胰腺炎是胰腺因胰蛋白酶的自身消化作用而引起的疾病,可分为急性胰腺炎和慢性胰腺炎。慢性胰腺炎是指由于各种不同原因所致的胰腺局部、阶段性或弥漫性的慢性进展性炎症,最终导致胰腺组织和 (或) 胰腺功能的不可逆损伤,甚至癌变。
胰腺癌是高度恶化的恶性肿瘤,其5年存活率 低于6%[1]。据Cancer Statistic报道,美国癌症死亡 患者中有7% 死于胰腺癌,胰腺癌死亡率位居肿瘤死亡率第四位; 至2015年底,美国预计新增胰腺癌患者48960人,其中40 560例将死于胰腺癌[2]。我国估计2015年新发胰腺癌103 428例,发病率呈上升趋 势,其中农村地区上升明显,城市地区上升速度略缓,到2015年总体上升趋势有所减缓,但短期内胰腺癌仍然是主要癌症之一[3]。胰腺位置较深,与胃、肝脏、胆囊和脾脏等相邻,其早期临床症状和上述脏器所致疾病较易混淆。特别是胰腺体尾部的肿瘤症状较为隐匿,早期缺乏特异性的临床表现,肿块不易被发现。因此,尽早洞察胰腺癌的临床表现,是诊断和治疗胰腺癌的关键。
慢性胰腺炎是导致胰腺癌的独立危险因素,吸烟、肥胖和胰腺癌家族史等是慢性胰腺炎癌变的危险因素。慢性胰腺炎恶性转化的危险性难以估计,目前的病例对照研究结果不一,其相对危险性从10.35[4]到28.264[5]不等。Lowenfels等[6]在1993年开展了一项涉及2 015例慢性胰腺炎患者的国际性大样本队列研究结果表明,慢性胰腺炎患者患胰腺癌的标化发病率 (SIR) 为14.8,慢性胰腺炎确诊后的10和20年,患者发生胰腺癌的累积风险分别为1.8% 和4.0%。Karlson等[7]对29 530例急、慢性胰腺炎和未明确类型的胰腺炎患者研究发现,慢性胰腺炎患者发展成胰腺癌的SIR为7.6。Malka等[8]对373例慢性胰腺炎患者平均随访9.2年,SIR为26.7。另对1 663例胰腺癌患者和2852例对照人群分析发现,具有胰腺炎病史的患者发展成胰腺癌的风险增加7.2倍,对于年龄不足55岁的患者,风险增加接近10倍[9]。国内研究报道慢性胰腺炎发生癌变的概率为8.1%,腹痛症状发生后的4年是胰腺癌发生的高峰期,腹痛第一次发生后的1、5和10年胰腺癌的累积发生率分别是1.5%、5.6% 和11.6%[10]。
1 慢性胰腺炎发病原因研究发现,不同原因所致的慢性胰腺炎虽然起始途径不同,但病理学改变相似,都是在持续发展的长期炎症环境作用下最终导致胰腺腺泡细胞和胰岛细胞的不可逆性损伤,造成胰腺组织纤维化,进而影响胰腺的正常分泌功能[11]。因此发病因素可归纳为以下3方面: ① 胰腺星状细胞 (pancreatic stellate cells,PSCs) 活化。PSCs有静止和活化两种表型[12]。在正常胰腺组织中呈静止状态,一旦胰腺受损,PSCs在各种因子的刺激作用下就会呈现活化状态。大量研究[13, 14]表明,PSCs的活化程度与胰腺组织的纤维化程度呈正相关,是慢性胰腺炎中各种细胞因子作用的靶点,也是胰腺炎病情恶化的核心。② 细胞因子。与急性胰腺炎的发病机制相似,炎症细胞释放的各种细胞因子在慢性胰腺炎炎症进程中发挥了重要作用。受 损胰腺的各种炎症细胞被激活,分泌 TGF-β (transforming growth factor-β)、TNF-α和IL-6等细胞因子刺激PSCs活化,活化的细胞又会自分泌这些细胞因子,进一步促进胰腺纤维化进程[15]。以TGF-β为例,它可与相应受体结合促进相关物质的磷酸化,通过Smad (Sma and Mad homologue) 途径或促分裂素原活化蛋白激酶 (mitogen-activated protein kinases,MAPK) 途径刺激细胞间质增生、调节细胞生长分化、促进细胞外基质 (ECM) 合成并抑制其降解[16]。③ 炎症细胞。细胞因子在胰腺炎病情恶化过程中的作用越来越受到重视,与细胞因子的产生直接相关的炎性细胞方面的研究也逐渐增多。学术界普遍认为,胰腺炎的持续发作伴随着大量的炎症细胞浸润,继而促进细胞因子的分泌和胰腺星状细胞的活化。
2 胰腺癌的发病原因胰腺癌可起源于胰腺腺泡、导管和胰岛,起源于胰腺导管上皮的胰腺肿瘤最为普遍,胰腺导管腺瘤又多发于胰头部位。位于胰头部位的肿瘤大多坚硬且与正常胰腺腺体组织无明显界限,可以广泛浸润于胰腺周围组织中,病理切片可见纤维化组织明显增多,与慢性胰腺炎的病理特征相似。胰腺癌是多因素引发的疾病,如吸烟、慢性炎症和某些炎症因子大量分泌等均可引起发病[17]。此外,胰腺癌发病相关基因如DPC4 (Smad4)、Kras、TP53基因等在胰腺癌的发展过程中也起着重要作用[18]。
2.1 Kras超过90% 的胰腺癌患者体内Kras基因都发生了突变,说明在胰腺癌的病变过程中,Kras起了重要的作用[19]。Kras基因涉及的通路大致如下: 在正常状态下,Ras与GDP结合,细胞外信号作用于生长因子受体使二者解离,Ras与Kras编码的GTP结合。Ras-GTP活性结合物会随着GTP的水解而逐渐减少。当Kras基因被激活发生突变后,Ras蛋白自身的GTP酶活性丧失,自身信号持续放大,进而激活数个信号通路,包括磷酸肌醇3激酶PI3K-AKT信号通路、Raf/丝裂原活化蛋白激酶MAP通路等[20]。
2.2 Smad4胰腺癌病变后期可能会出现Smad4的缺失,Smad4蛋白在通过TGF-β信号传导通路传导细胞外信号的过程中起着关键作用。TGF-β是正常细胞中重要的抑癌基因,可调节细胞的增殖和分化。它涉及的信号通路如下: 1/3型丝氨酸/苏氨酸表面受体与TGF-β配体结合后使受体二聚化,磷酸化的Smad2/ Smad3蛋白与Smad4蛋白结合转移到核内,与转录辅助因子联合调节细胞周期、分化和增长[21]。胰腺癌病变患者体内Smad4缺失,中断了上述信号通路,使其丧失了对细胞的调节作用。
2.3 TP53有文献[22]显示,高达85% 的胰腺癌患者TP53失活,它也在调控细胞周期、凋亡等方面发挥重要作用。TP53涉及的调节通路如下: DNA损伤激活TP53基因促进p21转录,后者与细胞周期蛋白- CDK复合物结合,使细胞周期停滞在G1期。
有报道显示,胚系突变与家族性胰腺癌也有关联,包括DNA错误匹配的修复基因MLH1[23]、阳性胰蛋白酶原基因PRSS1[24]以及某些肿瘤抑制因子的靶基因INK4A和LKB1[25]。越来越多的证据表明胰腺上皮、基质和细胞外基质蛋白的相互作用在胰腺癌的进程中发挥着重要作用。可见胰腺癌变是一个复杂的过程,其中分子通路机制有待阐明。
3 胰腺炎癌变的相关信号通路 3.1 TGF-β信号通路人的转化生长因子TGF-β 家族包括TGF-β亚型、激动素、骨形成蛋白 (bone morphogenetic proteins,BMPs)、生长和分化因子 (growth and differentiation factors,DGFs) 共33个成员[26]。TGF-β的作用有两相阶段特异性,在肿瘤发生阶段能抑制肿瘤生长,而在肿瘤后期能促进肿瘤侵袭和转移。TGF-β的作用机制是通过与细胞膜表面 受体结合形成异源三聚体,激活R-Smad蛋白将信 号传导入胞内,R-Smad与Co-Smad结合后转移至细胞核,与靶基因结合调节蛋白合成。TGF-β家族能调节细胞的生长、存活、分化和侵袭,对胚胎的发育和维持组织稳态有重要作用[27, 28]。TGF-β家族成员参 与了包括纤维化、自身免疫病和癌症等许多疾病的 发生发展[29]。上皮间质转化 (epithelial-mesenchymal transition,EMT) 是指上皮细胞在形态学上发生向间质细胞表型的转化并获得迁移的能力。肿瘤微环境 包括肿瘤相关巨噬细胞、树突状细胞和调节性T细胞等多种免疫细胞产生的TGF-β1均能有效诱导细 胞的EMT[30]。有研究表明过表达TGF-β1能诱导小鼠产生慢性胰腺炎[31],TGF-β1诱导的EMT是连接炎症和癌症的桥梁[32]。炎症微环境中过表达的TGF-β1通过下调miR-217促进慢性胰腺炎向胰腺癌转化的EMT过程,miR-217通过抑制SIRT1的表达促进这 一过程[33]。EMT发展过程中,Wnt信号通路为上皮 细胞提供EMT能力,TGF-β/BMP家族提供EMT适 合的微环境,两者协同诱导EMT[32]。在癌症中很多干细胞通路,例如Wnt、Ras、SHH (sonic hedgehog) 和Notch (Notch homolog) 信号通路都高度活化,并且为肿瘤细胞提供EMT能力。免疫浸润细胞分泌TGF-β1、IL8、IL6、MMPs和TNF-α等EMT促进因子诱导EMT的肿瘤微环境[33]。
Smad4作为TGF-β信号通路的主要效应器,在超过50% 的胰腺癌患者中失去活性[34]。Smad4单独失活不能导致胰腺的癌变,对正常胰腺的发展也可有可无[35, 36]。TGF-β信号通路的失活导致TGF-α的表达上调,Smad4失活协调TGF-α表达上调促进胰腺纤维化,促进胰腺上皮内瘤变,导致慢性胰腺炎向胰腺癌的转化[37]。慢性胰腺炎的标志是胰腺组织的纤维化,而持续活化的PSCs是促进胰腺纤维化的重要原因。
3.2 JAK/STAT通路Janus激酶/信号转导与转录激活子 (the Janus kinase/signal transducer and activator of transcriptions,JAK/STAT) 信号通路介导多种细胞因子信号转导途径,广泛参与调节细胞的增殖、分化和凋亡以及免疫应答等生物过程。JAK/STAT通路的激活参与多种疾病的发生发展,包括实体瘤、淋巴瘤、白血病和慢性炎症等多种疾病[38]。JAK/STAT信号通路基本传递过程是: 细胞因子与其受体结合后引起受体分子的二聚化,使得与受体偶联的JAKs相互接近并通过交互的酪氨酸磷酸化而活化,活化的JAKs催化受体本身的酪氨酸磷酸化并形成相应的STATs停靠位点,使STATs通过SH2结构域与受体结合并在JAKs的作用下实现其磷酸化活化,然后STATs形成同/异二聚体并进入细胞核内,与靶基因的启动子相结合以激活相应基因的转录和表达。
STAT3作为炎症信号通路的重要分子在肿瘤发生发展与侵袭方面也发挥了重要作用。JAK/STAT通路激活后能抑制细胞凋亡并促进肿瘤细胞增殖和侵袭的生物效应。活化的STAT3可以不同程度地破坏细胞外基质并且造成组织基底膜的降解和破坏,为肿瘤细胞发生早期转移提供适宜的环境。另外,STAT3也能促进EMT过程,促进慢性炎症向癌症的转化。
本课题组研究发现胰岛再生蛋白REG家族 (又称胰腺炎相关蛋白) 成员REG3A高表达协同SOCS3的甲基化能上调JAK/STAT3通路,促进人胰腺癌细胞恶性增殖[39]。IL-6也能激活JAK/STAT通路促进慢性炎症的恶变,导致多种恶性肿瘤的发生[40, 41]。进一步研究发现,REG3A的作用机制与IL-6相似,在IL-6诱导的炎性环境中,REG3A通过上调JAK2/STAT3通路的表达促进胰腺癌细胞系的增殖,而高表达的STAT3又能正反馈上调REG3A的表达,从而形成一个正反馈回路不断促进胰腺癌细胞的恶性增殖[42]。
3.3 MAPK通路多项研究表明胰腺星状细胞的活化促进胰腺肿瘤的生成和发展。长期的慢性炎症使 胰腺组织损伤,产生多种细胞因子激活PSCs,导致胰腺组织纤维化,促进胰腺细胞恶性增殖产生肿瘤,肿瘤生成后又促进胰腺肿瘤细胞增殖、迁移和侵袭,形成肿瘤的血管生成和化学耐药[43]。在胰腺损伤和炎症过程中,静息状态的PSCs转变成肌成纤维样细胞,并以表达α-平滑肌肌蛋白 (α-smooth muscle actin,α-SMA) 为一标志性活动。这一步称为“活化”。活化的PSCs失去脂滴,增生活跃,迁移增多,合成I、III型胶原及增强纤维连接蛋白能力,并能表达细胞因子、趋化因子和细胞黏附分子。
MAPK信号通路链由MAPK、MAPK激酶 (MAP kinase kinase,MAPKK) 和MAPK激酶激酶 (MAP kinase kinase kinase,MAPKKK) 参与构成,是真核生物信号传递网络中的重要途径之一,在基因表达调控和细胞质功能活动中发挥关键作用,参与细胞的增殖、分化、迁移及凋亡等过程。MAPK信号转导通路采用高度保守的三级激酶级联传递信号: 细胞外信号激活MAPKKK,进而活化MAPKK; 然后通过MAPK分子中特定的酪氨酸和丝氨酸残基的磷酸化激活MAPK。MAPK家族主要有4个亚族,主 要包括: 细胞外信号调节激酶1/2 (ERK1/2)、c-Jun 氨基末端激酶 (JNK)、p38丝裂原活化蛋白激酶(p38MAPK) 和细胞外信号调节激酶5 (ERK5)。
早期研究[44, 45]报道ERK1/2是PSCs活化和增殖的主要调控通路,血小板衍生生长因子 (PDGF) 能刺激PSCs的活化,其刺激作用依赖于ERK1/2通路的激活。ERK通路抑制剂PD98059作用于PSCs后阻断了PSCs细胞的增殖,对PSCs的迁移抑制作用可达50%[45]。另外硫化氢 (H2S) 的供体NaHS对PSCs的抑制作用也是通过ERK1/2通路实现的[46]。血管紧张素II能诱导PSCs细胞的增殖,在促进PSCs增殖的同时能激活JNK和ERK1/2,提示血管紧张素II可能通过JNK和ERK实现对PSCs的促增殖作用[47]。JNK抑制剂SP600125在抑制PDGF诱导PSCs活化的同时能抑制JNK和AP-1的活化,说明JNK通路的活化有促进PSCs的活化作用[48]。慢性胰腺炎小鼠模型中,BMP通过抑制TGF-β/Smad2和p38MAPK通路抑制PSCs的活化,起到逆转胰腺组织纤维化的作用[16]。有研究报道高糖摄取能显著激活大鼠PSCs的增殖,而p38MAPK的阻断剂SB203580则能降低α-SMA的表达,说明高糖环境诱导的PSCs增殖是通过p38MAPK通路介导的。
3.4 NF-κB通路核转录因子kappa B (NF-κB) 是存在于各种真核细胞的一类重要的转录激活因子,广泛参与包括免疫反应、细胞增殖和细胞凋亡等一 系列生物过程,与多种肿瘤的发生发展密切相关。NF-κB家族包括原癌基因C-Rel、NF-κB1 (p50/p105)、NF-κB2 (p52/p100)、Re1A (p65) 和RelB五个成员。正常情况下细胞中大部分的NF-κB二聚体通过与细胞质中3个NF-κB抑制蛋白 (IκBα、IκBβ、IκBε) 中的1个结合,以无活性的状态存在于细胞中。
近期越来越多的研究显示慢性炎症、NF-κB的激活和癌症的发展有密切关系[49]。NF-κB在慢性胰腺炎和胰腺癌中都显示异常激活,提示其在慢性胰腺炎向胰腺癌发展过程中的重要作用[50]。促炎因子如IL-1α的自分泌能诱导NF-κB的激活,而持续激活的NF-κB促进胰腺癌的发展[51]。激活的NF-κB及下游靶基因如促炎因子TNF-α和IL-1是炎症向肿瘤生成转化的关键因子。研究报道阿司匹林预防胰腺癌作用的机制是通过阻断炎症反应中NF-κB的激活以抑制胰腺癌的发生[52]。IKK2是κB激酶2的抑制剂,NF-κB通路的一员,IKK2协调Notch通路上调Notch靶基因HES1和HEY1,抑制抗炎因子的表达,促进胰腺癌的发生[53]。另外,NF-κB还能通过抑制E-钙黏蛋白的表达促进EMT[54],NF-κB对于TGF-β1诱导的EMT也是必需的[55]。
3.5 TLRs通路Toll样受体 (Toll-like receptors,TLRs) 是高度保守的一个受体家族,至少包括12个成员。TLRs能特异地识别病原相关的分子模式 (PAMPs),不仅能激活天然免疫,而且还在调节获得性免疫中发挥重要作用,是连接天然免疫和获得性免疫的桥梁。包括单核细胞/巨噬细胞、中性粒细胞和树突状细胞等许多免疫效应细胞均表达TLRs,TLRs识别并结合PAMPs后激活下游信号分子,诱导化学趋化因子、细胞因子和共刺激分子等一系列生物因子的表达。目前研究表明TLRs至少介导两种信号级联放大通路,一种是髓样分化因子 (myeloid differentiationfactor 88,MyD88) 依赖途径,另一种是MyD88非依赖途径。
TLRs一方面在免疫中发挥重要作用,过度的激活TLRs在促进肿瘤形成、迁移方面也至关重要。有研究表明TLR4和TLR7在人和小鼠的胰腺癌细胞中都有较高的表达[56, 57]。在急性和慢性胰腺炎模型中 持续激活的TLR不仅加重了胰腺的炎症,同时也加速了胰腺的肿瘤形成[57]。脂多糖能通过TLR4/MyD88通路诱导NF-κB的激活,从而产生促进细胞增殖、迁移和侵袭等一系列生物学效应[58]。TLR7在正常人和小鼠胰腺组织中不表达,但是在Kras突变的胰腺癌小鼠模型和人胰腺癌样本中,不论是在上皮导管瘤细胞还是在巨噬细胞、树突状细胞、T/B细胞中都有极高的表达量。在伴随有明显炎症的胰腺癌中,TLR7的激活加速了胰腺肿瘤的形成和恶化[59]。
3.6 microRNA和lncRNA参与的慢性胰腺炎恶变的通路microRNA (miRNA) 和长链非编码RNA (lncRNA) 是真核生物体内具有转录后调控功能的非编码RNA,在多种生物途径中均发挥了重要作用,正成为研究热点。已有文献[60, 61, 62]报道,在胰腺癌中miR-1290、miR-200a、miR-200b和miR-196a-2呈现高表达,并预示不良预后,未来可作为胰腺癌诊断指标。但关于microRNA的具体作用机制阐述较少。慢性胰腺炎中持续分泌的多种细胞因子激活PSCs,上调miR-210的表达并通过PI3K/AKT通路促进胰腺癌细胞迁移能力[63]。激活的PSCs还可介导CCN2上调miR-21,继而抑制凋亡通路促进胰腺癌的发生发展,同时miR-21通过正反馈调节机制上调CCN2并活化周边PSCs[64, 65, 66]。miR-21有望成为慢性胰腺炎恶性 转化的关键转录后调控者。正常组织中表达的miR-21对组织损伤有保护作用[67]。慢性胰腺炎的组织长期受损,持续激活miR-21,进一步活化TGF-β通路,同时TGF-β通路又增加miR-21的合成形成正反馈回路[68]。此外,miR-21还能促进EMT,通过介导ERK/MAPK通路抑制细胞凋亡,促进组织纤维化[69, 70]。
通过基因测序技术,研究人员发现多种lncRNA在胰腺癌中异常高表达,主要包括HOTTIP、PVT1和MALAT1,具体机制尚不清楚[71, 72, 73, 74]。关于慢性胰腺炎恶性转化过程中lncRNA的报道较少,但有研究[75]表明在膀胱癌中,肿瘤相关纤维原细胞分泌的TGF-β1介导lncRNA-ZBE2NT诱导EMT; 肝癌中lncRNA- ATB能上调TGF-β通路诱导EMT[76]。
4 总结慢性胰腺炎被认为是胰腺癌的高危因素,转化机制尚未完全阐明。长期慢性炎症过程中异常激活的TGF-β、JAK/STAT、MAPK、NF-κB和TLRs等通路能通过多种途径诱导胰腺细胞恶性转化 (图 1),最终导致胰腺肿瘤的生成并加速胰腺肿瘤的转移和侵袭。其中,慢性胰腺炎中被激活的PSCs在促进慢性胰腺炎向胰腺癌转化过程中的作用至关重要。MAPK通路被活化后转移至核内磷酸化下游的转录因子如NF-κB,启动特定基因的转录激活PSCs; NF-κB、TGF-β和JAK/STAT等通路被激活后形成转录复合物进入细胞核内,表达多种炎症信号分子激活PSCs,促进慢性胰腺炎向胰腺癌的转化。同时,慢性胰腺炎发生过程中分泌的多种细胞因子协同以上通路,为胰腺细胞提供诱导EMT的肿瘤微环境,促进慢性胰腺炎的恶性转化。此外,非编码RNA在慢性胰腺炎恶性转化过程中的调控作用也值得关注,例如长期炎症环境中过表达的miR-21通过与TGF-β通路形成正反馈回路诱导胰腺癌的产生。研究慢性胰腺炎恶性转化过程中的调控通路,并找到多条通路的关键节点,以其为靶点为临床寻找有效的治疗胰腺癌的药物提供新的思路和方向。
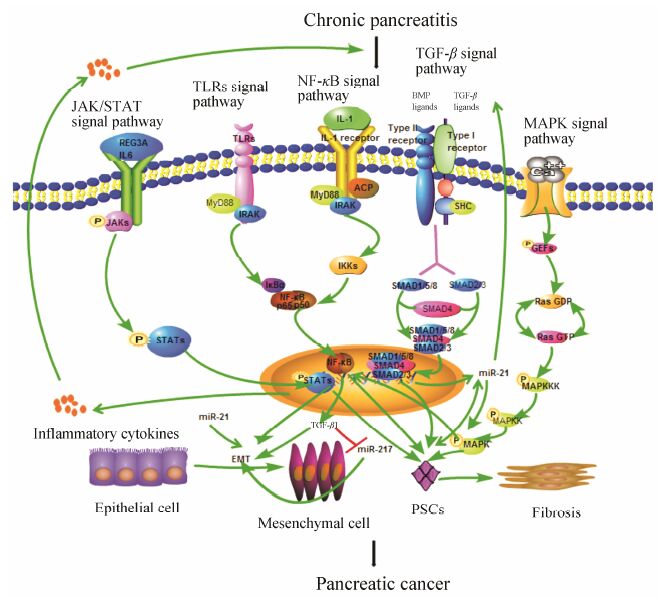
|
Figure 1 Relevant signal pathways in chronic inflammation-linked pancreatic carcinogenesis |
| [1] | Tang B, Li Y, Qi G, et al. Clinicopathological significance of CDKN2A promoter hypermethylation frequency with pancreatic cancer[J]. Sci Rep, 2015, 5:13563. |
| [2] | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015[J]. CA Cancer J Clin, 2015, 65:5-29. |
| [3] | Ma SJ, Qu ZL, Kong D, et al. Progress on epidemiology and diagnosis in pancreatic cancer[J]. Chin J Surg Integrated Tradit West Med (中国中西医结合外科杂志), 2015, 21:87-92. |
| [4] | Capurso G, Boccia S, Salvia R, et al. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas:a multicentre case-control study[J]. Am J Gastroenterol, 2013, 108:1003-1009. |
| [5] | Wu Q, Chen G, Wu WM, et al. Metabolic syndrome components and risk factors for pancreatic adenocarcinoma:a case-control study in China[J]. Digestion, 2012, 86:294-301. |
| [6] | Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group[J]. J Natl Cancer Inst, 1997, 89:442-446. |
| [7] | Karlson BM, Ekbom A, Josefsson S, et al. The risk of pancreatic cancer following pancreatitis:an association due to confounding?[J]. Gastroenterology, 1997, 113:587-592. |
| [8] | Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis[J]. Gut, 2002, 51:849-852. |
| [9] | Bracci PM, Wang FR, Hassan MM, et al. Pancreatitis and pancreatic cancer in two large pooled case-control studies[J]. Cancer Causes Control, 2009, 20:1723-1731. |
| [10] | Wang W, Wang J, Li ZS, et al. The incidence and risk factors of pancreatic cancer in adult patients with chronic pancreatitis[J]. Chin J Dig (中华消化杂志), 2009, 29:93-96. |
| [11] | Zhang SK, Tsui NC, Li DH, et al. Expression of trans-forming growth factor β1/Sma-and Mad-related proteins in rat with chronic pancreatitis induced by dibutyltin dichloride[J]. Pancreas, 2010, 39:252-253. |
| [12] | Apte M, Pirola R, Wilson J. New insights into alcoholic pancreatitis and pancreatic cancer[J]. J Gastroenterol Hepatol, 2009, 24 Suppl 3:S51-S56. |
| [13] | Shimizu K, Kobayashi M, Tahara J, et al. Cytokines and peroxisome proliferator-activated receptor γ ligand regulate phagocytosis by pancreatic stellate cells[J]. Gastroenterology, 2005, 128:2105-2118. |
| [14] | Tahara J, Shimizu K, Shiratori K. Engulfment of necrotic acinar cells by pancreatic stellate cells inhibits pancreatic fibrogenesis[J]. Pancreas, 2008, 37:69-74. |
| [15] | Madro A, Celiński K, Slomka M. The role of pancreatic stellate cells and cytokines in the development of chronic pancreatitis[J]. Med Sci Monit, 2004, 10:Ra166-Ra170. |
| [16] | Gao XX, Cao YN, Staloch DA, et al. Bone morphogenetic protein signaling protects against cerulein-induced pancreatic fibrosis[J]. PLoS One, 2014, 9:e89114. |
| [17] | Li DH, Xie KP, Wolff R, et al. Pancreatic cancer[J]. Lancet, 2004, 363:1049-1057. |
| [18] | Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are ge-netically similar and distinct from well-differentiated pancreatic neuro-endocrine tumors[J]. Am J Surg Pathol, 2012, 36:173-184. |
| [19] | Morris JP 4th, Wang SC, Hebrok M. Kras, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma[J]. Nat Rev Cancer, 2010, 10:683-695. |
| [20] | Huang H, Daniluk J, Liu Y, et al. Oncogenic K-Ras requires activation for enhanced activity[J]. Oncogene, 2014, 33:532-535. |
| [21] | Xia X, Wu WD, Huang C, et al. Smad4 and its role in pancreatic cancer[J]. Tumor Biol, 2015, 36:111-119. |
| [22] | Saiki Y, Horii A. Molecular pathology of pancreatic cancer[J]. Pathol Int, 2014, 64:10-19. |
| [23] | Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation:a prospective cohort study[J]. J Clin Oncol, 2012, 30:958-964. |
| [24] | Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe[J]. Clin Gastroenterol Hepatol, 2004, 2:252-261. |
| [25] | Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma[J]. Genes Dev, 2006, 20:1218-1249. |
| [26] | Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer[J]. FEBS Lett, 2012, 586:1959-1970. |
| [27] | Moustakas A, Heldin CH. The regulation of TGFβ signal transduction[J]. Development, 2009, 136:3699-3714. |
| [28] | Massagué J. TGFβ in cancer[J]. Cell, 2008, 134:215-230. |
| [29] | Gordon KJ, Blobe GC. Role of transforming growth factor β superfamily signaling pathways in human disease[J]. Biochim Biophys Acta, 2008, 1782:197-228. |
| [30] | Dumitriu IE, Dunbar DR, Howie SE, et al. Human den-dritic cells produce TGF-β1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells[J]. J Immunol, 2009, 182:2795-2807. |
| [31] | Truty MJ, Urrutia R. Basics of TGF-β and pancreatic cancer[J]. Pancreatology, 2007, 7:423-435. |
| [32] | Fuxe J, Karlsson MC. TGF-β-induced epithe-lial-mesenchymal transition:a link between cancer and inflammation[J]. Semin Cancer Biol, 2012, 22:455-461. |
| [33] | Deng SC, Zhu S, Wang B, et al. Chronic pancreatitis and pancreatic cancer demonstrate active epithelial-mesenchymal transition profile, regulated by miR-217-SIRT1 pathway[J]. Cancer Lett, 2014, 355:184-191. |
| [34] | Yang G, Yang X. Smad4-mediated TGF-β signaling in tumorigenesis[J]. Int J Biol Sci, 2010, 6:1-8. |
| [35] | Izeradjene K, Combs C, Best M, et al. Kras (G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas[J]. Cancer Cell, 2007, 11:229-243. |
| [36] | Kojima K, Vickers SM, Adsay NV, et al. Inactivation of Smad4 accelerates Kras (G12D)-mediated pancreatic neoplasia[J]. Cancer Res, 2007, 67:8121-8130. |
| [37] | Garcia-Carracedo D, Yu CC, Akhavan N, et al. Smad4 loss synergizes with TGFα overexpression in promoting pan-creatic metaplasia, PanIN development, and fibrosis[J]. PLoS One, 2015, 10:e0120851. |
| [38] | Recio C, Oguiza A, Mallavia B, et al. Gene delivery of suppressors of cytokine signaling (SOCS) inhibits inflammation and atherosclerosis development in mice[J]. Basic Res Cardiol, 2015, 110:8. |
| [39] | Wang J, Zhou H, Han Y, et al. SOCS3 methylation in synergy with Reg3A overexpression promotes cell growth in pancreatic cancer[J]. J Mol Med, 2014, 92:1257-1269. |
| [40] | Bromberg J, Wang TC. Inflammation and cancer:IL-6 and STAT3 complete the link[J]. Cancer Cell, 2009, 15:79-80. |
| [41] | Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 sig-nalling in cancer:new and unexpected biological functions[J]. Nat Rev Cancer, 2014, 14:736-746. |
| [42] | Liu XL, Wang J, Wang HJ, et al. REG3A accelerates pancreatic cancer cell growth under IL-6-associated inflammatory condition:involvement of a REG3A-JAK2/STAT3 positive feedback loop[J]. Cancer Lett, 2015, 362:45-60. |
| [43] | Bachem MG, Zhou SX, Buck K, et al. Pancreatic stellate cells——role in pancreas cancer[J]. Langenbecks Arch Surg, 2008, 393:891-900. |
| [44] | Jaster R, Sparmann G, Emmirich J, et al. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells[J]. Gut, 2002, 51:579-584. |
| [45] | Masamune A, Kikuta K, Satoh M, et al. Differential roles of signaling pathways for prolifsulfide on rat pancreatic stellate cells[J]. Pancreas, 2012, 41:74-83. |
| [46] | Schwer CI, Stoll P, Goebel U, et al.Acta Biochim eration and migration of rat pancreatic stellate cells[J]. Tohoku J Exp Med, 2003, 199:69-84. |
| [47] | Reinehr R, Zoller S, Klonowski-Stumpe H, et al. Effects of angiotensin Ⅱ on rat pancreatic stellate cells[J]. Pancreas, 2004, 28:129-137. |
| [48] | Masamune A, Kikuta K, Suzuki N, et al. A c-Jun NH2-terminal kinase inhibitor SP600125(anthra[1,9-cd]pyrazole-6(2H)-one) blocks activation of pancreatic stellate cells[J]. J Pharmacol Exp Ther, 2004, 310:520-527. |
| [49] | Dhillon N, Aggarwal BB, Newman RA, et al. Phase Ⅱ trial of curcumin in patients with advanced pancreatic cancer[J]. Clin Cancer Res, 2008, 14:4491-4499. |
| [50] | Arlt A, Muerköster SS, Schäfer H. Targeting apoptosis pathways in pancreatic cancer[J]. Cancer Lett, 2013, 332:346-358. |
| [51] | Niu JG, Li ZK, Peng BL, et al. Identification of an autoregu-latory feedback pathway involving interleukin-1α in induction of constitutive NF-κB activation in pancreatic cancer cells[J]. J Biol Chem, 2004, 279:16452-16462. |
| [52] | Sclabas GM, Uwagawa T, Schmidt C, et al. Nuclear factor kappa B activation is a potential target for preventing pancreatic carcinoma by aspirin[J]. Cancer, 2005, 103:2485-2490. |
| [53] | Nakashima H, Nakamura M, Yamaguchi H, et al. Nuclear factor-κB contributes to hedgehog signaling pathway activa-tion through sonic hedgehog induction in pancreatic cancer[J]. Cancer Res, 2006, 66:7041-7049. |
| [54] | Chua HL, Bhat-Nakshatri P, Clare SE, et al. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells:potential involvement of ZEB-1 and ZEB-2[J]. Oncogene, 2007, 26:711-724. |
| [55] | Huber MA, Azoitei N, Baumann B, et al. NF-κB is es-sential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression[J]. J Clin Invest, 2004, 114:569-581. |
| [56] | Ochi A, Graffeo CS, Zambirinis CP, et al. Toll-like re-ceptor 7 regulates pancreatic carcinogenesis in mice and humans[J]. J Clin Invest, 2012, 122:4118-4129. |
| [57] | Ochi A, Nguyen AH, Bedrosian AS, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells[J]. J Exp Med, 2012, 209:1671-1687. |
| [58] | Ikebe M, Kitaura Y, Nakamura M, et al. Lipopolysac-charide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway[J]. J Surg Oncol, 2009, 100:725-731. |
| [59] | Eigenbrod T, Dalpke AH. TLR7 inhibition:a novel strategy for pancreatic cancer treatment?[J]. JAKSTAT, 2013, 2:e23011. |
| [60] | Li A, Omura N, Hong SM, et al. Pancreatic cancers epige-netically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels[J]. Cancer Res, 2010, 70:5226-5237. |
| [61] | Li AG, Yu J, Kim H, et al. microRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls[J]. Clin Cancer Res, 2013, 19:3600-3610. |
| [62] | Bloomston M, Frankel WL, Petrocca F, et al. microRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis[J]. JAMA, 2007, 297:1901-1908. |
| [63] | Takikawa T, Masamune A, Hamada S, et al. miR-210 regulates the interaction between pancreatic cancer cells and stellate cells[J]. Biochem Biophys Res Commun, 2013, 437:433-439. |
| [64] | Liu R, Chen X, Du Y, et al. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer[J]. Clin Chem, 2012, 58:610-618. |
| [65] | Ma X, Conklin DJ, Li F, et al. The oncogenic microRNA miR-21 promotes regulated necrosis in mice[J]. Nat Commun, 2015, 6:7151. |
| [66] | Charrier A, Chen R, Chen L, et al. Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes[J]. J Cell Commun Signal, 2014, 8:147-156. |
| [67] | Hu LH, Ji JT, Li ZS. Potential application of miRNAs as diagnostic and therapeutic tools in chronic pancreatitis[J]. J Cell Mol Med, 2015, 19:2049-2057. |
| [68] | Zarjou A, Yang SZ, Abraham E, et al. Identification of a microRNA signature in renal fibrosis:role of miR-21[J]. Am J Physiol Renal Physiol, 2011, 301:F793-F801. |
| [69] | Thum T, Gross C, Fiedler J, et al. microRNA-21 con-tributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts[J]. Nature, 2008, 456:980-984. |
| [70] | Cufí S, Bonavia R, Vazquez-Martin A, et al. Silibinin suppresses EMT-driven erlotinib resistance by reversing the high miR-21/low miR-200c signature in vivo[J]. Sci Rep, 2013, 3:2459. |
| [71] | Li ZH, Zhao XH, Zhou Y, et al. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer[J]. J Transl Med, 2015, 13:84. |
| [72] | Zhang JS, Zhang PJ, Wang L, et al. Long non-coding RNA HOTAIR in carcinogenesis and metastasis[J]. Acta Biochim eration and migration of rat pancreatic stellate cells[J]. Tohoku J Exp Med, 2003, 199:69-84. |
| [73] | Jiao F, Hu H, Yuan CC, et al. Elevated expression level of long noncoding RNA MALAT-1 facilitates cell growth, migration and invasion in pancreatic cancer[J]. Oncol Rep, 2014, 32:2485-2492. |
| [74] | Huang C, Yu W, Wang Q, et al. Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients[J]. Minerva Med, 2015, 106:143-149. |
| [75] | Zhuang JL, Lu Q, Shen B, et al. TGFβ1 secreted by can-cer-associated fibroblasts induces epithelial-mesenchymal transi-tion of bladder cancer cells through lncRNA-ZEB2NAT[J]. Sci Rep, 2015, 5:11924. |
| [76] | Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma[J]. Cancer Cell, 2014, 25:666-681. |
 2016, Vol. 51
2016, Vol. 51


