绿原酸 (5-O-caffeoylquinic acid,5-CQA) 是由咖啡酸和奎宁酸缩合而成的一个酯类化合物,由于酯化的位点不同,还可形成3-O-咖啡酰奎宁酸 (3-O-caffeoylquinic acid,3-CQA,亦称新绿原酸) 和4-O-咖啡酰奎宁酸 (4-O-caffeoylquinic acid,4-CQA,亦称隐绿原酸),3个化合物的化学结构见图 1。CQA广泛分布于中药材和食物中,如杜仲、金银花、咖啡豆、越橘、苹果以及马铃薯等[1, 2],是植物体在有氧呼吸过程中经莽草酸途径产生的一种苯丙素类化合物[3],具有多种生物学活性,如抗菌消炎[4]、抗氧化[5]、糖脂代谢调控[6]和抗肿瘤[7]等作用。研究表明,3-、4-和5-CQA的抗氧化活性与酪氨酸酶抑制活性基本相当[8],对α-淀粉酶活性的抑制作用为5-CQA > 4-CQA > 3-CQA[9]; 对β-胡萝卜素褪色酶的抑制作用为4-CQA > 3-CQA > 5-CQA; 对DNA氧化损伤的保护作用为5-CQA > 4-CQA > 3-CQA[10]。
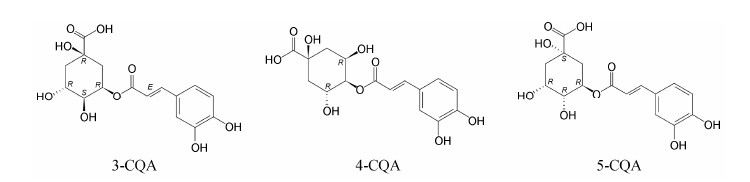 | Figure 1 Chemical structures of 3-O-caffeoylquinic acid (3-CQA), 4-O-caffeoylquinic acid (4-CQA), and 5-O-caffeoylquinic acid (5-CQA) |
5-CQA在酸性pH条件下十分稳定,但在中性和碱性pH条件以及在加热情况下,5-CQA很容易水解并生成4-CQA和3-CQA[11]。然而在中性及碱性pH条件下,三者如何转化,目前尚不清楚。本文研究了3-、4-和5-CQA在中性及碱性pH条件下的降解动力学及相互转化规律,对阐明CQA在食品、药品以及人体内的稳定性具有重要的指导意义。
材料与方法 药品和试剂绿原酸 (纯度≥98%,批号DR13313)、新绿原酸 (纯度≥98%,批号DR140331)、隐绿原酸 (纯度≥98%,批号DR140331) 和咖啡酸 (纯度≥98%,批号DR1300424) 购自上海历鼎生物技术有限公司; 葛根素 (纯度≥98%,批号753200007) 购自中国食品药品检定研究院。
仪器日本岛津高效液相色谱仪 (包括LC-20AD二元泵、SPD-20A紫外检测器、CTO-20AC柱温箱、SIL-20ACHT自动进样器、LC-Solution工作站); 德国Eppendorf 5810R型高速离心机; 德国Sartorius BP211D型电子天平; 上海伟业仪器厂pHs-2c型酸度计。
色谱条件Shim-pack ODS (150 mm × 2.0 mm,4.6 µm) 分析柱; 配Shim-pack (5 mm × 2.0 mm,4.6 µm) 保护柱; 甲醇-1% 乙酸水溶液流动相; 梯度洗脱 (0~3 min,15%甲醇; 3~11 min,20%甲醇; 11~13 min,15%甲醇); 流速0.3 mL·min-1; 柱温40 ℃; 进样量10 µL; 紫外检测波长325 nm; 样品分析时间 13 min。
缓冲液配制乙酸缓冲溶液 (pH 4.69): 精密称取NaOAc·3H2O 1.905 4 g,精密量取冰乙酸0.351 mL,用纯水定容至100 mL。磷酸缓冲液 (pH 7.05): 分别精密称取Na2HPO4·12H2O (4.370 0 g) 和NaH2PO4·2H2O (1.217 2 g),用纯水定容至100 mL。硼酸缓冲液 (pH7.96): 分别精密称取Na2B4O7·H2O (0.572 1 g) 和硼酸 (0.865 9 g),用纯水定容至100 mL。碳酸缓冲液 (pH 9.25): 分别精密称取Na2CO3·10H2O (0.286 2 g) 和NaHCO3(0.756 g),用纯水定容至100mL。
CQA降解条件取缓冲液900 µL和3-CQA (1 mg·mL-1) 甲醇溶液100 µL,振荡混匀后于37 ℃下反应。按如下时间点取样,每个时间点取反应液100µL,加入葛根素 (内标,2 mg·mL-1) 甲醇溶液 10 µL和流动相 (1% 乙酸水溶液-甲醇,4∶1) 890 µL,振荡混匀后于4 ℃、12 000 r·min-1离心10 min,取 上清液进样分析。在pH 7.05磷酸缓冲液中的取样时间点为: 0、0.5、1.5、3、4.5、6、7.5、9和10.5 h; 在pH 7.96硼酸缓冲液中的取样时间点为: 0、0.5、1、2.5、4、5、5.5、6和7.5 h; 在pH 9.25碳酸缓冲液中的取样时间点为: 0、10、30、60、90、120、150、180和210 min。4-CQA和5-CQA的处理同上。
方法学考察分别取不同浓度梯度的3-、4-和 5-CQA工作溶液10 µL,加入pH 4.69的乙酸缓冲液90 µL、葛根素 (内标,2 mg·mL-1) 10 µL和流动相 890 µL,配制8个不同浓度梯度的CQA样品,每个 浓度进行6个样本分析。以每个待测物浓度为横坐 标,待测物与内标物的峰面积之比为纵坐标,用加权 (W = 1/x2) 最小二乘法进行线性回归分析,制备标准曲线。同法,分别配制低、中、高浓度3-、4-和5-CQA质量控制 (QC) 样品,每个浓度进行6个样本分析,测定日间 (连续3天)、日内精密度和准确度值以及样品常温放置24 h后的稳定性。
结果 1 方法学考察3-、4-和5-CQA的线性回归方程、精密度、准确度和室温放置24 h后的稳定性结果见表 1,典型的色谱分离图见图 2。结果显示,3-、4-和5-CQA以及内标的分离度良好,降解物对目标化合物没有明显干扰。同时,所建方法的精密度、准确度与稳定性均满足定量分析的要求。
|
|
Table 1 Regression equation, precision, accuracy and stability of CQA in acetic buffer (pH 4.69). |
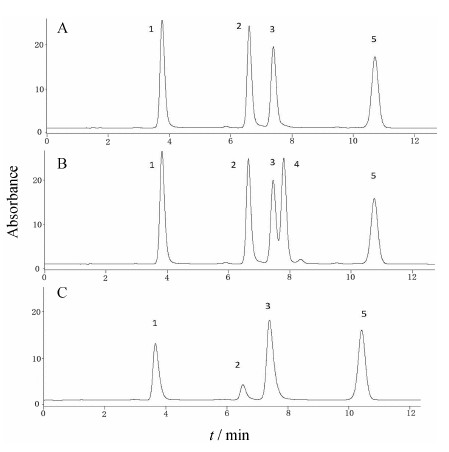 | Figure 2 Typical chromatograms of 3-, 4-, 5-CQA and puerarin (IS). A: CQA (5 µg·mL−1) and IS (20 µg·mL−1) in acetic buffer (pH 4.69); B: CQA (5 µg·mL−1), caffeic acid (5 µg·mL−1), and IS (20 µg·mL−1) in acetic buffer (pH 4.69); C: Degradation of 4-CQA (10 µg·mL−1) at 37 ℃ and pH 7.05 for 6 h. 1: 3-CQA; 2: 5-CQA; 3: 4-CQA; 4: Caffeic acid; 5: IS |
3-、4-和5-CQA在37 ℃、不同pH缓冲液 (pH 7.05、7.96和9.25) 中的降解曲线见图 3。图中实心曲线是应用Origin8.0软件,利用Weibull Model拟合得到的3-、4-和5-CQA在不同pH值缓冲液中的降解动力学曲线。Weibull分布是一个概率分布函数,可用来拟合化合物的降解动力学过程[12, 13, 14]。
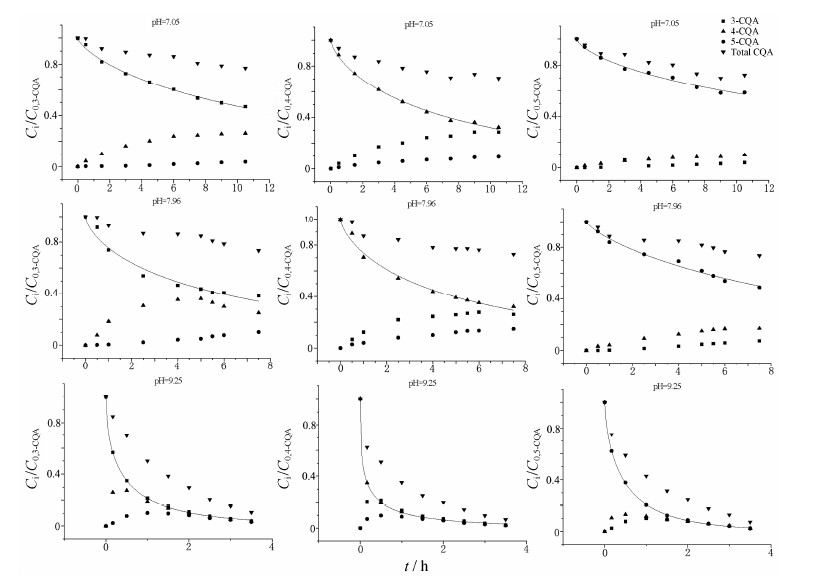 | Figure 3 Degradation of 3-CQA, 4-CQA and 5-CQA (all at 10 µg·mL−1) at 37 ℃ and pH 7.05, 7.96 and 9.25. Cirepresents concentration of 3-CQA at indicated incubation time (n = 3). C0 represents the initial concentration of them. Solid curves of the compounds were treated by Origin8.0 software using Weibull model to calculate kinetic parameters of k and n |
方程(1) 是常用来描述化合物降解动力学的Weibull方程:
| ${C_i}/{C_0} = \exp [ - {(kt)^n}]$ | (1) |
式中k是反应速率常数,表示化合物的降解快慢; n是形状常数,当n > 1时,该函数是一个增函数; 当n < 1时,该函数是一个减函数; 当n = 1时,该函 数满足指数分布[13]。Ci表示CQA在某一时刻的浓 度,C0表示CQA的初始反应浓度。根据方程 (1),应用Origin8.0软件进行非线性最小二乘拟合,可计算得到降解参数k和n值。根据方程 (2),可计算得到降解半衰期 (t1/2):
| ${t_{1/2}} = {(0.693)^{1/n}}/k$ | (2) |
在本实验条件下,3-、4-和5-CQA在不同pH值缓冲液中的降解动力学参数k、n、r2和t1/2见表 2。
|
|
Table 2 Kinetic parameters of CQA degraded at 37 ℃ and neutral and alkaline pH values |
本文研究表明,3-、4-和5-CQA的稳定性与pH值有关,在酸性条件下较为稳定,在中性和碱性条件下不稳定,易分解并转化成其他两个同分异构体,且随碱性的增强,三者的降解愈发强烈,转化成的CQA总量就越低,但三者的稳定性与相互转化各有特点。此外,3-、4-和5-CQA之间的相互转化趋势在pH 7.05~9.25时相似,即3-CQA和5-CQA在水解时更趋向于转化为4-CQA,而4-CQA水解时更趋向于转化为3-CQA,而非5-CQA。同时,3-、4-和5-CQA水解并没有产生咖啡酸,说明CQA降解生成了其他物质。
比较中性和碱性pH条件下三者的降解动力学参数k、n和t1/2值表明,在中性和碱性缓冲液中,随着pH值的增大,k值增大、t1/2值减小,说明随着pH值的增大,3-、4-和5-CQA的稳定性降低。同时,k值的大小顺序为4-CQA > 3-CQA > 5-CQA,t1/2的大小顺序则相反,说明在中性及碱性环境下,三者的稳定顺序为4-CQA < 3-CQA < 5-CQA。
| [1] | Clifford MN. Chlorogenic acids and other cinnamates-nature, occurrence and dietary burden [J]. J Sci Food Agric, 1999, 79: 362-372. |
| [2] | Gonthier MP, Verny MA, Besson C, et al. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats [J]. J Nutr, 2001, 131: 66-71. |
| [3] | Gao JM, Zhang AL, Zhang KJ, et al. Review of chlorogenic acid distribution, extraction and biological activity [J]. J Northwest For Univ (西北林学院学报), 1999, 14: 73-82. |
| [4] | Gao HS, Satsu H, Bae M, et al. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice [J]. Food Chem, 2015, 168: 167-175. |
| [5] | Yun N, Kang JW, Lee SM. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties [J]. J Nutr Biochem, 2012, 23: 1249-1255. |
| [6] | Ong KW, Hsu A, Tan BK. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation [J]. Biochem Pharmacol, 2013, 85: 1341-1351. |
| [7] | Kurata R, Adachi M, Yamakawa O, et al. Growth suppression of human cancer cells by polyphenolics from sweet potato (Ipomoea batatas L.) leaves [J]. J Agric Food Chem, 2007, 55: 185-190. |
| [8] | Iwai K, Kishimoto N, Kakino Y, et al. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans [J]. J Agric Food Chem, 2004, 52: 4893-4898. |
| [9] | Narita Y, Inouye K. Inhibitory effects of chlorogenic acids from green coffee beans and cinnamate derivatives on the activity of porcine pancreas α-amylase isozyme I [J]. Food Chem, 2011, 127, 1532-1539. |
| [10] | Xu JG, Hu QP, Liu Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers [J]. J Agric Food Chem, 2012, 60: 11625-11630. |
| [11] | Trugo LC, Macrae R. Chlorogenic acid composition of instant coffees [J]. Analyst, 1984, 109: 263-266. |
| [12] | Narita Y, Inouye K. Degradation kinetics of chlorogenic acid at various pH values and effects of ascorbic acid and epigallocatechin gallate on its stability under alkaline conditions [J]. J Agric Food Chem, 2013, 61: 966-972. |
| [13] | Cunha LM, Oliveira FA, Oliveira JC. Optimal experimental design for estimating the kinetic parameters of processes described by the Weibull probability distribution function [J]. J Food Eng, 1998, 37: 175-191. |
| [14] | Watanabe Y, Okayasu T, Idenoue K, et al. Degradation kinetics of catechin in aqueous solution in the presence of ascorbic acid or octanoyl ascorbate [J]. Jpn J Food Eng, 2009, 10: 117-124. |
 2016, Vol. 51
2016, Vol. 51


