeO-5'),3.79 (2H,dd,J = 12.0,5.5 Hz,H2-9),3.77 (MeO-3),3.51 (1H,m,H-8); 13C NMR (CD3OD,125 MHz) δ: 195.2 (C-9'),155.2 (C-7'),151.9 (C-4'),148.1 (C-3),146 .8 (C-4),145.0 (C-5'),132.9 (C-1),130.2 (C-3'),128.6 (C-1'),126.1 (C-8'),119.0 (C-6),118.8 (C-2'),115.2 (C-5),113.1 (C-6'),109.4 (C-2),89.1 (C-7),63.5 (C-9),55.7 (MeO-3),55.3 (MeO-3'),53.7 (C-8)。以上NMR数据与文献[32]报道 (+)-balanophonin [(+)-蛇菰脂醛素] 在CDCl3中的数据一致,差别可归属于溶剂效应所致。
化合物6 白色无定形粉末; [α] +15.6 (c 0.15, MeOH); UV (MeOH) λmax (logε) 205 (5.34),230 (5.07),280 (4.38) nm; CD (MeOH) 206 (Δε -0.91),228 (Δε +1.29),249 (Δε -0.06),277 (Δε +0.09) nm; ESI-MS m/z 327 [M-H]-; 1H NMR (DMSO-d6 500 MHz) δ: 7.14 (2H,d,J = 9.0 Hz,H-2',H-6'),6.87 (1H,brs,H-2),6.73 (1H,brd,J = 8.0 Hz,H-6),6.71 (2H,d,J = 9.0 Hz,H-3',5'),6.70 (1H,d,J = 8.0 Hz,H-5),4.60 (1H,d,J = 5.0 Hz,H-7),4.58 (1H,d,J = 5.0 Hz,H-7'),4.09 (H,dd,J = 8.5,6.5 Hz,H-9a),4.07 (1H,dd,J = 8.5,7.0 Hz,H-9'a),3.75 (3H,s,MeO-3),3.70 (1H,dd,J = 8.5,4.5 Hz,H-9b),3.68 (1H,dd,J = 8.5,4.5 Hz,H-9'b),3.00 (2H,m,H-8,H-8'); 13C NMR (DMSO-d6,125 MHz) δ: 156.8 (C-4'),147.6 (C-3),146.0 (C-4),132.2 (C-1),131.6 (C-1'),127.6 (C-2',C-6'),118.7 (C-6),115.2 (C-5),115.1 (C-3',C-5'),110.5 (C-2),85.3 (C-7'),85.1 (C-7),71.0 (C-9),70.8 (C-9'),55.6 (MeO-3),53.7 (C-8'),53.5 (C-8)。以上NMR数据与文献[33]报道 (+)- demethoxypinoresinol [(+)-去甲氧基松脂素] 在DMSO-d6中的数据一致,且发现文献中1H NMR数据归属有误。
化合物7 白色无定形粉末; [α] +26.9 (c 0.08, MeOH);UV (MeOH) λmax (logε) 205 (7.94),230 (4.51),275 (4.13),338 (3.89) nm; CD (MeOH) 242 (Δε +0.05),278 (Δε +0.03),296 (Δε -0.02) nm; ESI-MS m/z 381 [M+Na]+,357 [M-H]-; 1H NMR (CD3OD,500 MHz) δ: 6.89 (2H,brs,H-2,H-2'),6.75 (1H,brd,J = 8.0 Hz,H-6,H-6'),6.71 (1H,d,J = 8.0 Hz,H-5,H-5'),4.65 (2H,d,J = 3.5 Hz,H-7,H-7'),4.17 (2H,dd,J = 9.0,6.5 Hz,H-9a,H-9'a),3.79 (6H,s,MeO-3,MeO-3'),3.78 (2H,dd,J = 9.0,2.5 Hz,H-9b,H-9'b),3.08 (2H,m,H-8,H-8'); 13C NMR (CD3OD,125 MHz) δ: 149.4 (C-3,C-3'),147.6 (C-4,C-4'),134.1 (C-1,C-1'),120.4 (C-6,C-6'),116.4 (C-5,C-5'),111.3 (C-2,C-2'),87.8 (C-7,C-7'),72.9 (C-9,C-9'),56.7 (MeO-3,MeO-3'),55.7 (C-8,C-8')。以上NMR数据与文献[34, 35]报道 (+)- pinoresinol [(+)-松脂素] 在CDCl3中的数据一致,差别可归属于溶剂效应所致。
化合物8 白色无定形粉末; [α] +15.2 (c 0.10, MeOH); UV (MeOH) λmax (logε) 209 (5.12),232 (5.06),281 (4.65) nm; CD (MeOH) 218 (Δε +2.94),240 (Δε -0.21) nm;ESI-MS m/z 393 [M+Cl]-; 1H NMR (CD3OD,500 MHz) δ: 6.91 (1H,brs,H-2),6.89 (1H,d,J = 1.0 Hz,H-2'),6.76 (1H,dd,J = 8.0,1.0 Hz,H-6'),6.74 (1H,brd,J = 8.5 Hz,H-6),6.72 (1H,d,J = 8.0 Hz,H-5),6.70 (1H,d,J = 8.5 Hz,H-5'),4.80 (1H,J = 5.0 Hz,H-7),4.36 (1H,d,J = 6.5 Hz,H-7'),4.04 (1H,d,J = 9.0 Hz,H-9'a),3.80 (6H,s,MeO-3,MeO-3'),3.78 (1H,dd,J = 9.0,7.0 Hz,H-9'b),3.73 (1H,dd,J = 9.0,8.5 Hz,H-9a),3.32 (1H,m,H-8),3.22 (1H,dd,J = 9.0,8.5 Hz,H-9b),2.88 (1H,m,H-8'); 13C NMR (CD3OD,125 MHz) δ: 149.4 (C-3'),149.2 (C-3),147.7 (C-4'),146.9 (C-4),134.2 (C-1'),131.6 (C-1),120.5 (C-6'),119.7 (C-6),116.4 (C-5'),116.3 (C-5),111.2 (C-2'),110.8 (C-2),89.8 (C-7'),83.8 (C-7),72.3 (C-9'),70.9 (C-9),56.7 (MeO-3,MeO-3'),55.9 (C-8'),51.6 (C-8)。以上NMR数据与文献[35]报道 (+)-epipinoresinol [(+)-表松脂酚] 在CDCl3数据一致,差别可归属于溶剂效应所致。该化合物的结构及其NMR数据归属通过2D NMR图谱解析得到确证。
化合物9 白色无定形粉末; [α] -6.5 (c 0.23, MeOH); UV (MeOH) λmax (logε) 216 (7.62),240 (4.83),274 (4.10) nm; CD (MeOH) 214 (Δε -0.79),223 (Δε +0.41),235 (Δε -0.19),280 (Δε -0.07) nm; ESI-MS m/z 441 [M+Na]-,417 [M-H]-; 1H NMR (CD3OD,600 MHz) δ : 6.65 (4H,s,H-2,H-2',H-6,H-6'),4.71 (2H,d,J = 4.2 Hz,H-7,H-7'),4.26 (2H,dd,J = 6.6,9.0 Hz,H-9a),3.87 (2H,dd,J = 3.0,9.0 Hz,H-9b),3.83 (12H,s,MeO-3,MeO-3',MeO-5,MeO-5'),3.13 (2H,m,H-8,H-8'); 13C NMR (CD3OD,150 MHz) δ: 149.4 (C-3,C-3',C-5,C-5'),136.2 (C-4,C-4'),133.1 (C-1,C-1'),104.5 (C-2,C-2',C-6,C-6'),87.6 (C-7,C-7'),72.8 (C-9,C-9'),56.8 (MeO-3,MeO-3',MeO-5,MeO-5'),55.5 (C-8,C-8')。以上1H NMR数据与文献[36, 37]报道(-)-丁香脂素 [(-)-syringaresinol] 在丙酮- d6中的数据一致,差别可归属于溶剂效应所致。
化合物10 白色无定形粉末; [α] -32.8 (c 0.09, MeOH); UV (MeOH) λmax (logε) 233 (4.62),274 (4.21) nm; CD (MeOH) 207 (Δε -0.48),222 (Δε +0.40),237 (Δε -0.29) nm; ESI-MS m/z 411 [M+Na]-,387 [M-H]-; 1H NMR (DMSO-d6,600 MHz) δ: 6.89 (1H,d,J = 1.2 Hz,H-2),6.76 (1H,dd,J = 8.4,1.2 Hz,H-6),6.73 ( 1H,d,J = 8.4 Hz,H-5),6.60 (2H,s,H-2',H-6'),4.62 (2H,brs,H-7,H-7'),4.15 (2H,m,H-9a,H-9'a),3.76 (9H,s,MeO-3,MeO-3',MeO-5'),3.75 (2H,m,H-9b,H-9'b),3.05 (2H,s,H-8,H-8'); 13C NMR (DMSO-d6,150 MHz) δ: 147.9 (C-3',C-5'),147.5 (C-3),145.9 (C-4),134.8 (C-4'),132.2 (C-1),131.4 (C-1'),118.6 (C-6),115.1 (C-5),110.4 (C-2),103.6 (C-2',C-6'),85.3 (C-7'),85.1 (C-7),71.0 (C-9),70.9 (C-9'),56.0 (MeO-3',MeO-5'),55.6 (MeO-3),53.7 (C-8),53.5 (C-8')。以上NMR数 据[37]与文献报道 (-)-皮树脂醇 [medioresinol] 在丙 酮-d6中的数据一致,差别可归属于溶剂效应所致。
化合物11 白色无定形粉末; [α] -7.6 (c 1.36, MeOH); UV (MeOH) λmax (logε) 208 (5.35),232 (5.11),282 (4.70) nm; CD (MeOH) 210 (Δε -2.47),225 (Δε +0.29),236 (Δε -0.98),287 (Δε +0.24) nm; ESI-MS m/z 361 [M+H]+,383 [M+Na]+,399 [M+K]+,395 [M+ Cl]-; 1H NMR (CD3OD,500 MHz) δ: 6.85 (1H,brs,H-2),6.73 (H,brs,H-2'),6.70 (2H,brd ,J = 8.0 Hz,H-5,H-5'),6.65 (1H,d,J = 8.0 Hz,H-6),6.58 (1H,d,J = 8.0 Hz,H-6'),4.69 (1H,d,J = 6.5 Hz,H-7),3.92 (1H,dd,J = 8.0,6.5 Hz,H-9'a),3.77 (1H,m,H-9a),3.78 (3H,s,MeO-3),3.77 (3H,s,MeO-3'),3.66 (1H,dd,J = 8.0,6.5 Hz,H-9'b),3.57 (1H,dd,J = 11.0,6.5 Hz,H-9b),2.87 (1H,dd,J = 4.0,13.0 Hz,H-7'a),2.67 (1H,m,H-8'),2.43 (1H,dd,J = 7.5,13.0 Hz,H-7'b),2.31 (1H,m,H-8); 13C NMR (CD3OD,125 MHz) δ: 149.3 (C-3,C-3'),147.3 (C-4),146.1 (C-4'),136.0 (C-1'),133.8 (C-1),122.5 (C-6'),120.1 (C-6),116.5 (C-5'),116.3 (C-5),113.7 (C-2'),110.9 (C-2),84.3 (C-7),73.8 (C-9'),60.7 (C-9),56.7 (MeO-3,MeO-3'),54.3 (C-8),44.1 (C-8'),33.9 (C-7')。除比旋光值为负外,该化合 物的以上NMR数据与文献[37]报道 (+)-落叶松脂醇 [(+)-lariciresinol] 在丙酮-d6中的数据一致,差别可归属于溶剂效应所致。
化合物12 无色油状物; [α] -13.5 (c 6.3, CH3OH); UV (MeOH) λmax (logε) 226 (4.92),282 (4.61) nm; CD (MeOH) 216 (Δε +1.48),243 (Δε -0.25),288 (Δε -0.29) nm; ESI-MS m/z 385 [M+Na]+,401 [M+K]+,361 [M-H]-,397 [M+Cl]-; 1H NMR (CD3OD,500 MHz) δ: 6.60 (2H,d,J = 8.5 Hz,H-5,H-5'),6.53 (2H,d,J = 1.5 Hz,H-2,H-2'),6.48 (2H,dd,J = 1.5,8.5 Hz,H-6,H-6'),3.67 (6H,s,MeO-3,MeO-3'),3.54 (2H,dd,J = 5.0,11.0 Hz,H-9a,H-9'a),3.50 (2H,dd,J = 5.5,11.0 Hz,H-9b,H-9'b),2.60 (2H,dd,J = 7.0,14.0 Hz,H-7a,H-7'a),2.50 (2H,dd,J = 7.5,14.0 Hz,H-7b,H-7'b),1.85 (2H,m,H-8,H-8'); 13C NMR (CD3OD,125 MHz) δ: 149.1 (C-3,C-3'),145.8 (C-4,C-4'),134.2 (C-1,C-1'),123.0 (C-6,C-6'),116.1 (C-5,C-5'),113.6 (C-2,C-2'),62.4 (C-9,C-9'),56.4 (MeO-3,MeO-3'),44.4 (C-8,C-8'),36.3 (C-7,C-7')。以上NMR数据与文献[38]报道 (-)-开环异落叶松脂醇 [(-)-secoisolariciresinol] 在CD3OD中的数据一致,差别可归属于仪器系统误差所致。
化合物13 黄色油状物; [α] -9.7 (c 3.68, MeOH); UV (MeOH) λmax (logε) 213 (5.08),233 (4.85,sh),285 (4.60) nm; CD (MeOH) 206 (Δε +3.43),223 (Δε -2.02),275 (Δε -0.56),293 (Δε +0.55),234 (Δε +0.18) nm; ESI-MS m/z 383 [M+Na]+,399 [M+K]+,359 [M-H]-,395 [M+Cl]-; 1H NMR (DMSO-d6,500 MHz) δ: 6.69 (1H,d,J = 8.0 Hz,H-5'),6.64 (1H,brs,H-2'),6.60 (1H,s,H-3),6.49 (1H,brd,J = 8.0 Hz,H-6'),6.09 (1H,s,H-6),3.74 (1H,d,J = 10.0 Hz,H-7'),3.70 (3H,s,MeO-3'),3.69 (3H,s,MeO-3),3.57 (1H,dd,J = 3.5,10.5 Hz,H-9a),3.43 (1H,m,H-9b),3.41 (2H,m,H2-9'),2.69 (1H,dd,J = 5.5,15.5 Hz,H-7a),2.69 (1H,dd,J = 9.5,15.5 Hz,H-7b),1.84 (1H,m,H-8'),1.62 (1H,m,H-8); 13C NMR (DMSO-d6,125 MHz) δ: 147.4 (C-5),145.6 (C-3'),144.7 (C-4),144.2 (C-4'),137.2 (C-2),132.8 (C-1'),127.3 (C-1),121.6 (C-6'),116.4 (C-3),115.4 (C-5'),113.3 (C-2'),111.9 (C-6),63.7 (C-9),59.9 (C-9'),55.8 (MeO-3'),55.6 (MeO-3),46.0 (C-7',C-8'),38.2 (C-8),32.4 (C-7)。以上NMR数据与文献[39]报道 (-)-对映-异落叶松脂醇 [(-)-enti- isolariciresinol] 在CD3OD中的数据一致,差别可归属于溶剂效应所致。
化合物14 无色油状物; [α] +5.0 (c 0.10, MeOH); UV (MeOH) λmax (logε) 235 (4.96),282 (4.49) nm; CD (MeOH) 234 (Δε -0.53),272 (Δε +0.11),330 (Δε -0.02) nm; ESI-MS m/z 369 [M+Na]+,385 [M+K]+,381 [M+Cl]-; 1H NMR (CD3OD,500 MHz) δ: 6.94 (1H,d,J = 1.5 Hz,H-2),6.83 (1H,dd,J = 1.5,8.0 Hz,H-6),6.80 (1H,d,J = 7.5 Hz,H-5'),6.78 (1H,d,J = 8.0 Hz,H-5),6.69 (1H,d,J = 1.5 Hz,H-2'),6.64 (1H,dd,J = 1.5,7.5 Hz,H-6'),4.81 (1H,d,J = 8.0 Hz,H-7),3.95 (1H,m,H-8),3.81 (MeO-3),3.61 (1H,dd,J = 2.5,12.5 Hz,H-9a),3.49 (2H,t,J = 6.5 Hz,H2-9'),3.41 (1H,dd,J = 5.0,12.5 Hz,H-9b),2.52 (2H,t,J = 6.5 Hz,H2-7'),1.74 (2H,quin,J = 6.5 Hz,H2-8'); 13C NMR (CD3OD,125 MHz) δ: 149.5 (C-3),148.6 (C-3'),145.3 (C-4),143.3 (C-4'),136.8 (C-1'),130.0 (C-1),122.7 (C-6'),121.9 (C-6),118.0 (C-2',C-5'),116.5 (C-5),112.3 (C-2),80.1 (C-7),78.0 (C-8),62.5 (C-9,C-9'),56.7 (MeO-3),35.9(C-8'),32.7 (C-7')。以上NMR数据与文献[40]报道 (+)- (7S,8S)-3-甲氧基-3',7-环氧-8,4'-氧新木脂素-4,9,9'-三醇 [(+)-(7S,8S)-3-methoxy-3',7-expoxy-8,4'-ox yneolignan- 4,9,9'-triol] 在丙酮-d6中的数据一致,差别可归属于溶剂效应所致。
化合物15 白色无定形粉末; [α] +2.6 (c 0.17, MeOH); UV (MeOH) λmax (log ε) 209 (5.03),225 (4.92,sh),281 (4.06) nm; CD (MeOH) 222 (Δε +1.78),236 (Δε -0.65),250 (Δε +0.35),270 (Δε -0.33),290 (Δε -0.30); ESI-MS m/z 387 [M+Na]+,363 [M-H]-,399 [M+Cl]-; 1H NMR (CD3OD,600 MHz) δ: 6.99 (1H,d,J = 1.8 Hz,H-2),6.82 (1H,dd,J = 1.8,8.4 Hz,H-6),6.74 (1H,d,J = 8.4 Hz,H-5),6.70 (1H,d,J = 8.4 Hz,H-5'),6.65 (1H,d,J = 2.4 Hz,H-2'),6.51 (1H,dd,J = 2.4,8.4 Hz,H-6'),4.85 (1H,与水峰重叠,H-7),4.12 (1H,m,H-8),3.83 (1H,dd,J = 5.4,12.0 Hz,H-9a),3.80 (3H,s,MeO-3),3.72 (1H,dd,J = 12.0,3.6 Hz,H-9b),3.53 (2H,t,J = 6.6 Hz,H2-9'),2.53 (2H,t,J = 6.6 Hz,H2-7'),1.76 (2H,quin,J = 6.6 Hz,H2-8'); 13CNMR (CD3OD,150 MHz) δ: 149.5 (C-3'),148.8 (C-3),147.1 (C-4),145.7 (C-4'),138.7 (C-1'),133.8 (C-1),120.8 (C-6'),120.6 (C-6),120.2 (C-5'),117.2 (C-2'),115.8 (C-5),111.7 (C-2),87.7 (C-8),74.1 (C-7),62.3 (C-9'),62.0 (C-9),56.3 (MeO-3),35.5 (C-8'),32.5 (C-7')。除比旋光值为正外,该化合物的以上NMR数据与文献[41]报道(-)-(7S,8R)-3',4-二羟基-3-甲氧基- 8,4'-氧新木脂素在CD3OD中的数据一致,差别可归属于仪器系统误差所致。
化合物16 白色无定形粉末; [α] -2.4 (c 0.13, MeOH); UV (MeOH) λmax (logε) 208 (4.86),224 (4.83,sh),282 (3.99) nm; CD (MeOH) 209 (Δε -0.93),218 (Δε +0.70),225 (Δε +0.74),238 (Δε -0.60),262 (Δε +0.13),280 (Δε -0.22),300 (Δε +0.21); ESI-MS m/z 387 [M+Na]+,363 [M-H] -,399 [M+Cl]-; 1H NMR (CD3OD,600 MHz) δ: 6.95 (1H,d,J = 1.8 Hz,H-2),6.85 (1H,d,J = 8.4 Hz,H-5'),6.79 (1H,d,J = 8.4 Hz,H-6),6.69 (1H,dd,J = 1.8,8.4 Hz,H-5),6.63 (1H,d,J = 2.4 Hz,H-2'),6.50 (1H,dd,J = 2.4,8.4 Hz,H-6'),4.84 (1H,d,J = 6.6 Hz,H-7),4.01 (1H,m,H-8),3.77 (3H,s,MeO-3),3.67 (1H,dd,J = 4.2,12.0 Hz,H-9a),3.48 (2H,t,J = 6.6 Hz,H2-9'),3.41 (1H,dd,J = 12.0,5.4 Hz,H-9b),2.50 (2H,t,J = 6.6 Hz,H2-7'),1.72 (2H,quin,J = 6.6 Hz,H2-8'); 13C NMR (CD3OD,150 MHz) δ: 149.3 (C-3'),148.9 (C-3),147.3 (C-4),146.0 (C-4'),138.6 (C-1'),134.0 (C-1),120.7 (C-6),120.5 (C-6'),119.6 (C-5'),117.2 (C-2'),115.9 (C-5),111.5 (C-2),87.8 (C-8),74.2 (C-7),62.3 (C-9'),61.7 (C-9),56.3 (MeO-3),35.5 (C-8'),32.5 (C-7')。该化合物的以上NMR数据显示其为化合物15的非对映异构体。两个化合物的CD谱在238 nm处均显示特征的负Cotton效应,表明二者具有相同的8R构型[41]。因此,化合物16的结构确定为 (-)-(7R,8R)-3',4-二羟基-3-甲氧基- 8,4'-氧新木脂素。
2 生物活性本文报道的16个木脂素类化合物均为首次从党参中分离得到,其中包括2个新的天然产物。这些成分的发现是对常用中药党参化学成分多样性的有益补充。在初步药理活性筛选中[18, 19],只有化合物12在1×10-5 mol·L-1浓度下对半胱氨酸亚铁 (Fe2+_Cys) 诱导大鼠肝微粒体脂质过氧化表现出一定抑制活性,抑制率为 (63.4 ± 8.3) % [阳性对照谷胱甘肽在相同浓度下的抑制率为 (32.3 ± 9.1) %],其他化合物均未显示明显活性。在1×10-5 mol·L-1浓度下,以上化合物对胃癌 (BGC-823)、卵巢癌 (A2780)、肺癌 (A549)、结肠癌 (HCT-8) 和肝癌 (Bel7402) 细胞株均无细胞毒性; 在1×10-5 mol·L-1浓度下,对HIV复制和蛋白酪氨酸磷脂酶1B也无抑制活性。由于药理活性评价模型所限,这些化合物作为常用传统中药党参的多样性化学成分,对党参临床功效的贡献和影响,尚需进一步深入探究。
实验部分P-2000 polarimeter旋光仪、J-810型圆二色谱仪 (日本JASCO公司),Inova 500和SYS 600型核磁共振仪 (美国Varian公司),Bruker 600型核磁共振仪 (德国Bruker公司),AccuToFCS JMS-T100CS型质谱仪、Agilent HP 1100型高效液相色谱仪 (美国Agilent公司),Grace C18半制备色谱柱 (250 mm× 10 mm,5 μm,美国Grace公司),YMC-Pack苯基半制备色谱柱 (250 mm× 10 mm,5 μm,日本YMC公司),HPD100型大孔树脂 (河北沧州宝恩化工有限公司),MCI吸附树脂 (CHP 20P,日本三菱公司生产),羟丙基葡聚糖凝胶Sephadex LH-20 (瑞士Amersham Bio-sciences公司),色谱用硅胶和硅胶预制板(青岛海洋化工集团公司)。所用试剂若无特别说明均购自北京化工厂,级别为分析纯或色谱纯。
人工种植党参药材于2012年10月购自甘肃渭源县,由中国医学科学院药物研究所马林副研究员鉴定为桔梗科党参属植物党参的干燥根,标本现存于中国医学科学院药物研究所标本室 (ID-S-2503)。
1 提取与分离干燥党参药材 (50 kg) 切碎后用水煎煮提取3次,每次用水约150 L煎煮时间0.5 h,提取液合并减压浓缩得到棕色提取膏 (26 kg)。提取膏用水100 L溶解后,装载至大孔吸附树脂 (HPD-110,20L) 柱 (20 cm× 200 cm) 上,依次用水 (100 L)、50% EtOH (120 L) 和95% EtOH (80 L) 洗脱,收集洗脱液并减压浓缩得到相应的洗脱部分A~C。
B部分 (270 g) 用20 L水溶解后,装载至MCI树脂 (CHP 20P,5.5 L) 上,先后用水 (20 L)、30% EtOH (30 L)、50% EtOH (30 L) 和95% EtOH (8 L) 洗脱,收集相应的洗脱液并减压浓缩得到B1~B4部分。B3部分(22 g) 经反相中压液相色谱分离,用水-甲醇 (40%~100%) 梯度洗脱,薄层色谱检测合并成分相似的洗脱液,减压回收溶剂得到5个组分B3-1~B3-5。组分B3-1 (10 g) 经硅胶柱色谱分离,用氯仿-甲醇 (0~100%) 梯度洗脱,薄层色谱检测合并成分相似洗脱液,减压回收溶剂后得到10个组分B3-1-1~ B3-1-10。B3-1-4 (0.5 g) 经硅胶柱色谱分离,以氯仿-甲醇 (0~100%) 梯度洗脱,得到B3-1-4-1~B3-1-4-3; 其中,B3-1-4-2 (300 mg) 经反相半制备HPLC (C18柱,MeOH-H2O,45∶55,V/V,3.0 mL·min-1) 分离得到13(41 mg,tR = 12 min)、3 (44 mg,tR = 20 min) 和12 (65 mg,tR = 24 min)。组分B3-1-6 (0.8 g) 经硅胶柱色谱分离,以氯仿-甲醇 (0~100%) 梯度洗脱得到B3-1-6-1~B3-1-6-4,其中B3-1-6-4 (90 mg) 再经反相半制备HPLC (C18柱,CH3CN-H2O,26:74,V/V,1.5 mL·min-1) 分离得到15 (4.0 mg; tR = 25 min) 和16(3.0 mg; tR = 26 min)。组分B3-1-10 (1.0 g) 经凝胶HW-40F柱色谱甲醇洗脱分离得到B3-1-10-1~B3-1-10-6; 其中,B3-1-10-5 (100 mg) 用反相半制备HPLC (Ph柱,MeOH-H2O,23∶77,V/V,3.0 mL·min-1) 分离,得到2 (4.0 mg,tR = 26 min)。组分B3-4 (2.5 g) 经硅胶柱色谱分离,以氯仿-甲醇 (100∶0~0∶100,V/V) 梯度洗脱,得到B3-4-1~B3-4-10。B3-4-1 (100 mg) 经反相半制备HPLC分离 (Ph柱,MeOH-H2O,69∶31,V/V,1.5 mL·min-1) 得到 1 (1.1 mg,tR = 38 min)。
C部分 (31.0 g) 经硅胶柱色谱用石油醚-丙酮梯度洗脱 (0~100%),薄层色谱检测合并成分相似的洗脱液,减压回收溶剂得到16个组分C1~C16。组分C9 (0.8 g) 经Sephadex LH-20柱色谱 (石油醚- CHCl3-MeOH,5∶5∶1,V/V/V) 分离得到C9-1~C9-7; 其中,C9-5 (80 mg) 经制备薄层色谱 (石油醚-丙酮,2∶1,V/V) 和反相半制备HPLC (Ph柱,MeOH-H2O,40∶60,V/V,1.5 mL·min-1) 分离纯化得到7 (2.0 mg,tR = 20 min)。组分C12 (0.8 g) 用Sephadex LH-20柱色谱 (石油醚-CHCl3-MeOH,5∶5∶1,V/V/V) 分离得到C12-1~C12-8; 其中,C12-8 (85 mg) 经制备薄层色谱 (石油醚-丙酮,1∶1,V/V) 和反相半制备HPLC (Ph柱,MeOH-H2O,49∶51,V/V,1.5 mL·min-1) 分离得到8 (2.0 mg,tR = 25 min)。组分C13 (1.0 g) 用Sephadex LH-20柱色谱 (石油醚-CHCl3-MeOH,5∶5∶1,V/V/V) 分离得到C13-1~C13-12; 其中,C13-12 (45 mg) 经反相半制备HPLC分离 (C18柱,MeOH- H2O,V/v,42∶58,1.5 mL·min-1) 得到6(1.7 mg,tR = 37 min)。C14 (1.0 g) 用Sephadex LH-20柱色谱 (石油醚-CHCl3-MeOH,5∶5∶1,V/V/V) 分离得到C14-1~C14-8; 其中,C14-4 (80 mg) 经制备薄层色谱 (石油醚-丙酮,2∶1,V/V) 分离得到C14-4-1~C14-4-3; C14-4-3 (20 mg) 再经反相半制备HPLC (C18柱,MeOH- H2O,54∶46,V/V,1.5 mL·min-1) 分离得到9 (1.2 mg,tR = 31 min) 和10 (1.1 mg,tR = 33 min)。C14-7 (100 mg) 经制备薄层色谱 (石油醚-丙酮,1∶1,V/V) 和反相半制备HPLC (Ph柱,MeCN-H2O,47∶53,V/V,1.5 mL·min-1) 分离得到5 (1.6 mg,tR = 26 min)。C14-8 (120 mg) 用制备薄层色谱 (石油醚-丙酮,1∶1,V/V) 分离得到C14-8-1~C14-8-5; 其中,C14-8-1 (8 mg) 和C14-8-3 (30 mg) 分别经反相半制备HPLC (C18柱,1.5 mL·min-1) 分离,前者以MeOH-H2O (55∶45,V/V) 为流动相得到14 (1.8 mg,tR = 28 min),后者用MeOH- H2O (50∶50,V/V) 为流动相得到11 (7.5 mg,tR = 31 min)。C15 (1.0 g)经Sephadex LH-20柱色谱分离 (石油醚-CHCl3-MeOH,5∶5∶1,V/V/V) 得到C15-1~C15-6,C15-4 (300 mg) 进一步经硅胶柱色谱用氯 仿-甲醇(0~100%) 梯度洗脱分离得到C-15-4-1~C-15-4-8。C-15-4-5 (48 mg) 经反相半制备HPLC (C18柱,MeOH-H2O,45∶55,V/V,1.5 mL·min-1) 分离得到4 (6.7 mg,tR = 52 min)。
2 化合物2的酶水解化合物2 (3.0 mg) 溶于2 mL的蒸馏水中,加入3.0 mg的蜗牛酶,37 ℃水浴温孵24 h。反应液用乙酸乙酯萃取2次,每次2 mL。乙酸乙酯相经HPLC分离纯化得到苷元,水相经硅胶柱色谱 (乙腈-水,8∶1,V/V) 分离得到糖。
3 化合物对Fe2+_半胱氨酸 (Cys) 诱导大鼠肝微粒体丙二醛 (MDA) 生成的影响以0.1 mol·L-1 PBS (pH 7.4) 为缓冲液,每0.5 mol反应体系中含微粒体蛋白0.8 mg和不同浓度的待筛样品 (对照组加入等体积溶剂),37 ℃水浴15 min后,加入FeSO4 50 μmol·L-1和Cys 200 μmol·L-1。37 ℃ 水浴反应15 min,加入20% 三氯乙酸0.5 mL,振荡混匀,3 000 r·min-1离心10 min。取上清液0.5 mL加入0.67% 硫代巴比妥酸1 mL,沸水浴10 min,自来水冷却后用半自动生化仪测定532 nm处的光密度值。按下列公式计算送筛样品对Fe2+-Cys诱导大鼠肝微粒体丙二醛(MDA) 生成的抑制率: 抑制率 (%) = (模型测定值-样品测定值)/模型测定值×100%。化合物12在1×10-5 mol·L-1浓度下,表现出一定的抑制活性,抑制率为63.4%。
| [1] | Jiangsu New Medical College. Dictionary of Traditional Chinese Medicine (中药大辞典)[M]. Shanghai:Shanghai Science and Technology Publishing House, 1986, 1:1837-1839. |
| [2] | Wang ZT, Du Q, Xu GJ, et al. Investigations on the protective action of Condonopsis pilosula (Dangshen) extract on experimentally-induced gastric ulcer in rats[J]. Gen Pharmacol, 1997, 28:469-473. |
| [3] | Shan BE, Yaoshida Y, Sugiura T, et al. Stimulating activity of Chinese medicinal herbs on human lymphocytes in vitro[J]. Int J Immunopharm, 1999, 21:149-159. |
| [4] | Singh B, Song H, Liu XD, et al. Dangshen (Codonopsis pilosula) and Baiguo (Gingko biloba) enhancing learning and memory[J]. Altern Ther Health Med, 2004, 10:52-56. |
| [5] | Yoo CS, Kim SJ. Methanol extract of Codonopsis pilosula inhibits inducible nitric oxide synthase and protein oxidation in lipopolysaccharide-stimulated raw cells[J]. Trop J Pharm Res, 2013, 12:705-710. |
| [6] | Tsai KH, Lee NH, Chen GY, et al. Dung-shen (Codonopsis pilosula) attenuated the cardiac-impaired insulin-like growth factor II receptor pathway on myocardial cells[J]. Food Chem, 2013, 138:1856-1867. |
| [7] | Yang CX, Gou YQ, Chen JY, et al. Structural characterization and antitumor activity of a pectic polysaccharide from Codonopsis pilosula[J]. Carbohydr Polym, 2013, 98:886-895. |
| [8] | Qi HY, Wang R, Liu Y, et al. Studies on the chemical constituents of Codonopsis pilosula[J]. J Chin Med Mater (中药材), 2011, 34:546-548. |
| [9] | He Q, Zhu EY, Wang ZT, et al. Studies on chemical constituents of Codonopsis pilosula[J]. Chin Pharm J (中国药学杂志), 2006, 41:10-12. |
| [10] | Wakana D, Kawahara N, Goda Y. Three new triterpenyl esters, codonopilates A-C, isolated from Codonopsis pilosula[J]. J Nat Med, 2011, 65:18-23. |
| [11] | Wakana D, Kawahara N, Goda Y. Two new pyrrolidine alkaloids, codonopsinol C and codonopiloside A, isolated from Codonopsis pilosula[J]. Chem Pharm Bull, 2013, 61:1315-1317. |
| [12] | Wang F, Jiang YP, Wang XL, et al. Chemical constituents from flower buds of Lonicera japonica[J]. China J Chin Mater Med (中国中药杂志), 2013, 38:1378-1385. |
| [13] | Song WX, Yang YC, Shi JG. Two new β-hydroxy amino acidcoupled secoiridoids from the flower buds of Lonicera japonica:isolation, structure elucidation, semisynthesis, and biological activities[J]. Chin Chem Lett, 2014, 25:1215-1219. |
| [14] | Jiang ZB, Song WX, Shi JG. Two 1-(6'-O-acyl-β-D-glucopyranosyl) pyridinium-3-carboxylates from the flower buds of Lonicera japonica[J]. Chin Chem Lett, 2015, 26:69-72. |
| [15] | Song WX, Guo QL, Yang YC, et al. Two homosecoiridoids from the flower buds of Lonicera japonica[J]. Chin Chem Lett, 2015, 26:517-521. |
| [16] | Yu Y, Jiang Z, Song W, et al. Glucosylated caffeoylquinic dcid derivatives from the flower buds of Lonicera japonica[J]. Acta Pharm Sin B, 2015, 5:210-214. |
| [17] | Jiang ZB, Meng XH, Jiang BY, et al. Two 2-(quinonylcarboxamino) benzoates from the lateral roots of Aconitum carmichaelii[J]. Chin Chem Lett, 2015, 26:653-656. |
| [18] | Guo QL, Wang YN, Zhu CG, et al. 4-Hydroxybenzylsubstituted glutathione derivatives from Gastrodia elata[J]. J Asian Nat Prod Res, 2015, 17:439-454. |
| [19] | Guo QL, Wang YN, Lin S, et al. 4-Hydroxybenzylsubstituted amino acid derivatives from Gastrodia elata[J]. Acta Pharm Sin B, 2015, 5:350-357. |
| [20] | Liu YF, Chen MH, Wang XL, et al. Antiviral enantiomers of a bisindole alkaloid with a new carbon skeleton from the roots of Isatis indigotica[J]. Chin Chem Lett, 2015, 26:931-936. |
| [21] | Liu YF, Chen MH, Guo QL, et al. Antiviral glycosidic bisindole alkaloids from the roots of Isatis indigotica[J]. J Asian Nat Prod Res, 2015, 17:689-704. |
| [22] | Jiang YP, Liu YF, Guo QL, et al. Acetylenes and fatty acids from Codonopsis pilosula[J]. Acta Pharm Sin B, 2015, 5:215-222. |
| [23] | Jiang YP, Liu YF, Guo QL, et al. C14-Polyacetylene glucosides from Codonopsis pilosula[J]. J Asian Nat Prod Res, 2015, 17:601-604. |
| [24] | Jiang YP, Liu YF, Guo QL, et al. Sesquiterpene glycosides from the roots of Codonopsis pilosula[J]. Acta Pharm Sin B, 2016, 6:46-54. |
| [25] | Jiang YP, Guo QL, Liu YF, et al. Codonopiloneolignanin A, a polycyclic neolignan with a new carbon skeleton from the roots of Codonopsis pilosula[J]. Chin Chem Lett, 2016, 27:55-58. |
| [26] | Jiang YP, Liu YF, Guo QL, et al. C14-Polyacetylenol glycosides from the dried roots of Codonopsis pilosula[J]. J Asian Nat Prod Res, 2015, 17:1166-1179. |
| [27] | Ying BP, Qin GW, Xu RS. Application of CD exciton chirality method in organic chemistry[J]. Chin J Org Chem (有机化学), 1987, 7:165-173. |
| [28] | Wu HH, Li ZF, Zhang QH, et al. Application of CD in study of the absolute configuration of lignans[J]. J Shenyang Pharm Univ (沈阳药科大学学报) 2010, 27:587-594. |
| [29] | Nishiyama A, Eto H, Terada Y, et al. Anodic oxidation of some propenylphenols:synthesis of physiologically active neolignans[J]. Chem Pharm Chem, 1983, 31:2834-2844. |
| [30] | Matsutomo T, Stark TD, Hofmann T. In vitro activity-guided identification of antioxidants in aged garlic extract[J]. J Agric Food Chem, 2013, 61:3059-3067. |
| [31] | Moss GP. Nomenclature of lignans and neolignans (IUPAC recommendations 2000)[J]. Pure Appl Chem, 2000, 72:1493-1523. |
| [32] | Yuen MSM, Xue F, Mak TCW, at al. On the absolute structure of optical active neolignans containing a dihydrobenzo[b]furan skeleton[J]. Tetrahedron, 1998, 54:12429-12444. |
| [33] | Mohamed KM. Chemical constituents of Gladiolus segetum Ker-Gawl[J]. Bull Pharm Sci, Assiut Univ, 2005, 28:71-78. |
| [34] | Miyazawa M, Kasahara H, Kameoka H. Phenolic lignans from flower buds of Magnolia fargesii[J]. Phytochemistry, 1992, 31:3666-3668. |
| [35] | Okuyama E, Suzumura K, Yamazaka M. Pharmacologically active components of Todopon Puok (Fagraea racemosa), a medicinal plant from Borneo[J]. Chem Pharm Bull, 1995, 43:2200-2204. |
| [36] | Ouyang MA, Wein YS, Zhang ZK, et al. Inhibitory activity against Tobacco Mosaic Virus (TMV) replication of pinoresinol and syringaresinol lignans and their glycosides from the root of Rhus javanica var. roxburghiana[J]. J Agric Food Chem, 2007, 55:6460-6465. |
| [37] | Wang LQ, Zhao YX, Zhou L, et al. Lignans from Gnetum montanum Markgr. f. megalocarpua[J]. Chem Nat Comp, 2009, 45:424-426. |
| [38] | Xie LH, Akao T, Hamasaki K, et al. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol[J]. Chem Pharm Bull, 2003, 51:508-515. |
| [39] | Urones JG, Teresa JDP, Marcos IS, et al. Ent-isolariciresinol in Reseda suffruticosa[J]. Phytochemistry, 1987, 26:1540-1541. |
| [40] | Fang JM, Lee CK, Chang YS. Lignans from leaves of Juniperus chinensis[J]. Phytochemistry, 1992, 31:3659-3661. |
| [41] | Gan M, Zhang Y, Lin S, et al. Glycosides from the root of Iodes cirrhosa[J]. J Nat Prod, 2008, 71:647-654. |
 2016, Vol. 51
2016, Vol. 51


