帕金森病 (Parkinson’s disease, PD) 是居于阿 兹海默病后的第2位最常见的神经退行性疾病, 其 主要病理学特征是在黑质中多巴胺能神经元细胞 死亡, 黑质纹状体通路退化[1]。此外, 受损的多巴胺能神经元胞浆内存在着路易小体 (Lewy body, LB), 其内主要包含异常的或者聚集体形式的α-突触核蛋白(α-synuclein)[2]。脑内产生多巴胺的细胞逐渐丧失了影响神经系统的功能, 使患者控制肌肉的能力受限。在临床上, PD具有一些核心运动症状, 统称为震颤性麻痹, 包括静止性震颤、运动迟缓、肌强直、姿势不稳和步态障碍。此外, 临床描述的PD还包括几种非运动性症状, 如执行功能障碍、自主神经系统功能障碍、睡眠障碍、行为和精神方面的改变以及嗅觉障碍等[2, 3, 3]。据2013年美国国立PD基金会统计, 全世界有400万~600万名PD患者, 在工业化国家中流行度接近总人口的0.3%。据估计, 中国PD患者超过200万名, 其中65岁以上发病率约为1.7%。基于世界人口的老龄化, 未来PD流行度将有明显的增加, 对世界10个人口最多国家和西欧5个人口最多国家的研究表 明, 这15个国家2030年PD患者的人数将达到870万~930万, 约为目前发病人数的两倍[5]。PD是一种进行性的、无法治愈的神经系统疾病, 尚未发现有延缓或停止疾病进展的确切疗法。现今PD治疗手段包括药物治疗和非药物治疗, 非药物治疗包括手术、教育和运动康复等策略, 有助于持续缓解症状并维持功能。在药物治疗领域现已有较大进展, 这些药物能够缓解症状和疾病进展, 如作用于谷氨酸受体的药物和儿茶酚-O-甲基转移酶 (catechol-O- methyltransferase , COMT) 抑制剂等[6]。
2 自噬与PD自噬是一个进化上保守的过程, 能够介导细胞内长寿命蛋白以及受损或多余细胞器的降解。自噬可由多种因素诱发, 包括有限的营养物质、低氧水平以及能量供给不足等, 其结果导致降解产物的释放, 例如氨基酸可被重新释放到细胞质内用于必需的生物合成途径[7]。根据内容物运送到溶酶体的途径的不同, 自噬可分为三种类型: 大自噬 (macroautophagy)、小自噬 (microautophagy) 以及分子伴侣介导的自噬 (chaperone-mediated autophagy, CMA), 其中最引人注目的是大自噬过程, 以下简称为自噬。在自噬过程中, 部分细胞质被双层膜的吞噬泡吞噬, 随后扩大成为自噬小体, 最后完整的自噬小体被运送到溶酶体进行降解[8]。近年来的研究已揭示了自噬的主要分子机制, 相关的主要自噬蛋白可被分为四组: ① Atg1/ unc-51-like kinase (ULK) 复合物[9, 10]; ② 两种泛素样蛋白共轭系统 (Atg12和Atg8/LC3)[11, 12]; ③ III 型磷脂酰肌醇3-激酶 (class III phosphatidylinositol 3-kinase, PtdIns3K)/Vps34 复合物I (含Beclin-1、Atg14和Vps15)[13]; ④ Atg9/mATG9跨膜蛋白系统[14]。雷帕霉素(rapamycin) 的靶标mTOR (mammalian target of rapamycin) 是主要调控自噬的关键组分之一[15]。此外, 一些其他激酶如蛋白激酶A、AMPK (AMP- activated protein kinase)/Snf1和Pho85也能在其他条件下调节自噬[16]。近年来的证据表 明自噬或许在PD的发病机制中有着重要作用, 在许多PD患者或者动物模型中都观察到了异常的自噬水平[17, 18, 19, 20]。此外, 一些PD相关蛋白, 如α-synuclein、Parkin和PINK1等都被发现能够参与自噬的调控[21]。
3 PD中的自噬相关通路 3.1 α-Synuclein与自噬细胞质内的α-synuclein聚集体是PD最显著的病理特征之一。α-Synuclein的水平高低是其细胞毒性的主要决定因素, 它的过表 达或缺乏降解将导致多巴胺能神经元的退行性病变。编码α-synuclein的基因的错义突变如A53T、A30P、E46K和H50Q, 以及一些翻译后修饰如磷酸化、泛素化、硝化、氧化和多巴胺依赖的加合物的形成等都会产生不同程度的神经毒性[ [22, 23, 24, 25, 26, 27, 28, 29, 30]]。α-Synuclein主要由泛素蛋白酶复合体系统(ubiquitin-proteasome system, UPS) 和自噬溶酶体途径(autophagy-lysosomal pathway, ALP) 降解[31], 后者包括CMA和大自噬。然而过多的α-synuclein以及该蛋白的突变或修饰形式会阻遏UPS以及CMA降解途径, 从而导致细胞质内毒性累积[ [32, 33]]。此时, 自噬能够作为一个补偿途径来降解α- synuclein, 从而具有神经保护作用; 然而一些研究也显示, 在压力条件下/span>, 有可能导致自噬性死亡[34, 35, 36]。此外, 异常的α-synuclein也被发现能够抑制自噬的发生。α-Synuclein通过抑制Rab1a的活性, 导致Atg9定位异常以及抑制自噬小体的形成, 从而抑制自噬发生[37]。在SH-SY5Y细胞模型中, A53T α-synuclein被发现能够上调mTOR信号, 导致自噬被抑制。同时, 通过siRNA使mTOR表 达沉默, 能够恢复自噬水平并且减少α-synuclein的积累[38]。在另一些细胞模型中, E46K突变能抑制JNK1的激活, 导致Bcl-2磷酸化减少, 更多的Bcl-2与Beclin-1结合, 进一步阻止了Beclin-1/hVps34的形成, 从而抑制了自噬。研究还发现E46K突变细胞更易遭受毒性损伤, 猜测其原因是自噬的受损[39]。此外, 利用A53T转基因小鼠研究发现, α-synuclein能够定位到线粒体, 并导致线粒体自噬的发生, 此时的线粒体自噬会导致线粒体包涵物 (mitochondrial inclusions) 的形成, 而这些受损的线粒体不能通过自噬被有效清除, 最终可能导致神经退行性变化[40]。之前有研究表 明, α-synuclein在正常情况下不会损伤线粒体的功能, 但是随着年龄增长或在病理条件下, α-synuclein会影响线粒体的正常功能[41], 而线粒体失调也是PD的另一显著特征, 后文中将会进行介绍。
3.2 线粒体自噬和PINK1-Parkin最近的研究表 明, 线粒体功能失调可能同时与家族性PD和散发性PD的发病机制有关。在所有真核细胞中, 线粒体通过氧化磷酸化作用提供90%以上的细胞所需能量, 是主要的供能细胞器[42]; 此外, 线粒体的功能还涉及到体内钙平衡和凋亡的调控[43]。然而, 线粒体也是细胞内活性氧簇 (reactive oxygen species, ROS) 的主要来源。由于细胞内抗氧化剂的存在, 可以容许正常水平的ROS产生, 然而在线粒体呼吸缺陷的病理条件下, 会产生超过抗氧化剂保护能力的ROS水平, 并导致线粒体等许多细胞组分的损伤, 而这些损伤与衰老、癌症以及包括PD在内的神经退行性疾病密切相关[44], 许多证据已表 明过高的氧化损伤会导致多巴胺能神经元的死亡。在家族性PD中, 一些PD相关基因 (如PARK2、PARK6和PARK7等) 已被报道与线粒体功能和线粒体自噬密切相关。线粒体自噬即是选择性地通过自噬降解线粒体, 尤其是受损的线粒体, 而在一些PD模型中, 线粒体功能是受损的, 这可能与PD的病理发生有关[45, 46, 47]。PINK1是PARK6基因编码的产物, 而Parkin是PARK2基因编码的产物, 二者都与线粒体功能密切相关, PINK1处于Parkin的上游, 能够诱导线粒体自噬[48, 49, 50, 51]。PINK1的C端有一段预测的激酶结构域, N端有一段线粒体靶向序列。PINK1特异性地积累在受损的线粒体上。在健康的线粒体中, PINK1被快速地降解, 而受损的线粒体则不能降解PINK1, 使之聚集在受损线粒体的外膜。PINK1积累后, 它能够磷酸化Parkin并激活Parkin的E3连接酶活性[52]。被PINK1招募的Parkin如何能够促进线粒体的自噬降解仍然未知, 有研究表 明Parkin或许能够使线粒体上的某些特定底物泛素化, 这些泛素化的底物将作为p62/SQSTM1识别的靶标, 从而使线粒体被招募到自噬泡, 并最终通过自噬被降解[53]。一些随后的研究还表 明, 编码PINK1和Parkin的基因突变会导致线粒体自噬受到抑制, 随之受损的线粒体累积在细胞内, 可能会启动凋亡事件, 最终导致神经退行性变化[54]。此外, 其他证据也表 明了PINK1参与自噬途径, 通过与Beclin-1结 合, PINK1显著增强了基础水平以及饥饿诱导的自噬, 而PINK1的突变形式则不能促进自噬, 因为它们不能与Beclin-1结合或者缺乏酶活性[55]。
3.3 其他PD相关基因和自噬除了SNCA (编码α-synuclein)、PARK2和PARK6之外, 还有一些PD相关基因也在自噬或者线粒体功能中有重要作用。PARK8 (编码LRRK2) 的突变与最常见的常染色体显性遗传PD有关, 一些散发性的PD患者中也发现有该基因的突变[56, 57]。有研究表 明LRRK2在自噬中发挥作用, 然而不同突变形式的LRRK2对自噬的影响可能是不同的。R1441C突变会损害自噬平衡, 而LRRK2敲减能够提高自噬活性, 并且在饥饿条件下通过诱导自噬而抑制细胞死亡[58]。此外, G2019S突变能够通过与线粒体膜蛋白Bcl-2反应来诱导线粒体自噬[59]。另自噬缺陷会引起LRRK2蛋白水平的提高并使之聚集在脑内[60]。LRRK2与α-synuclein在一些情况下是相关的。一方面, 在Y1699C或G2019S突变患者诱导的iPS多巴胺能神经元中检测到α-synuclein表 达上升; 另一方面, LRRK2过表 达会促进α-synuclein异常聚集, 从而增强α-synuclein介导的病理发生[61, 62]。然而LRRK2相关的PD并不总是以α-synuclein聚 集为特征, 也可表 现为沉积的MAPT/tau蛋白和泛素阳性包涵体[63]。有理论推测LRRK2失调可能作用于SNCA和MAPT的上游, 而哪一种病理状态会随后发展则取决于其他遗传因素或者环境因素[64], 但是这种理论还为时过早, 可能需要更多论证。
PARK7/DJ-1的突变是造成常染色体隐性遗传PD的原因之一。早期的研究表 明DJ-1的缺失会损伤基础水平的自噬, 同时造成有缺陷的线粒体积累[65, 66]。然而随后研究的发现与之相悖, DJ-1的缺失会促进自噬活性, 并且观察到了增多的自噬标志物[67, 68, 69]。另一项研究还表 明DJ-1或许是通过维持线粒体的功能来间接地影响自噬, DJ-1的缺失降低了NF-κB信号, 减少mTOR对自噬的抑制[70]。此外, DJ-1敲减诱导的自噬能够通过抑制JNK而被抑制, 说明DJ-1还有可能是通过JNK通路来调控自噬的[71]。除此之外, 还有一些PD相关基因的编码产物涉及到自噬的调控中, 如UCL-L1、ATP13A2和GBA[72, 73, 74] (图 1)。
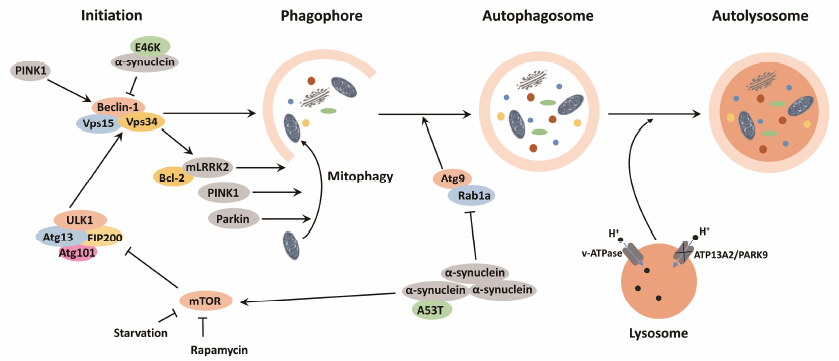
|
Figure 1 Role of PD-associated proteins in autophagy and targeting autophagy pathway for PD therapy. As illustrated, the process of autophagy can be divided into four steps: ① autophagy initiation; ② formation of phagosome; ③ formation of autophagosome; ④ fusion with lysosome and degradation. PD-related proteins can affect the process of autophagy via different pathways: over-expression of α- synuclein compromises autophagy via Rab1a inhibition, which results in the alteration of the autophagy protein Atg9 localization and the inhibition of autophagosome formation[37]. A53Tα-synuclein impairs autophagy in SH-SY5Y cells by upregulating mTOR signaling[38]. Overexpressed E46K α-synuclein inhibits JNK1 activation, leading to a reduced Bcl-2 phosphorylation and increased association between Bcl-2 and Beclin-1, further disrupting the formation of Beclin-1/hVps34 complex, which is essential for autophagy initiation[39]. PINK1 and Parkin play crucial roles in clearing damaged mitochondria, and mutations of PINK1 and Parkin may impede mitophagy[54]. PINK1 can enhance autophagy by interacting with Beclin-1, but mutations of PINK1 lose this function[55]. LRRK2 can regulate autophagy either positively or negatively, while its molecular mechanism still remains unclear. According to a recent study, LRRK2 G2019S mutation can induce mitophagy by interacting with Bcl-2[59]. ATP13A2 mutations cause a pH elevation in lysosome, which leads to decreased activity of lysosomal protease, thus inhibiting the fusion of autophagosome and lysosome[73] |
目前, PD仍旧是一个不可治愈的影响全球数百万人的疾病。现在, 有许多治疗手段被应用于PD, 包括药物、手术和深部脑刺激等[6]。由于PD的病因仍然不明, 可采取的疾病治疗手段主要集中于通过提高相关脑内区域的多巴胺水平来实现神经细胞的保护和症状控制, 然而基于多巴胺的治疗策略常伴随着一系列的不良反应或是无明显治疗效果[75]。现在, 由于治疗手段的缺乏和日益上升的全球患病人数, 对新的能够有效治疗PD的策略的需求极为迫切。随着不断有证据表 明自噬在PD中的重要角色, 靶向自噬来治疗PD提供了一个新的有前途的方向。
目前, 在临床前研究中, 已有大量化合物被报道能够在PD模型中通过调节自噬而达到缓解症状的目的 (表 1)。首先, 自噬促进剂已在一些研究中展现出其应用前景。在SH-SY5Y细胞中, 天然化合物curcumin能够通过下调mTOR信号恢复自噬, 从而有效减少A53T α-synuclein的积累[38]。Rapamycin通过抑制mTOR和诱导Bcl-2来引起自噬, 并且在蛋白 酶体抑制剂诱导的神经退行性病变中展现了神经保护的作用[76]。两种葡糖苷 (脂) 酰鞘氨醇合成酶抑制剂PPMP和Genz-123346通过AKT-mTOR依赖的 途径增强了自噬, 显著降低神经元中的α-synuclein水平[77]。KYP-2047, 一种脯氨酰寡肽 (PREP) 抑制剂, 通过Beclin-1依赖的途径来增强自噬以清除α- synuclein[78, 79]。酪氨酸激酶Abl抑制剂尼洛替尼 (nilotinib), 一种被用于治疗白血病的药物, 在小鼠中能够刺激α-synuclein的自噬性清除 (Beclin-1依赖) 并保护神经元, 提高多巴胺水平, 从而提高小鼠的运动机能[80]。在鱼藤酮诱导的SH-SY5Y细胞和过表 达A30P或A53T α-synuclein的PC12细胞系中, 白藜芦醇 (resveratrol) 通过激活AMPK/SIRT1通路来增强自噬, 以促进α-synuclein降解[81]。除以上已有明确自噬作用机制的化合物之外, 还有许多化合物也能激活自噬而展现细胞保护作用, 使之成为PD治疗的候选药物。海藻糖 (trehalose) 作为不依赖mTOR的自噬诱导剂, 能够加强对A30P和A53T α-synuclein的清除能力, 同时保护神经元免于十字孢碱诱导的细胞死亡[82]。甲哌氟丙嗪 (trifluoperazine), 一种吩噻嗪类抗精神病药, 激活了LUHMES神经元中的自噬, 并使其免于α-synuclein介导的细胞死亡[83, 84]。类似地, latrepirdine 缓解了分化的SH-SY5Y神经元和小鼠大脑中的α-synuclein降解情况, 与之同时伴随有增强的自噬现象[85]。两种自噬促进剂丙戊酸钠 (VPA) 和卡马西平 (CBZ) 可以增强SH-SY5Y细胞在鱼藤酮毒性下的存活能力[86]。一种从山葡萄中分离的化合物amurensin G , 也通过诱导自噬增强了SH-SY5Y细胞在鱼藤酮毒性下的存活能力[87]。Chebulagic acid被报道通过增强自噬来保护SH-SY5Y细胞免受MPP+的毒性[88]。类似地, 天然化合物Paeoniflorin也通过诱导自噬增强了PC12细胞在MPP+下的存活能力[89]。此外, 在C57BL/6小鼠PD模型中联合使用VPA和碳酸锂 (lithium carbonate), 减少MPTP导致的多巴胺能神经元的死亡, 而该治疗效果很可能是由于自噬途径的激活[90]。以上研究表 明, 通过自噬激活来增强对异常α-synuclein的清除, 进而保护神经元细胞免于死亡, 可能成为一种有效的治疗手段来缓解PD症状。
|
|
Table 1 Autophagy-modulating compounds in PD models |
除自噬促进剂之外, 在其他的研究中, 也有少数化合物与PD中的死亡性自噬相关。PD相关蛋白引起的自噬通路异常也包括细胞的死亡性自噬, 因此自噬抑制剂将可能对该种条件下的PD症状有治疗 效果。一种Drp抑制剂P110可通过降低死亡性自噬来提高细胞的生存能力, 同时减少了PD模型中多巴胺能神经元的损失[91, 92]。在6-OHDA诱导的PD模 型中, N-{3-[2-(4-phenyl-piperazin-1-yl)-ethyl]-phenyl}- picolinamide激活AKT, 从而抑制了由6-OHDA导致的自噬性死亡和凋亡[93]。β-Asarone通过下调JNK通路, 间接抑制Beclin-1, 进而保护细胞免于6-OHDA导致的自噬性死亡[94]。
以上证据有力地表 明了自噬调节化合物将在PD治疗中具有极好的应用前景, 然而从目前的研究看, 大部分化合物还未被确认其精确作用机制。就关键自噬药物靶点而言, mTOR通路和Beclin-1复合物这两个重要自噬开关可能成为良好靶点, 目前已有明确机制的化合物也大多靶向它们, 如curcumin等。靶点的评估可从两方面入手: ① 作为重要的自噬调节子, mTOR和Beclin-1均在自噬中起不可或缺的作用。② 目前已阐明的PD相关蛋白在自噬通路中的作用多涉及这两个蛋白, 如A53T α-synuclein上调mTOR来抑制自噬, E46K α-synuclein通过JNK通路抑制Beclin-1复合物, 靶向这类蛋白更具有PD针对性。因此, 从这两方面入手, 在自噬和PD蛋白相关通路中寻找关键药物靶点是药物开发的关键。同时随着研究的深入, 自噬在PD中的相关机制将会进一步被阐明, 更多的自噬靶点将会被发现。然而, 靶向自噬治疗PD仍处于萌芽阶段, 目前正在研发中的PD治疗药物主要包括COMT抑制剂、信号转导调节剂和基因治疗药物等, 并没有直接靶向自噬通路的药物处于临床研究; 而已经上市的PD药物中也并未包括自噬靶向药物。尽管如此, 已有的PD模型中自噬调节化合物已经为自噬靶向药物开发提供了极为有利的基础。与其他种类药物相比, 对于这类化合物的开发较为稀少, 研究者应当投入更多的精力来设计和开发靶向自噬的PD治疗候选化合物。
5 小结PD是世界上最常见的神经退行性疾病之一, 目前并没有非常有效的治疗手段。最新的证据表 明自噬与PD的发病机制密切相关, 许多自噬相关基因编码的蛋白如α-synuclein、PINK1和Parkin等都被发现涉及自噬的调控。许多PD模型研究表 明, 自噬可作为一种补偿性的途径降解异常的α-synuclein积累, 从而缓解PD相关症状, 一些化合物也已被报道可通过调节自噬来缓解症状。由此, 靶向自噬途径来治疗PD为研究者提供了一个新的方向, 尽管目前并没有直接靶向于自噬关键分子来治疗PD的药物被报道。同时由于死亡性自噬也可能存在, 靶向自噬途径则需要更加慎重。此外, PD的发病机制尚不明确, 一些PD相关基因在自噬中的角色也尚不可知。随着新兴技术如基因组学和蛋白质组学的发展, 或许更多的PD相关蛋白和生物标志物会被发现, 可能为PD药物设计提供更多的靶标。总之, 在自噬领域存在着许多可能性, 将为PD治疗提供新的希望。
| [1] | Damier P, Hirsch EC, Agid Y, et al. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease [J]. Brain, 1999, 122: 1437- 1448. |
| [2] | Shulman JM, De Jager PL, Feany MB. Parkinson's disease: genetics and pathogenesis [J]. Annu Rev Pathol, 2011, 6: 193-222. |
| [3] | Fahn S. Description of Parkinson's disease as a clinical syndrome [J]. Ann N Y Acad Sci, 2003, 991: 1-14. |
| [4] | Driver-Dunckley E, Adler CH, Hentz JG, et al. Olfactory dysfunction in incidental Lewy body disease and Parkinson's disease [J]. Parkinsonism Relat Disord, 2014, 20: 1260- 1262. |
| [5] | Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030 [J]. Neurology, 2007, 68: 384- 386. |
| [6] | Thomson Reuters. Disease briefing: Parkinson's disease [J]. J Int Pharm Res (国际药学研究杂志), 2015, 42: 338-345. |
| [7] | Huang J, Klionsky DJ. Autophagy and human disease [J]. Cell Cycle, 2007, 6: 1837-1849. |
| [8] | Majeski AE, Dice JF. Mechanisms of chaperone-mediated autophagy [J]. Int J Biochem Cell Biol, 2004, 36: 2435- 2444. |
| [9] | Mercer CA, Kaliappan A, Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy [J]. Autophagy, 2009, 5: 649-662. |
| [10] | Jung CH, Jun CB, Ro SH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery [J]. Mol Biol Cell, 2009, 20: 1992-2003. |
| [11] | Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. ‘Protein modifications: beyond the usual suspects' review series [J]. EMBO Rep, 2008, 9: 859-864. |
| [12] | Mizushima N, Kuma A, Kobayashi Y, et al. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate [J]. J Cell Sci, 2003, 116: 1679-1688. |
| [13] | Yuan HX, Russell RC, Guan KL. Regulation of PIK3C3/ VPS34 complexes by mTOR in nutrient stress-induced autophagy [J]. Autophagy, 2013, 9: 1983-1995. |
| [14] | Suzuki SW, Yamamoto H, Oikawa Y, et al. Atg13 HORMA domain recruits Atg9 vesicles during autophagosome formation [J]. Proc Natl Acad Sci U S A, 2015, 112: 3350-3355. |
| [15] | Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1 [J]. Nat Cell Biol, 2011, 13: 132-141. |
| [16] | Klionsky DJ, Baehrecke EH, Brumell JH, et al. A comprehensive glossary of autophagy-related molecules and processes (2nd ed) [J]. Autophagy, 2011, 7: 1273-1294. |
| [17] | Zavodszky E, Seaman MN, Moreau K, et al. Mutation in VPS35 associated with Parkinson's disease impairs WASH complex association and inhibits autophagy [J]. Nat Commun, 2014, 5: 3828. |
| [18] | Ahmed I, Liang Y, Schools S, et al. Development and characterization of a new Parkinson's disease model resulting from impaired autophagy [J]. J Neurosci, 2012, 32: 16503- 16509. |
| [19] | Shen YF, Tang Y, Zhang XJ, et al. Adaptive changes in autophagy after UPS impairment in Parkinson's disease [J]. Acta Pharmacol Sin, 2013, 34: 667-673. |
| [20] | Manzoni C, Mamais A, Dihanich S, et al. Pathogenic Parkinson's disease mutations across the functional domains of LRRK2 alter the autophagic/lysosomal response to starvation [J]. Biochem Biophys Res Commun, 2013, 441: 862-866. |
| [21] | Li J, Li S, Zhang L, et al. Deconvoluting the complexity of autophagy and Parkinson's disease for potential therapeutic purpose [J]. Oncotarget, 2015, 6: 40480-40495. |
| [22] | Auluck PK, Caraveo G, Lindquist S. α-Synuclein: membrane interactions and toxicity in Parkinson's disease [J]. Annu Rev Cell Dev Biol, 2010, 26: 211-233. |
| [23] | Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease [J]. Science, 1997, 276: 2045-2047. |
| [24] | Krüger R, Kuhn W, Müller T, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease [J]. Nat Genet, 1998, 18: 106-108. |
| [25] | Zarranz JJ, Alegre J, Gómez-Esteban JC, et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia [J]. Ann Neurol, 2004, 55: 164-173. |
| [26] | Chartier-Harlin MC, Kachergus J, Roumier C, et al. α-Synuclein locus duplication as a cause of familial Parkinson's disease [J]. Lancet, 2004, 364: 1167-1169. |
| [27] | Singleton AB, Farrer M, Johnson J, et al. α-Synuclein locus triplication causes Parkinson's disease [J]. Science, 2003, 302: 841. |
| [28] | Maraganore DM, de Andrade M, Elbaz A, et al. Collaborative analysis of α-synuclein gene promoter variability and Parkinson disease [J]. JAMA, 2006, 296: 661-670. |
| [29] | Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation [J]. Nature, 2011, 477: 107-110. |
| [30] | Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease [J]. Mov Disord, 2013, 28: 811-813. |
| [31] | Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, et al. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein [J]. J Neurosci, 2011, 31: 14508-14520. |
| [32] | Webb JL, Ravikumar B, Atkins J, et al. α-Synuclein is degraded by both autophagy and the proteasome [J]. J Biol Chem, 2003, 278: 25009-25013. |
| [33] | Cuervo AM, Stefanis L, Fredenburg R, et al. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy [J]. Science, 2004, 305: 1292-1295. |
| [34] | Stefanis L, Larsen KE, Rideout HJ, et al. Expression of A53T mutant but not wild-type α-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death [J]. J Neurosci, 2001, 21: 9549-9560. |
| [35] | Xilouri M, Vogiatzi T, Vekrellis K, et al. Alpha-synuclein degradation by autophagic pathways: a potential key to Parkinson's disease pathogenesis [J]. Autophagy, 2008, 4: 917-919. |
| [36] | Ebrahimi-Fakhari D, McLean PJ, Unni VK. Alpha-synuclein's degradation in vivo: opening a new (cranial) window on the roles of degradation pathways in Parkinson disease [J]. Autophagy, 2012, 8: 281-283. |
| [37] | Lynch-Day MA, Mao K, Wang K, et al. The role of autophagy in Parkinson's disease [J]. Cold Spring Harb Perspect Med, 2012, 2: a009357. |
| [38] | Jiang TF, Zhang YJ, Zhou HY, et al. Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson's disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy [J]. J Neuroimmune Pharmacol, 2013, 8: 356-369. |
| [39] | Yan JQ, Yuan YH, Gao YN, et al. Overexpression of human E46K mutant α-synuclein impairs macroautophagy via inactivation of JNK1-Bcl-2 pathway [J]. Mol Neurobiol, 2014, 50: 685-701. |
| [40] | Chen L, Xie Z, Turkson S, et al. A53T human α-synuclein overexpression in transgenic mice induces pervasive mitochondria macroautophagy defects preceding dopamine neuron degeneration [J]. J Neurosci, 2015, 35: 890-905. |
| [41] | Bendor JT, Logan TP, Edwards RH. The function of α-synuclein [J]. Neuron, 2013, 79: 1044-1066. |
| [42] | McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse [J]. Curr Biol, 2006, 16: R551- R560. |
| [43] | Celsi F, Pizzo P, Brini M, et al. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration [J]. Biochim Biophys Acta, 2009, 1787: 335-344. |
| [44] | Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine [J]. Annu Rev Genet, 2005, 39: 359-407. |
| [45] | Hedrich K, Marder K, Harris J, et al. Evaluation of 50 probands with earlyonset Parkinson's disease for Parkin mutations [J]. Neurology, 2002, 58: 1239-1246. |
| [46] | Hattori N, Kitada T, Matsumine H, et al. Molecular genetic analysis of a novel Parkin gene in Japanese families with autosomal recessive juvenile parkinsonism: evidence for variable homozygous deletions in the Parkin gene in affected individuals [J]. Ann Neurol, 1998, 44: 935-941. |
| [47] | Valente EM, Bentivoglio AR, Dixon PH, et al. Localization of a novel locus for autosomal recessive early-onset parkinsonism, PARK6, on human chromosome 1p35-p36 [J]. Am J Hum Genet, 2001, 68: 895-900. |
| [48] | Shimura H, Hattori N, Kubo S, et al. Familial Parkinson disease gene product, parkin, is an ubiquitin-protein ligase [J]. Nat Genet, 2000, 25: 302-305. |
| [49] | Li Y, Tomiyama H, Sato K, et al. Clinicogenetic study of PINK1 mutations in autosomal recessive early-onset parkinsonism [J]. Neurology, 2005, 64: 1955-1957. |
| [50] | Rohé CF, Montagna P, Breedveld G, et al. Homozygous PINK1 C-terminus mutation causing early-onset parkinsonism [J]. Ann Neurol, 2004, 56: 427-431. |
| [51] | Kann M, Jacobs H, Mohrmann K, et al. Role of parkin mutations in 111 community-based patients with early-onset parkinsonism [J]. Ann Neurol, 2002, 51: 621-625. |
| [52] | Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease [J]. Neuron, 2015, 85: 257-273. |
| [53] | Koyano F, Matsuda N. Molecular mechanisms underlying PINK1 and Parkin catalyzed ubiquitylation of substrates on damaged mitochondria [J]. Biochim Biophys Acta, 2015, 1853: 2791-2796. |
| [54] | Vives-Bauza C, Zhou C, Huang Y, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy [J]. Proc Natl Acad Sci U S A, 2010, 107: 378-383. |
| [55] | Michiorri S, Gelmetti V, Giarda E, et al. The Parkinson- associated protein PINK1 interacts with Beclin1 and promotes autophagy [J]. Cell Death Differ, 2010, 17: 962-974. |
| [56] | Gilks WP, Abou-Sleiman PM, Gandhi S, et al. A common LRRK2 mutation in idiopathic Parkinson's disease [J]. Lancet, 2005, 365: 415-416. |
| [57] | Verstraeten A, Theuns J, Van Broeckhoven C. Progress in unraveling the genetic etiology of Parkinson disease in a genomic era [J]. Trends Genet, 2015, 31: 140-149. |
| [58] | Alegre-Abarrategui J, Christian H, Lufino MM, et al. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model [J]. Hum Mol Genet, 2009, 18: 4022-4034. |
| [59] | Su YC, Guo X, Qi X. Threonine 56 phosphorylation of Bcl-2 is required for LRRK2 G2019S-induced mitochondrial depolarization and autophagy [J]. Biochim Biophys Acta, 2015, 1852: 12-21. |
| [60] | Lachenmayer ML, Yue Z. Genetic animal models for evaluating the role of autophagy in etiopathogenesis of Parkinson disease [J]. Autophagy, 2012, 8: 1837-1838. |
| [61] | Daher JP, Volpicelli-Daley LA, Blackburn JP, et al. Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats [J]. Proc Natl Acad Sci U S A, 2014, 111: 9289-9294. |
| [62] | Lin X, Parisiadou L, Gu XL, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant α-synuclein [J]. Neuron, 2009, 64: 807-827. |
| [63] | Houlden H, Singleton AB. The genetics and neuropathology of Parkinson's disease [J]. Acta Neuropathol, 2012, 124: 325-338. |
| [64] | Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson's disease [J]. Nat Rev Neurosci, 2010, 11: 791-797. |
| [65] | Krebiehl G, Ruckerbauer S, Burbulla LF, et al. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson's disease-associated protein DJ-1 [J]. PLoS One, 2010, 5: e9367. |
| [66] | González-Polo R, Niso-Santano M, Morán JM, et al. Silencing DJ-1 reveals its contribution in paraquat-induced autophagy [J]. J Neurochem, 2009, 109: 889-898. |
| [67] | Irrcher I, Aleyasin H, Seifert EL, et al. Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics [J]. Hum Mol Genet, 2010, 19: 3734-3746. |
| [68] | Thomas KJ, McCoy MK, Blackinton J, et al. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy [J]. Hum Mol Genet, 2011, 20: 40- 50. |
| [69] | Vasseur S, Afzal S, Tardivel-Lacombe J, et al. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses [J]. Proc Natl Acad Sci U S A, 2009, 106: 1111-1116. |
| [70] | McCoy MK, Cookson MR. DJ-1 regulation of mitochondrial function and autophagy through oxidative stress [J]. Autophagy, 2011, 7: 531-532. |
| [71] | Ren H, Fu K, Mu C, et al. DJ-1, a cancer and Parkinson's disease associated protein, regulates autophagy through JNK pathway in cancer cells [J]. Cancer Lett, 2010, 297: 101- 108. |
| [72] | Pukass K, Richter-Landsberg C. Inhibition of UCH-L1 in oligodendroglial cells results in microtubule stabilization and prevents α-synuclein aggregate formation by activating the autophagic pathway: implications for multiple system atrophy [J]. Front Cell Neurosci, 2015, 9: 163. |
| [73] | Ramirez A, Heimbach A, Gründemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase [J]. Nat Genet, 2006, 38: 1184-1191. |
| [74] | Murphy KE, Gysbers AM, Abbott SK, et al. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson's disease [J]. Brain, 2014, 137: 834-848. |
| [75] | Valadas JS, Vos M, Verstreken P. Therapeutic strategies in Parkinson's disease: what we have learned from animal models [J]. Ann N Y Acad Sci, 2015, 1338: 16-37. |
| [76] | Pan T, Kondo S, Zhu W, et al. Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement [J]. Neurobiol Dis, 2008, 32: 16-25. |
| [77] | Shen W, Henry AG, Paumier KL, et al. Inhibition of glucosylceramide synthase stimulates autophagy flux in neurons [J]. J Neurochem, 2014, 129: 884-894. |
| [78] | Savolainen MH, Richie CT, Harvey BK, et al. The beneficial effect of a prolyl oligopeptidase inhibitor, KYP-2047, on alpha-synuclein clearance and autophagy in A30P transgenic mouse [J]. Neurobiol Dis, 2014, 68: 1-15. |
| [79] | Savolainen MH, Yan X, Myohanen TT, et al. Prolyl oligopeptidase enhances α-synuclein dimerization via direct protein-protein interaction [J]. J Biol Chem, 2015, 290: 5117-5126. |
| [80] | Hebron ML, Lonskaya I, Moussa CE. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α-synuclein in Parkinson's disease models [J]. Hum Mol Genet, 2013, 22: 3315-3328. |
| [81] | Wu Y, Li X, Zhu JX, et al. Resveratrol-activated AMPK/ SIRT1/autophagy in cellular models of Parkinson's disease [J]. Neurosignals, 2011, 19: 163-174. |
| [82] | Sarkar S, Davies JE, Huang Z, et al. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein [J]. J Biol Chem, 2007, 282: 5641-5652. |
| [83] | Höllerhage M, Goebel JN, de Andrade A, et al. Trifluoperazine rescues human dopaminergic cells from wild-type α-synuclein-induced toxicity [J]. Neurobiol Aging, 2014, 35: 1700-1711. |
| [84] | Zhang L, Yu J, Pan H, et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen [J]. Proc Natl Acad Sci U S A, 2007, 104: 19023- 19028. |
| [85] | Steele JW, Ju S, Lachenmayer ML, et al. Latrepirdine stimulates autophagy and reduces accumulation of α-synuclein in cells and in mouse brain [J]. Mol Psychiatry, 2013, 18: 882-888. |
| [86] | Xiong N, Jia M, Chen C, et al. Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y [J]. Neuroscience, 2011, 199: 292-302. |
| [87] | Ryu HW, Oh WK, Jang IS, et al. Amurensin G induces autophagy and attenuates cellular toxicities in a rotenone model of Parkinson's disease [J]. Biochem Biophys Res Commun, 2013, 433: 121-126. |
| [88] | Kim HJ, Kim J, Kang KS, et al. Neuroprotective effect of chebulagic acid via autophagy induction in SH-SY5Y cells [J]. Biomol Ther, 2014, 22: 275-281. |
| [89] | Cao BY, Yang YP, Luo WF, et al. Paeoniflorin, a potent natural compound, protects PC12 cells from MPP+ and acidic damage via autophagic pathway [J]. J Ethnopharmacol, 2010, 131: 122-129. |
| [90] | Li XZ, Chen XP, Zhao K, et al. Therapeutic effects of valproate combined with lithium carbonate on MPTP-induced parkinsonism in mice: possible mediation through enhanced autophagy [J]. Int J Neurosci, 2013, 123: 73-79. |
| [91] | Su YC, Qi X. Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation [J]. Hum Mol Genet, 2013, 22: 4545-4561. |
| [92] | Qi X, Qvit N, Su YC, et al. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity [J]. J Cell Sci, 2013, 126: 789-802. |
| [93] | Tovilovic G, Zogovic N, Soskic V, et al. Arylpiperazine- mediated activation of Akt protects SH-SY5Y neuroblastoma cells from 6-hydroxydopamine-induced apoptotic and autophagic death [J]. Neuropharmacology, 2013, 72: 224-235. |
| [94] | Zhang S, Gui XH, Huang LP, et al. Neuroprotective effects of β-asarone against 6-hydroxy dopamine-induced parkinsonism via JNK/Bcl-2/Beclin-1 pathway [J]. Mol Neurobiol, 2014. DOI: 10.1007/s12035-014-8950-z. |
 2016, Vol. 51
2016, Vol. 51


