2. 华侨大学生物医学学院、华侨大学分子药物研究院厦门市疾病预防与控制中心, 福建厦门 361021
2. Xiamen Municipal Center for Disease Control and Prevention, College of Biomedical Science, Institutes of Molecular Medicine, Huaqiao University, Xiamen 361021, China
有数据统计[1],全球53%的肝细胞癌 (HCC) 患者与乙型肝炎病毒 (hepatitis B virus,HBV) 感染有密切关系。大量研究预测乙肝病毒表面抗原(HBsAg) 携带者最终患HCC的风险是正常人的25~37倍[2]。HCC患者有非常不明确的预后状况,平均只有不到6个月的存活期和6.5% 的5年存活率。
慢性乙肝患者的血清检测中,HBV X蛋白 (hepatitis B virus X protein,HBx) 的检出率均较其他乙肝标志物低,仅为10%~20%,在肝细胞的检出量也较低,而由乙型肝炎演变成HCC的肝癌细胞中表现出高表达,其HBx基因与HBx表达的阳性率约为50%~90%[3],而且分离培养的人肝癌细胞仍保持表达HBx的特性。此外,约40%~60% 表达HBx基因或HBx的肝细胞癌患者的血清中HBsAg为阴性[4]。在一定程度上,这反映出HBx与传统乙肝标志物不呈平行关系,而HBx检出率升高也可能反映患者病情恶化或是具有慢性肝炎向HCC发展的趋势。
在由HBV诱发HCC的患者中发现有HBV DNA整合到肝细胞染色质中,这也被认为是HCC发生的关键点。HBx基因的整合会导致正常肝细胞的基因组异常,在其他致癌因素或辅助因子的共同作用下可激活癌基因表达,或HBx的反式激活作用使癌基因转录活性增强而最终导致肝细胞恶性转化。因此HBx的高表达可能预示着HCC的发生。
1 HBx基因与HBxX-开放阅读框 (hepatitis B virus X protein open reading frame,X-ORF) 是HBV DNA中最小的一个开放式阅读框架,在不同亚型的HBV中其序列大小会有所不同。编码基因区位于C-ORF上游,定位于nt 1 374~1838,编码154 aa的碱性多肽,分子质量约为17 kDa。由于缺少高分辨率晶体的X衍射图和核磁共振成像,HBx的三维结构尚未知。据预测HBx的空间结构与DNA糖基化酶的中央区域极其相似(图 1)。

|
Figure 1 The picture is taken from the web of National Center of Biotechnology Information (NCBI). A: Whole-genome of hepatitis B virus; B: Three-dimensional structure of HBx binding protein |
HBx基因在所有哺乳动物嗜肝病毒中高度保 守。在HCC细胞中,80% 以上是整合型病毒,整合的HBx基因部分缺失,导致读码框变化,C端截短的HBx检出率较高。Chen等[5]在40% 肝癌组织和70% HCC患者血清中发现,HBx基因在20 4位上插入突 变 (插入204AGGCCC) 常伴随260 (G→A) 和264 (G/C/T→A) 位上点突变,在同一样本中会发现多种类型突变。最新统计学研究,HBx基因的8个突变 位点能作为对HCC患者术后存活的独立预测因子: 1 383、1 461、1 485、1 544、1 613、1 653、1 719和1 753。此外在显著性临界线的4个突变位点: 1 527、1 637、1 674和1 762/1 764[6]。
HBx基因至少编码Mr 17 000、8 000和6 600三种蛋白,从5' AUG起始密码开始的序列表达完整长度的Mr 17 000蛋白,即HBx。Mr 8 000和Mr 6 600是由X-ORF内起始密码开始的缺少氨基末端的两条部分重叠的序列所编码的。这三种蛋白在功能上具有 不同活性,Mr 17 000蛋白能激活NF-κB依赖性启动子序列,Mr 8 000和Mr 6 600转录激活SV40的增强子/启动子序列。有研究表明HBx的aa 72~117是结合细胞色素C氧化酶 (cytochrome C oxidase,COXIII) 的关键部位[7]。
相比只患有肝炎的患者,在HCC患者中能频繁检测出HBx抗体。病毒复制过程中HBx属于调节性蛋白。在HBx转基因鼠实验中可以看到其提高了细胞对化学致癌物的敏感性。体内实验指出HBx可能作为HCC发生的辅因子。此外,HBx的85%~90%基因整合入由HBV诱导的肝癌细胞中,这也表明HBx与肝细胞癌变的过程关系密切。HBx基因翻译出的氨基酸序列在已知的蛋白质中无同源性。在众多病毒子型中HBx高度保守。HBx在肝细胞内的分布与其表达水平有关,低水平表达的HBx主要分布在核内,高水平时多聚集在胞浆中,与线粒体密切相关,与内质网、溶酶体等细胞器无关。HBx主要与宿主 细胞的转录因子 (如Ras、c-Jun、NF-κB、c-Fos、c-Myc、MAPK、FAK、Jak-STAT和p53) 形成蛋白-蛋白复合物,通过多种信号转导通路,激活或改变转录因子的活性,影响细胞凋亡和DNA修复,从而促使肝细胞癌变或提高癌细胞侵袭和转移能力。
2 HBx的反式激活HBx是复杂多样的反式激活因子,它并不直接作用于DNA,而是通过蛋白与蛋白之间的相互作用和相互联系实现激活或抑制的多样性能,包括作用于胞质内的信号通路和核内的DNA结合蛋白上 (图 2)。据报道,HBx的转录活性对病毒的复制是必需的。Sung等[8]运用染色质免疫共沉淀芯片 (chromatin immune coprecipitation chip,CHIP-chip) 技术确定了HBx直接调控184个基因靶点,它能将蛋白-DNA的间接调控转化成直接的基因靶点调控,同时证明了HBx与一些预测转录因子相联系。

|
Figure 2 The interaction of hepatitis B virus X protein and signal pathways |
HBx能上调一系列的细胞和病毒基因,包括HBV增强子、第二第三激活子和原癌基因 (c-Jun、c-Fos、c-Myc)。HBx对转录因子也表现出激活活性,如激活蛋白-1 (activator protein-1,AP-1)、激活转录因子 (activating transcription factor,ATF)/cAMP-应答、环磷腺苷效应元件结合蛋白 (cAMP-response element binding protein,CREB)。一些研究已报道,由HBx引起NF-κB的反式激活间接上调一些宿主基因。
2.1 NF-κB信号通路HBx通过反式激活诱导型一氧化氮合成酶 (inducible nitric oxide synthase,iNOS) 启动子,此外,HBx通过反式激活一氧化氮合成酶启动子,并在肿瘤转移相关基因1 (metastasis-associated genes 1,MTA1) 的辅助下,提高iNOS的活性,促进NO的释放[9],促使炎症发生。在转染了HBx基因的多种肝细胞中发现,HBx与Fas配体作用密切,抑制Fas介导的细胞凋亡[10],也通过上调SAPK/JNK通路影响细胞存亡[11]。表达在胞浆中的HBx也被验证能激活信号转导通路,如Ras/Raf-1/MAP、JAK/STAT。HBx驱动5-脂氧合酶(5-lipoxygenase,5-Lox)、骨桥蛋白、钙蛋白酶小亚基1 (calpain small subunit,Capn4) 和Ras/Raf-1/MAP信号级联,能激活AP-1和NF-κB等转录因子[12, 13],从而解除细胞周期性相关因子的控制,促进细胞增殖和迁移。NF-κB信号通道在HBx调节作用下,上调血管内皮生长因子 (vascular endothelial growth factor,VEGF)、金属蛋白酶2 (metal matrix proteinase,MMP2)、MMP9、MMP14和MTA-1表 达[14, 15],这些激酶能破坏癌细胞的组织学屏障,有助于癌细胞的转移。通过激活c-Myc,HBx 能上调热休克蛋白90α (heat shock protein,HSP90α)[16],而HSP90α已经作为一种全新的肿瘤标记物。这些因子均受NF-κB信号通路调控,因而HBx间接或直接干预和影响了通路下游的信号传递,也调节 (-κB位点) 结合基因的转录。
2.2 Notch信号通路HBx能与Notch1胞内区域 (ICN1) 结合,减少其沟裂区域形成空间位阻,从而降低ICN1的内源蛋白和下游目标基因的mRNA水平,并通过早老素1 (presenilin-1,Psen1) 的转录抑制,避免ICN1的清除,最终减弱肝癌细胞衰老样生长停滞[17]。转染HBx基因的LO2细胞实验也验证HBx通过下调cyclin D1/细胞周期蛋白依赖性激酶 (cyclin- dependent kinase,CDK4),上调p21和Rb,减少Notch1 shRNA生成,抑制Notch1信号通路[18]。 此外,Wnt能与Notch[19]、NF-κB/Notch[20]协同作用,诱导肝细胞癌变。
2.3 Wnt/β-链蛋白信号通路在肝癌细胞中HBx对Wnt/β-链蛋白的激活是必需的[21],通过Wnt信号通路,沉默SFRP1和SFRP5基因[22],激活Src激酶和屏蔽SIRT1脱乙酰酶[23],激活胞外信号调节激酶ERK,使糖原合成酶激酶-3β (glycogen synthase kinase-3β,GSK-3β) 失活[24],上调原癌基因gankyrin。此外,截短的HBx抑制β-蛋白/TCF4转录能力 (N端1~84 aa,达95.5% 的抑制率,C端85~154 aa,达84.7% 的抑制率),C端缺失的HBx调节Wnt-5a的表达[25]。
2.4 促炎因子HBx引起激活T细胞的核因子 (nuclear factor of activated T cell,NF-AT) 的脱磷酸作用而发生迁移,导致肝内NF-AT量增多,调节IL-32[26]、IL-6[27]、IL-8和CXCL-2分泌,促使炎症发生。在肝脏中,IL-6调节一系列急性蛋白的合成,其高表达最终会促使肝硬化和HCC的发生。此外,HBx抑制STAT1的磷酸化,抑制IFN-α的抗病毒活性[28]。
低氧诱导因子-1 (hypoxia inducible factor-1,HIF-1) 是HIF-1α和HIF-1β两个亚基的二聚体,作为血管生长因子表达的关键转录因子,能在低氧状态下上调血管生长的相关基因。HBx在正常和缺氧情况下都能提高HIF-1α的表达,而HIF-1α能迅速降解pVHL调控的蛋白分解体,因而保护HIF-1二聚体的完整性。此外,HIF-1也是碳酸酐酶-9的转录激活剂,这样能提高肿瘤细胞在低氧环境中的生存能力[29]。HBx提高活性氧水平,调节线粒体的Ca2+ 摄取,激活细胞溶质钙信号[30],引起内质网 (ER) 应激,促使ATF4作用于COX-2,从而提高其表达[31]。
2.5 胞外信号激酶HBx通过激活ERK和c-jun氨基末端激酶 (JNKs),使AP-1/c-Jun二聚体复合物积累增多,AP-1控制着分化、增殖和凋亡等细胞进程,AP-1的持续上调会导致肝细胞癌变和癌细胞转移,此外C端缺失的HBx能通过激活c-Jun/MMP10,提高癌细胞的侵入能力[32]。在90名乙型肝炎患者的肝脏活组织切片中,Murata等[33]发现在早期致癌过程中,HBx将TGF-β从抑癌的pSmad3C通路向JNK依赖的促癌pSmad3L通路转化,抑制免疫活性细胞的增殖,能诱导c-Sis的表达,却抑制c-Myc的表达。统计由HBV引起HCC的患者中发现男性居多,有学者推 测与雄性激素水平有关,实验也验证HBx通过激活c-Src信号通路,增强雄性激素受体的基因应答,提高激素水平[34],而雄性激素如何诱导肝细胞癌变尚未知。
2.6 细胞周期和细胞功能调节数据表明在肝癌细胞系中,HBx能促使p53的功能丧失,诱导关键的下游调节,反式转录激活p21wafl/cipl[35]; 此外HBx通过使GSK-3β失活,提高细胞周期蛋白结构稳定性[36, 37],促使其在核内积累,最终延长了G1到S期时间。HBx反式激活DNA甲基化酶,下调p16、p15、p8[38]和p21,抑制全反式视黄酸 (all-trans retinoic acid,ATRA) 诱导的细胞衰老[39]。同时HBx绑定DNA损伤结合蛋白1 (DNA damage-binding protein 1,DDB1),使其重新定位于CUL4-DDB1泛素连接酶,而影响泛素化修饰系统的正常功能[40]。
HBx通过增强早期生长响应因子 (EGR-2和EGR-3) 的反式转录活性,诱导Fas配体基因的表达,在肿瘤细胞中Fas配体能促使Fas耐受T细胞提早凋亡,从而帮助细胞躲避免疫监视。
Feng等[41]运用iTRAQ偶联2D LC-MS/MS方法对HBx诱导的蛋白表达谱进行蛋白组学分析,发现HBx能上调微管微丝交联因子1 (microtubule actin cross-linking factor 1,MACF1)、高迁移率族蛋白B (high mobility group box 1,HMGB1) 和膜联蛋白 A2,下调Lamin A/C,影响一些胞外蛋白包括Ca2+ 结合蛋白 (S100A11、 S100A6和S100A4) 和蛋白酶 (PSMA3)。HBx也提高Snail蛋白[42]的稳定性,下调蛋白ZNF198和SUZ12表达[43],通过PI3K通路,调节LASP-1[44],提高AIB1蛋白结构稳定性[45],上调着丝粒蛋白抗体 (CENP-A),抑制微囊蛋白1表达[46]。以上作用的综合结果是降低细胞的黏着力,提高癌细胞的侵袭和转移能力。
2.7 人端粒酶和端粒酶逆转录酶端粒酶在保持端粒稳定、基因组完整、细胞长期的活性和潜在的继续增殖能力等方面有重要作用。一般,在正常人体组织中端粒酶的活性是被抑制。而近几年不断发现肿瘤 细胞中存在较复杂的端粒酶调控网络,其参与了对肿瘤细胞的凋亡和基因组稳定的调控过程。Liu等[47]在表达HBx的不同肝癌细胞系 (HepG2、SMMC7721、HepG2.2.15、Bel7402和COS-7) 研究中发现HBx通过反式激活提高端粒酶和端粒酶逆转录酶 (human telomerase reverse transcriptase,hTERT) 的活性。HBx对端粒酶的激活作用能在一定程度上防止癌细胞衰老和凋亡,也致使癌细胞的进一步迁移与恶化。
3 促凋亡HBx通过激活肿瘤坏死因子受体 (tumor necrosis factor receptors,TNFR) 和NF-κB信号通路引起肝细胞脂肪变性和凋亡[48]。HBx上调凋亡相关因子Bax,靶向抗凋亡蛋白Bcl-xL,使肝细胞敏感于TRAIL诱导的凋亡[49]。UVDDB和B1-CDK1是细胞凋亡过程中不可或缺的影响分子。实验表明,在转染了HBx基因序列的细胞系中,HBx能持续激活UVDDB和B1- CDK1激酶。HBx上调Fas/FasL、p53蛋白,减弱蛋白酶-8、Bax/Bcl-2、c-Myc和UV-DDB1的活性,与TNFR1和NF-κB相互作用,持续激活B1-CDK1激酶和CCL13[50] (图 3)。
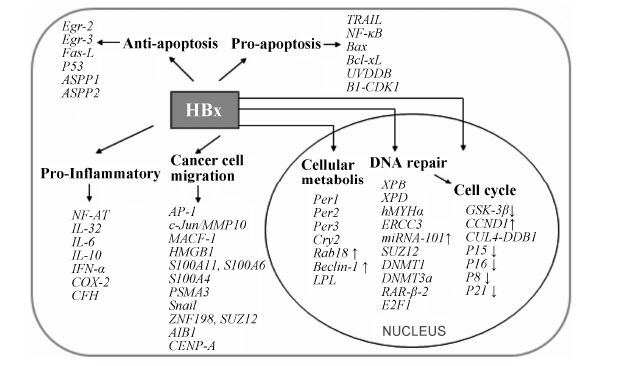
|
Figure 3 The function of hepatitis B virus X protein |
HBx能与p53的C端特异性结合,抑制p53介导的凋亡,同时下调c-Myc,诱导丝氨酸蛋白酶基因,促进p53凋亡刺激蛋白 (ASPP1和ASPP2) 基因的甲基化[51],抑制半胱天冬酶通路[52],因而可能导致HCC形成。在HCC患者的肝组织和邻近组织的样本中,利用免疫共沉淀检测到HBx和生存素的表达水平较高,又在H7402和LO2细胞中验证HBx与生存素相互作用。此外HBx也能通过上调和激活依赖p21活化的激酶1,抑制癌细胞的失巢式凋亡[53]。
5 胞内代谢异常HBV感染的肝细胞逐渐呈现出代谢异常,线粒体活性氧和脂质过氧化物生成增加[54],线粒体外膜孔蛋白3抗体(voltage-dependent anion channel,VDAC3) 减少,实验结果揭示HBx使生物钟基因 (Per1、Per2、Per3和Cry2 ) 的表达紊乱[55],上调致癌基因Rab18[56],与HSP60、HSP70形成复合物[57],改变线粒体跨膜 蛋白活性。在基因鼠模型中,HBx能显著激活脂质相关基因脂蛋白脂肪酶和脂肪酸结合蛋白4等,脂质代谢基因的调节异常可能指示着HCC的发展进程[58]。HBx也能上调Beclin-1基因,下调c-Myc,且促使microRNA-30a过表达而抑制HBV诱导的自噬体形成[59]; 同时破坏溶酶体的成熟,抑制自噬泡的降解作用[60],与c-Myc共同上调核糖体生物合成,提高细胞转化功能[61]。
6 表观遗传变异HBx促癌作用还表现在抑制核内DNA的修复,Lee等[62]研究发现HBx能使细胞易受UV损伤,抑 制DNA损伤修复。不仅如此,HBx还能作用于p53依赖的转录因子TFIIH[63],下调XPB、XPD和hMYHα,使DNA突变加和物8-羟基脱氧鸟苷 (8-OhdG) 积累增加[64],抑制DNA损伤修复酶ERCC3。此外,HBx结合于DDB1,阻止转录偶联的核苷酸切除修复,上调microRNA-101,诱导畸变DNA的甲基化[65]。在HBV复制和癌变过程,SUZ12/PRC2目标基因被激活[66],SUZ12蛋白利用其锌指结构域与HOTAIR非编码RNA分子进行结合,一起参与到染色质沉默之中。
在转录水平HBx通过作用于许多胞内因子,下调某些基因表达。Jung等[67]已证明HBx通过上调DNA甲基转移酶 (DNMT1和DNMT3a),使视黄酸β受体2 (RAR-β-2) 的启动子甲基化,使得RAR-β表达的下降。这样就削弱了RAR对p16、p21和p27的下调能力,导致细胞转录因子E2F1的激活。因此,HBx过表达的细胞对RAR诱导的细胞生长抑制不敏感,这也可能意味着RAR-β-2的低表达是癌变过程中的重要一步。HBx也被报道能激活DNMT,使钙黏蛋白启动子甲基化,限制了钙黏蛋白的转录作用,从而影响Wnt的激活[68]。
此外,HBx对抑癌因子p16INK4A的甲基化有两 个不同方面表现。Zhu等[69]揭示HBx与DNMT1和DNMT3a作用联系密切,却在肿瘤组织中发现HBx的表达对p16INK4A启动子的甲基化和p16的表达没有显著影响。Kim等[70]实验研究为HBx下调p16INK4A提供证据,HBx能激活G1-CDKs和E2F1,使Rb磷酸化,产生不成熟的衰老诱导物和过氧化氢,从而逃脱了G1期的清除。
7 HBx变异HBx的天然变异已在肝癌患者的血清和肝组织中得到验证[71, 72]。HBx氨基酸序列 (154 aa) 根据它的同源性划分成6个区域 (A~F),其中A、C和E区相对保守。HBx通过其缺失突变修饰的生物学功能,在原发性肝癌发生、发展过程中起着重要作用。
报道最频繁的突变是HBx的3'末端。肝癌组织中HBx 3'末端缺失较非癌组织频发,这可能意味着这个突变在癌变进程中扮演重要角色。在HBsAg阴性、HBV DNA阳性患者的肿瘤和非肿瘤组织切片中分析了HBx基因结构和表达,并发现在大多数的肝癌细胞中HBx基因是被修复的。C端缺失的HBx在肝癌细胞中不断被检测到 (79.3%,n = 111),体内外实验也揭示有缺失的蛋白比全长蛋白更具促进肝癌细胞迁移的能力。Ma等[73]研究也表明C端缺失的HBx具有激活细胞增殖和丧失促凋亡的能力,进而促使肝细胞癌变 (57.2%,n = 194)。敲除了382~401碱基对 (HBxΔ127) 表达出的HBx能激活依赖于5-脂氧合酶的固醇调节元件结合蛋白-1c (sterol regulatory element binding protein 1c,SREBP-1c) 的表达,从而促进肝细胞生长[74]。HBxΔ127促进肝癌细胞迁移的作用是通过p-ERK1/2上调Capn4实现的。这一发现对进一步揭示HBx突变体HBxΔ127促进肝癌细胞转移的分子机制具有重要意义。而HBx突变体削弱了反式激活和促凋亡的功能,但仍保留对p53调节的凋亡的抑制能力[75]。
8 microRNA表达microRNA在HCC的发展中发挥着重要作用,并直接参与了肝癌细胞周期、分化、凋亡、侵袭和远距离转移等过程,其作用具有网络化、双向性及组织特异性的特点。同时microRNA逐渐作为HCC及慢性乙肝肝损伤的分子标记物,其表达谱能预测HCC病理类型、恶性程度、分期和分级等病理特征。microRNA与肝癌之间的关系也是目前学者们研究的热点。其中,microRNA-122是关键的肝特异性microRNA,占肝microRNA总量的70%,可调节cyclin G1抑制癌细胞增殖,从而影响Cip/Kip家族中p27、p21和p57蛋 白,直接抑制DNA复制。然而在HCC患者的肝癌细
胞中发现microRNA-122表达较低,Song等[76]利用microRNA微阵列和实时PCR技术分析验证了HBx是通过结合过氧化物酶体增殖物激活受体 (PPARγ),达到抑制microRNA的作用。microRNA-205是众所周知的抑癌microRNA,而HBx通过甲基化作用,使microRNA-205丧失抑癌功能[77]。HBx能影响一些胞内microRNA的表达,表现出上调microRNA-7[78]、microRNA-146a、microRNA-101和microRNA-29a[79],下调microRNA-11、microRNA-148[80]、microRNA-132、microRNA-16和microRNA-520b。以上调节的最终结果都是诱使炎症活化物的产生,加重炎症,从抑癌效果逐步转化成促癌作用。其中HBx上调microRNA- 146a[81],下调补体因子H (complement factor H,CFH),促发补体系统,HBx-microRNA146a-CFH复合物的活化将触发炎症反应,而炎症是肝脏持续受损,演化成肝硬化HCC的第一步。
9 结语HBx诱导肝癌发生涉及多方面,与多种细胞因子相关,它能与胞内外不同信号分子直接或间接进行协同作用,促使肝细胞癌变或癌细胞的恶性转移。最新的研究正在揭露HCC发生的不同分子机制和途径,而HBx诱导肝癌发生本身是具有争议性的,因为其最严密、最精确的作用机制还不能被揭示。但是,也不可否认HBx对肝癌发生有着确切的影响,不管是体外或体内、细胞系还是转基因鼠,HBx的过表达均能显著地出现促癌效果,在不同层次上激活或抑制、上调或下调相关基因、激酶和转录因子等。本文总结和分析的分子机制有望发展成治疗HBV关联的HCC药物的新靶点和治疗策略。
| [1] | Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture[J]. Eur J Cancer, 2001, 37 Suppl 8:S4-S66. |
| [2] | Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma:synergism of alcohol with viral hepatitis and diabetes mellitus[J]. Hepatology, 2002, 36:1206-1213. |
| [3] | Su Q, Schröder CH, Hofmann WJ, et al. Expression of hepatitis B virus X protein in HBV-infected human livers and hepatocellular carcinomas[J]. Hepatology, 1998, 27:1109-1120. |
| [4] | Paterlini P, Poussin K, Kew M, et al. Selective accumula-tion of the X transcript of hepatitis B virus in patients negative for hepatitis B surface antigen with hepatocellular carcinoma[J]. Hepatology, 1995, 21:313-321. |
| [5] | Chen GG, Li MY, Ho RL, et al. Identification of hepatitis B virus X gene mutation in Hong Kong patients with hepato-cellular carcinoma[J]. J Clin Virol, 2005, 34:7-12. |
| [6] | Xie Y. X protein mutations in hepatitis B virus DNA predict postoperative survival in hepatocellular carcinoma[J]. Tumour Biol, 2014, 35:10325-10331. |
| [7] | Li D, Ding J, Chen Z, et al. Accurately mapping the location of the binding site for the interaction between hepatitis B virus X protein and cytochrome c oxidase Ⅲ[J]. Int J Mol Med, 2015, 35:319-324. |
| [8] | Sung WK, Lu Y, Lee CW, et al. Deregulated direct targets of the hepatitis B virus (HBV) protein, HBx, identified through chromatin immunoprecipitation and expression microarray profiling[J]. J Biol Chem, 2009, 284:21941-21954. |
| [9] | Bui-Nguyen TM, Pakala SB, Sirigiri DR, et al. Stimulation of inducible nitric oxide by hepatitis B virus transactivator protein-HBx requires MTA1 coregulator[J]. J Biol Chem, 2010, 285:6980-6986. |
| [10] | Pan J, Duan LX, Sun BS, et al. Hepatitis B virus X protein protects against anti-Fas-mediated apoptosis in human liver cells by inducing NF-κB[J]. J Gen Virol, 2001, 82:171-182. |
| [11] | Diao J, Khine AA, Sarangi F, et al. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway[J]. J Biol Chem, 2001, 276:8328-8340. |
| [12] | Um HR, Lim WC, Chae SY, et al. Raf-1 and protein kinase B regulate cell survival through the activation of NF-κB in hepatitis B virus X-expressing cells[J]. Virus Res, 2007, 125:1-8. |
| [13] | Zhang X, You X, Wang Q, et al. Hepatitis B virus X pro-tein drives multiple cross-talk cascade loops involving NF-κB, 5-LOX, OPN and Capn4 to promote cell migration[J]. PLoS One, 2012, 7:e31458. |
| [14] | Liu LP, Liang HF, Chen XP, et al. The role of NF-κB in hepatitis B virus X protein-mediated upregulation of VEGF and MMPs[J]. Cancer Invest, 2010, 28:443-451. |
| [15] | Bui-Nguyen TM, Pakala SB, Sirigiri RD, et al. NF-κB signaling mediates the induction of MTA1 by hepatitis B virus transactivator protein HBx[J]. Oncogene, 2010, 29:1179-1189. |
| [16] | Li W, Miao X, Qi Z, et al. Hepatitis B virus X protein upregulates HSP90alpha expression via activation of c-Myc in human hepatocarcinoma cell line, HepG2[J]. Virol J, 2010, 7:45. |
| [17] | Xu J, Yun X, Jiang J, et al. Hepatitis B virus X protein blunts senescence-like growth arrest of human hepatocellular carcinoma by reducing Notch1 cleavage[J]. Hepatology, 2010, 52:142-154. |
| [18] | Sun Q, Wang R, Wang Y, et al. Notch1 is a potential therapeutic target for the treatment of human hepatitis B virus X protein-associated hepatocellular carcinoma[J]. Oncol Rep, 2014, 31:933-939. |
| [19] | Luo J, Zhou H, Wang F, et al. The hepatitis B virus X protein downregulates NF-κB signaling pathways through decreasing the Notch signaling pathway in HBx-transformed L02 cells[J]. Int J Oncol, 2013, 42:1636-1643. |
| [20] | Sun Q, Wang R, Luo J, et al. Notch1 promotes hepatitis B virus X protein-induced hepatocarcinogenesis via Wnt/β-catenin pathway[J]. Int J Oncol, 2014, 45:1638-1648. |
| [21] | Shen L, Zhang X, Hu D, et al. Hepatitis B virus X (HBx) play an anti-apoptosis role in hepatic progenitor cells by activating Wnt/β-catenin pathway[J]. Mol Cell Biochem, 2013, 383:213-222. |
| [22] | Xie Q, Chen L, Shan X, et al. Epigenetic silencing of SFRP1 and SFRP5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway[J]. Int J Cancer, 2014, 135:635-646. |
| [23] | Srisuttee R, Koh SS, Kim SJ, et al. Hepatitis B virus X (HBX) protein upregulates β-catenin in a human hepatic cell line by sequestering SIRT1 deacetylase[J]. Oncol Rep, 2012, 28:276-282. |
| [24] | Cha MY, Kim CM, Park YM, et al. Hepatitis B virus X protein is essential for the activation of Wnt/β-catenin signaling in hepatoma cells[J]. Hepatology, 2004, 39:1683-1693. |
| [25] | Liu X, Wang L, Zhang S, et al. Mutations in the C-terminus of the X protein of hepatitis B virus regulate Wnt-5a expression in hepatoma Huh7 cells:cDNA microarray and proteomic analyses[J]. Carcinogenesis, 2008, 29:1207-1214. |
| [26] | Pan X, Cao H, Lu J, et al. Interleukin-32 expression in-duced by hepatitis B virus protein X is mediated through activation of NF-κB[J]. Mol Immunol, 2011, 48:1573-1577. |
| [27] | Luo MX, Wong SH, Chan MT, et al. Autophagy mediates HBx-induced nuclear factor-κB activation and release of IL-6, IL-8 and CXCL2 in hepatocytes[J]. J Cell Physiol, 2015, 230:2382-2389. |
| [28] | Yue X, Wang H, Zhao F, et al. Hepatitis B virus-induced calreticulin protein is involved in IFN resistance[J]. J Immunol, 2012, 189:279-286. |
| [29] | Holotnakova T, Tylkova L, Takacova M, et al. Role of the HBx oncoprotein in carbonic anhydrase 9 induction[J]. J Med Virol, 2010, 82:32-40. |
| [30] | Yang B, Bouchard MJ. The hepatitis B virus X protein elevates cytosolic calcium signals by modulating mitochon-drial calcium uptake[J]. J Virol, 2012, 86:313-327. |
| [31] | Cho HK, Cheong KJ, Kim HY, et al. Endoplasmic reticu-lum stress induced by hepatitis B virus X protein enhances cyclo-oxygenase 2 expression via activating transcription factor 4[J]. Biochem J, 2011, 435:431-439. |
| [32] | Sze KM, Chu GK, Lee JM, et al. C-terminal truncated hepatitis B virus X protein is associated with metastasis and enhances invasiveness by C-Jun/matrix metalloproteinase protein 10 activation in hepatocellular carcinoma[J]. Hepa-tology, 2013, 57:131-139. |
| [33] | Murata M, Matsuzaki K, Yoshida K, et al. Hepatitis B virus X protein shifts human hepatic transforming growth factor (TGF)-β signaling from tumor suppression to oncogenesis in early chronic hepatitis B[J]. Hepatology, 2009, 49:1203-1217. |
| [34] | Chiu CM, Yeh SH, Chen PJ, et al. Hepatitis B virus X protein enhances androgen receptor responsive gene expression depending on androgen level[J]. Proc Natl Acad Sci U S A, 2007, 104:2571-2578. |
| [35] | Iyer S, Groopman JD. Interaction of mutant hepatitis B X protein with p53 tumor suppressor protein affects both transcription and cell survival[J]. Mol Carcinog, 2011, 50:972-980. |
| [36] | Chen X, Zhang L, Zheng S, et al. Hepatitis B virus X protein stabilizes cyclin D1 and increases cyclin D1 nuclear accumula-tion through ERKs-mediated inactivation of GSK-3β[J]. Cancer Prev Res, 2015, 8:455-463. |
| [37] | Zeng Z, Tu J, Cheng J, et al. Influence of CCND1 G870A polymorphism on the risk of HBV-related HCC and cyclin D1 splicing variant expression in Chinese population[J]. Tumour Biol, 2015, 36:6891-6900. |
| [38] | Du Y, Kong G, You X, et al. Elevation of highly up-regulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18[J]. J Biol Chem, 2012, 287:26302-26311. |
| [39] | Park SH, Jung JK, Lim JS, et al. Hepatitis B virus X protein overcomes all-trans retinoic acid-induced cellular senescence by downregulating levels of p16 and p21 via DNA methylation[J]. J Gen Virol, 2011, 92:1309-1317. |
| [40] | Li T, Robert EI, van Breugel PC, et al. A promiscuous α-helical motif anchors viral hijackers and substrate receptors to the CUL4-DDB1 ubiquitin ligase machinery[J]. Nat Struct Mol Biol, 2010, 17:105-111. |
| [41] | Feng H, Li X, Niu D, et al. Protein profile in HBx trans-fected cells:a comparative iTRAQ-coupled 2D LC-MS/MS analysis[J]. J Proteomics, 2010, 73:1421-1432. |
| [42] | Liu H, Xu L, He H, et al. Hepatitis B virus X protein promotes hepatoma cell invasion and metastasis by sta-bilizing Snail protein[J]. Cancer Sci, 2012, 103:2072-2081. |
| [43] | Wang WH, Studach LL, Andrisani OM. Proteins ZNF198 and SUZ12 are down-regulated in hepatitis B virus (HBV) X protein-mediated hepatocyte transformation and in HBV replication[J]. Hepatology, 2011, 53:1137-1147. |
| [44] | Tang R. Role of hepatitis B virus X protein in regulating LIM and SH3 protein 1(LASP-1) expression to mediate proliferation and migration of hepatoma cells[J]. Virol J, 2012, 9:163. |
| [45] | Liu Y, Tong Z, Li T, et al. Hepatitis B virus X protein stabilizes amplified in breast cancer 1 protein and cooperates with it to promote human hepatocellular carcinoma cell invasiveness[J]. Hepatology, 2012, 56:1015-1024. |
| [46] | Yan J, Lu Q, Dong J, et al. Hepatitis B virus X protein suppresses caveolin-1 expression in hepatocellular carcinoma by regulating DNA methylation[J]. BMC Cancer, 2012, 12:353. |
| [47] | Liu H, Shi W, Luan F, et al. Hepatitis B virus X protein upregulates transcriptional activation of human telomerase reverse transcriptase[J]. Virus Genes, 2010, 40:174-182. |
| [48] | Kim JY, Song EH, Lee HJ, et al. HBx-induced hepatic steatosis and apoptosis are regulated by TNFR1 and NF-κB-dependent pathway[J]. J Mol Biol, 2010, 397:917-931. |
| [49] | Geng X, Huang C, Qin Y, et al. Hepatitis B virus X protein targets Bcl-2 proteins to increase intracellular calcium, re-quired for virus replication and cell death induction[J]. Proc Natl Acad Sci U S A, 2012, 109:18471-18476. |
| [50] | Kuo CY, Tsai JI, Chou TY, et al. Apoptosis induced by hepatitis B virus X protein in a CCL13-HBx stable cell line[J]. Oncol Rep, 2012, 28:127-132. |
| [51] | Zhao J, Wu G, Bu F, et al. Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region-containing protein 1(ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma[J]. Hepatology, 2010, 51:142-153. |
| [52] | Liu H, Yuan Y, Guo H, et al. Hepatitis B virus encoded X protein suppresses apoptosis by inhibition of the caspase-independent pathway[J]. J Proteome Res, 2012, 11:4803-4813. |
| [53] | Xu J, Liu H, Chen L, et al. Hepatitis B virus X protein confers resistance of hepatoma cells to anoikis by up-regulating and activating p21-activated kinase 1[J]. Gastroenterology, 2012, 143:199-212. |
| [54] | Tan C, Guo H, Zheng M, et al. Involvement of mitochon-drial permeability transition in hepatitis B virus replication[J]. Virus Res, 2009, 145:307-311. |
| [55] | Yang SL, Yu C, Jiang JX, et al. Hepatitis B virus X protein disrupts the balance of the expression of circadian rhythm genes in hepatocellular carcinoma[J]. Oncol Lett, 2014, 8:2715-2720. |
| [56] | You X, Liu F, Zhang T, et al. Hepatitis B virus X protein upregulates oncogene Rab18 to result in the dysregulation of lipogenesis and proliferation of hepatomacells[J]. Carcino-genesis, 2013, 34:1644-1652. |
| [57] | Zhang SM, Sun DC, Lou S, et al. HBx protein of hepatitis B virus (HBV) can form complex with mitochondrial HSP60 and HSP70[J]. Arch Virol, 2005, 150:1579-1590. |
| [58] | Teng CF, Hsieh WC, Yang CW, et al. A biphasic response pattern of lipid metabolomics in the stage progression of hepatitis B virus X tumorigenesis[J]. Mol Carcinog, 2015. DOI:10.1002/mc.22266. |
| [59] | Kumar S, Gupta P, Khanal S, et al. Overexpression of microRNA-30a inhibits hepatitis B virus X protein-induced autophagosome formation in hepatic cells[J]. FEBS J, 2015, 282:1152-1163. |
| [60] | Liu B, Fang M, Hu Y, et al. Hepatitis B virus X protein inhibits autophagic degradation by impairing lysosomal maturation[J]. Autophagy, 2014, 10:416-430. |
| [61] | Shukla SK, Kumar V. Hepatitis B virus X protein and c-Myc cooperate in the upregulation of ribosome biogenesis and in cellular transformation[J]. FEBS J, 2012, 279:3859-3871. |
| [62] | Lee AT, Ren J, Wong ET, et al. The hepatitis B virus X protein sensitizes HepG2 cells to UV light induced DNA damage[J]. J Biol Chem, 2005, 280:33525-33535. |
| [63] | Qadri I, Fatima K, Abdel-Hafiz H. Hepatitis B virus X protein impedes the DNA repair via its association with transcription factor, TFIIH[J]. BMC Microbiol, 2011, 11:48. |
| [64] | Cheng B, Zheng Y, Guo X, et al. Hepatitis B viral X pro-tein alters the biological features and expressions of DNA repair enzymes in LO2 cells[J]. Liver Int, 2010, 30:319-326. |
| [65] | Wei X, Xiang T, Ren G, et al. miR-101 is down-regulated by the hepatitis B virus X protein and induces aberrant DNA methylation by targeting DNA methyltransferase 3A[J]. Cell Signal, 2013, 25:439-446. |
| [66] | Studach LL, Menne S, Cairo S, et al. Subset of Suz12/PRC2 target genes is activated during hepatitis B virus replication and liver carcinogenesis associated with HBV X protein[J]. Hepatology, 2012, 56:1240-1251. |
| [67] | Jung JK, Park SH, Jang KL. Hepatitis B virus X protein overcomes the growth-inhibitory potential of retinoic acid by downregulating retinoic acid receptor-β2 expression via DNA methylation[J]. J Gen Virol, 2010, 91:493-500. |
| [68] | Lee JO, Kwun HJ, Jung JK, et al. Hepatitis B virus X protein represses E-cadherin expression via activation of DNA methyltransferase 1[J]. Oncogene, 2005, 24:6617-6625. |
| [69] | Zhu YZ, Zhu R, Fan J, et al. Hepatitis B virus X protein induces hypermethylation of p16INK4A promoter via DNA methyltransferases in the early stage of HBV associated hepatocarcinogenesis[J]. J Viral Hepat, 2010, 17:98-107. |
| [70] | Kim YJ, Jung JK, Lee SY, et al. Hepatitis B virus X protein overcomes stress-induced premature senescence by re-pressing p16INK4a expression via DNA methylation[J]. Cancer Lett, 2010, 288:226-235 |
| [71] | Guo X, Jin Y, Qian G, et al. Sequential accumulation of the mutations in core promoter of hepatitis B virus is associated with the development of hepatocellular carcinoma in Qidong, China[J]. J Hepatol, 2008, 49:718-725. |
| [72] | Kim JK, Chang HY, Lee JM, et al. Specific mutations in the enhancer Ⅱ/core promoter/precore regions of hepatitis Bvirus subgenotype C2 in Korean patients with hepatocellular carcinoma[J]. J Med Virol, 2009, 81:1002-1008. |
| [73] | Ma NF, Lau SH, Hu L, et al. COOH-terminal truncated HBV X protein plays key role in hepatocarcinogenesis[J]. Clin Cancer Res, 2008, 14:5061-5068. |
| [74] | Wang Q, Zhang WY, Ye LH, et al. A mutant of HBx (HBxDelta127) promotes hepatoma cell growth via sterol regulatory element binding protein 1c involving 5-lipoxy-genase[J]. Acta Pharmacol Sin, 2010, 31:367-374. |
| [75] | Jiang W, Wang XW, Unger T, et al. Cooperation of tumor-derived HBx mutants and p53-249(ser) mutant in regulating cell proliferation, anchorage-independent growth and ane-uploidy in a telomerase-immortalized normal human hepatocyte-derived cell line[J]. Int J Cancer, 2010, 127:1011-1020. |
| [76] | Song K, Han C, Zhang J, et al. Epigenetic regulation of microRNA-122 by peroxisome proliferator activated recep-tor-gamma and hepatitis B virus X protein in hepatocellular carcinoma cells[J]. Hepatology, 2013, 58:1681-1692. |
| [77] | Zhang T, Zhang J, Cui M, et al. Hepatitis B virus X protein inhibits tumor suppressor miR-205 through inducing hyper-methylation of miR-205 promoter to enhance carcinogenesis[J]. Neoplasia, 2013, 15:1282-1291. |
| [78] | Wang Y, Lu Y, Toh ST, et al. Lethal-7 is down-regulated by the hepatitis B virus X protein and targets signal transducer and activator of transcription 3[J]. J Hepatol, 2010, 53:57-66. |
| [79] | Kong G, Zhang J, Zhang S, et al. Upregulated microRNA-29a by hepatitis B virus X protein enhances hepatoma cell migration by targeting PTEN in cell culture model[J]. PLoS One, 2011, 6:e19518. |
| [80] | Xu X, Fan Z, Kang L, et al. Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis[J]. J Clin Invest, 2013, 123:630-645. |
| [81] | Li JF, Dai XP, Zhang W, et al. Upregulation of microRNA-146a by hepatitis B virus X protein contributes to hepatitis development by downregulating complement factor H[J]. MBio, 2015. DOI:10.1128/mBio.02459-14. |
 2016, Vol. 51
2016, Vol. 51


