2. 陕西中医药大学, 陕西省中药资源产业化协同创新中心, 陕西省中药基础与新药研究重点实验室, 陕西省风湿与肿瘤类中药制剂工程技术研究中心, 陕西咸阳 712083
2. Shaanxi Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, Shaanxi Province Key Laboratory of New Drugs and Chinese Medicine Foundation Research, Shaanxi Rheumatism and Tumor Center of TCM Engineering Technology Research, Shaanxi University of Chinese Medicine, Xian Yang 712083, China
The epidermal growth factor receptor (EGFR,erbB1) is a member of the erbB family including erbB2/HER2,erbB3/HER3,and erbB4/HER4[1, 2]. It is over-expressed in a large number of human tumors,and associated with cancer cell proliferation,apoptosis,angiogenesis,and metastasis[3, 4]. The receptor exists as inactive monomer. Upon ligand binding,EGFR forms homo- or hetero-dimer with other members of the erbB family and undergoes autophosphorylation. This initiates a cascade of intracellular signal transduction[5, 6]. Deregulation of the EGFR signaling pathway has been observed in many human solid cancers,such as lung,breast,and bladder cancers[7, 8]. Therefore,inhibitors of EGFR have emerged as promising anticancer agents and have been investigated extensively.
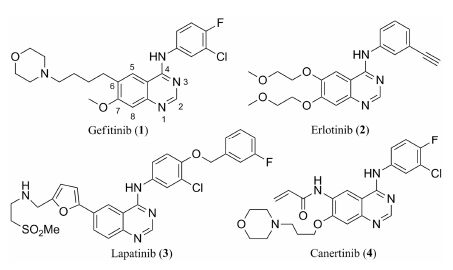
In the past decade,a series of EGFR inhibitors have been discovered. Some of them have been used in clinical treatments. As show in Figure 1,gefitinib and erlotinib have been used to treat non-small-cell lung cancer (NSCLC)[9, 10]. Lapatinib was approved for the treatment of breast cancer in 2007[11]. Canertinib was the first irreversible kinase inhibitor to enter clinical trials. The development of canertinib was stopped at the end of Phase II,due to thrombocytopenia,but its chemical structure has been used as a template to design new compounds[12].
|
Figure 1 Chemical structures of tyrosine kinase inhibitors |
All of the above agents belong to the 4-anilinoquinazoline class of inhibitors and the structure-activity relationship (SAR) has been revealed as follows[13]: ① the quinazoline moiety fits into the ATP binding pocket of the kinase domain,② the N-1 of the quinazoline ring interacts with the backbone NH of Met-769 via a hydrogen bond,and water mediated hydrogen bonding is observed between the N-3 of the quinazoline ring and the Thr-766 side chain,③ the aniline moiety lies in a deep and hydrophobic pocket,and ④ the substitution at C-6 or C-7 of the quinazoline ring conveys a more favorable pharmacokinetic profile and improves the physical properties.
In our ongoing search for functional groups that possessing antitumor activities,we focused on the Schiff base for its inhibition activity on tumor proliferation[14]. Consequently,thirteen Schiff base derivatives were synthesized by combining N4-(3-chloro-4-fluorophenyl) quinazoline-4,6-diamine (compound 12) as a driving portion and benzaldehyde derivatives to form the Schiff bases warheads.
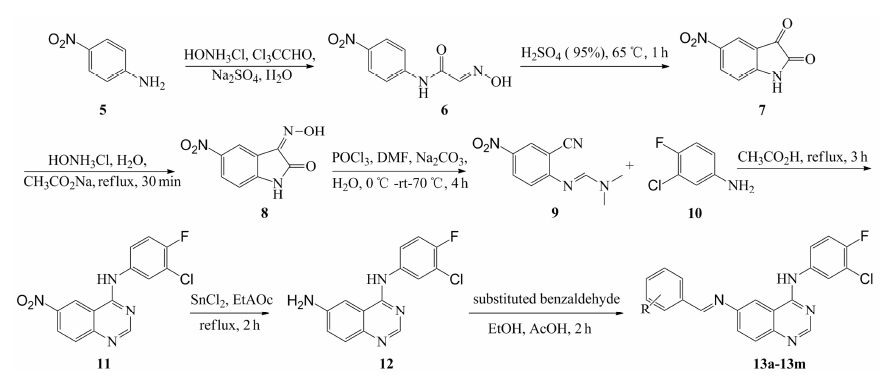
Results and discussion 1 ChemistryThirteen compounds were synthesized using p-nitroaniline as a starting material (Scheme 1). It reacted with chloral hydrate and hydroxylamine hydrochloride to produce compound 6 firstly. Then Beckmann rearrangement was carried out in 95% of H2SO4 to form 5-nitroisatin (7). Compound 7 underwent condensation with hydroxylamine hydrochloride followed by heating in the solution of DMF and POCl3 to form compound 9[15]. Then,compound 9 reacted with 3- chloro-4-fluoro-benzenamin to produce the compound 11. The nitro group of 11 was reduced to amine with SnCl2 to form compound 12[16]. Thirteen Schiff base derivatives were synthesized by reacting compound 12 with substituted benzaldehydes to form 13a-13m[17, 18]. The IR,1H NMR,UV,MS and elemental analysis data of compounds 13a-13m was elucidated in Table 1.
|
Scheme 1The synthetic route of compounds 13a-13m |
|
|
Table 1 IR,1H NMR,UV,MS and elemental analysis data of compounds 13a-13m |
The key step of the synthetic route was the reduction of nitro group to amine. In order to increase the total yields of final products,different reductants were tested (Table 2) and the reaction time and temperature were investigated to ensure the highest yield in each reaction system. Though three reductants of Fe,H2 and Na2S/Na2S2O3 could give acceptable yields,the post-processing was not satisfied. The system using hydrazine hydrate as reductant could easily get pure product by employing recrystallization,but the yield was too low. There were two main reasons resulted in the low yield. Firstly,there were two main intermediates in the procession of reducing nitro to amino,nitroso and hydroxyimino group. That meant two main by product existed in the reaction system. Secondly,the low yield was caused by the post purification for the use of active carbon and two times of recrystallization. Finally,the use of SnCl2 gave the highest yield among the five reductants and the post-processing was also very convenient.
|
|
Table 2 The yield and reaction conditions of different reductants |
Compounds 13a-13m were evaluated on cell- based assays for their ability to inhibit the proliferation of tumor cells (A549 human lung cancer cells,human hematoma carcinoma cells HepG2 and SMMC7721) with EGFR highly expressed (Table 3),gefitinib was used as a positive control. To test the effect of Schiff base on the antitumor activity of 4-anilinoquinazolines,compound 14 without imine group,was synthesized. The synthetic detail of compound 14 was reported in previous work[15].
|
|
Table 3 In vitro antitumor activity of the designed 4-anilinoquinazoline derivatives. aIC50, compound concentration required to inhibit tumor cell proliferation by 50%; bHuman lung cancer cell line (A549); cHuman hematoma carcinoma cell line (HepG2); dHuman hematoma carcinoma cell line (SMMC7721) |
As shown in Table 3,all the compounds exhibited moderate inhibitor potency. Nine compounds among those tested indicated a concentration-dependent decrease in surviving fraction of the three cell lines. Compound 13i and 13j exhibited the best inhibitor potency with IC50 values equivalent to or less than those of gefitinib. The IC50 values of 13j on A549 and SMMC7721 were 3.06 and 3.9 µmol·L-1 respectively,less than those of gefitinib (5.51 and 4.16 µmol·L-1 respectively). Compounds 13a,13m and 13h had poor inhibitor potency with IC50 values higher than 50 µmol·L-1. Other compounds displayed moderate inhibitor potency and IC50 values were all in the range of 10-50 µmol·L-1. By comparing the IC50 values of 13b-13e,which all possessed a hydroxyl group at different positions on the benzene ring,it could be concluded that para- hydroxyl was better than that in the ortho- and meta-position. The same conclusion was also drawn by comparing the IC50 values of 13f-13h that the chlorine at para- position was better than at the ortho- and meta- positions.
The IC50 value of compound 14 on A549 cell line was better than most of other twelve compounds except for 13j. But the IC50 values on the HepG2 and SMMC-7721 were all lower than other compounds. Above all,it was concluded that Schiff base could affect the antitumor potency of 4-anilinoquinazoline moderately. Compound 13j displayed notably inhibitor potency on A549 even better than that of gefitinb and it was worthy to do a further study.
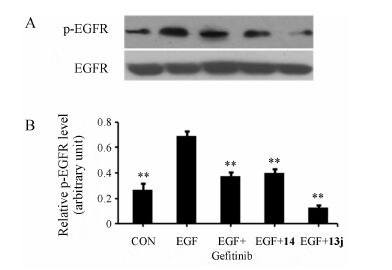
3 Inhibition of EGFR autophosphorylationOn the basis of the MTT assay,compounds 13j and 14 were chosen to perform Western blotting analysis in order to examine their ability to inhibit EGFR autophosphorylation in the A549 cell line. The results of Western blotting analysis were shown in Figure 2. Group 1 was the blank control without EGF stimulation. The other four groups were all stimulated with 10 nmol·L-1 of EGF. Group 3 included 2 µmol·L-1 of gefitinib as a positive control. Group 4 and 5 included 2 µmol·L-1 of compounds 13j and 14,respectively. The results showed that the protein expression level of phosphorylated EGFR in group 2 with EGF stimulation was higher than that of group 1,but it was lower than that of groups 3,4 and 5. This indicates that the inhibitor potency of compound 14 on the protein expression level of phosphorylated EGFR was equivalent
|
Figure 2 Inhibitory effect of compounds 14 and 13j upon EGFR autophosphorylation. (A) A549 cells treated with or without gefitinib, 13j and 14. The change of p-EGFR was analyzed by Western blotting. (B) The result of Western blotting was ana-lyzed by Image J. The X-axis represents different groups and the Y-axis represents the ratio of the gray value of p-EGFR to that of EGFR. **P < 001 vs EGF group |
Figure 2 Inhibitory effect of compounds 14 and 13j upon EGFR autophosphorylation. (A) A549 cells treated with or without gefitinib,13j and 14. The change of p-EGFR was analyzed by Western blotting. (B) The result of Western blotting was analyzed by Image J. The X-axis represents different groups and the Y-axis represents the ratio of the gray value of p-EGFR to that of EGFR. **P < 001 vs EGF group
Conclusions
Thirteen of 4-anilinoquinazoline derivatives were synthesized and their EGFR inhibitor activities were evaluated by MTT assay and Western blotting analysis. Among them,compounds 13a-13l were reported firstly. MTT assay was carried out on three human cancer cell lines with EGFR highly expressed and the results exhibited that most of the compounds showed moderate inhibitor potency. Compounds 13i and 13j displayed notable inhibitor potency with IC50 values on three cell lines and these was equivalent to or less than those of gefitinib. By comparing the IC50 values of 13b-13e and 13f-13h,which possessed the same substituent on the benzene ring,it was concluded that the para- substituent was better than the ortho- and meta-position. The results of Western blotting analysis indicated that the inhibitor potency of compound 13j was better than that of gefitinib. It was concluded that Schiff base could affect the antitumor potency of 4-anilinoquinazoline moderately. Compound 13j exhibited dramatically inhibitor potency in MTT assay and Western blotting analysis.
Experimental sections
All chemicals and solvents were analytical grade and used without further purification. Analytical TLC was performed on pre-coated silica gel plates (HG/ T2354-92). Melting points were determined on an X-5 micro melting point apparatus and were uncorrected. The UV spectra were recorded on a 2600UV/VIS Spectrophotometer using methanol as blank. IR spectra were recorded on a FT-IR spectrometer (Spectrum Two). 1H NMR spectra were recorded at 400 MHz on a Bruker Avance-400 MHz spectrometer with TMS as an internal standard; chemical shifts were given in ppm and coupling constants in Hertz. Mass spectra were performed on LTQ-XL with direct sample injection. Elemental analysis was performed on a Vario EL III CHNOS analyzer.
1 Procedures for the synthesis of compound 7A 500 mL,three-necked,round-bottomed flask fitted with a condenser and a thermometer was charged with chloral hydrate (16.50 g,0.1 mol) and 220 mL of water. Then anhydrous sodium sulfate (15.00 g),p-nitroaniline (0.1 mol),HCl solution (5.2%,70 mL) and hydroxylamine hydrochloride (20.81 g,0.3 mol,in 95 mL of water) were added in successively. After being heated to reflux for 1 h,the reaction mixture was cooled to room temperature. The intermediate compound (anilide derivative) was collected by filtration and dried,which was used for next step without further purification.
A 100 mL,three-necked,round-bottomed flask fitted with a thermometer was charged with concentrated sulfuric acid (53 mL). After heating to 50 ℃ with stirring,the dried intermediate (20.5 g,0.098 mol) from the above step was added in over a period of 20 min. The resulting solution was heated to 65 ℃ and kept for 1 h and then cooled to room temperature with an ice bath. The precipitate was filtered out and dried to get 14.74 g of pale yellow crystal solid with a yield of 76.4%. mp 254-257 ℃; IR (KBr): 3334.61,3 095.36,1 770.03,1 751.21,1 619.29,1 533.45,1 470.52,1 336.55,903.33,852.46 cm-1; UV-VIS (CH3OH),λ/nm: 210,321 nm; 1H NMR (400 MHz,CDCl3): δ 10.74 (s,1H),8.25 (d,1H),7.97 (s,1H),7.69 (d,1H); ESI-MS m/z (%) [M+H]+: 193.08; EA (Elemental analysis) Calcd. for C8H4N2O4: C,50.01; H,2.10; N,14.58. Found: C,50.05; H,2.06; N,14.56.
2 Procedures for the synthesis of compound 8 and 9A mixture of compound 7 (0.05 mol,9.6 g) and hydroxylamine hydrochloride (0.065 mol) in water (100 mL) was heated to reflux for 30 min,then sodium acetate (7.5 g) was added and the solution was continued to reflux for another 30 min. The mixture was cooled to room temperature. The solid was filtered and dried for the next step. The obtained yield of compound 8 was 97%.
Compound 8 (6.73 g,0.031 mol) was added to the mixture of POCl3 (4.3 mL,0.046 mol) and DMF (20 mL) that was iced to 0 ℃ slowly. The resulting mixture was stirred at room temperature for 10 min,and then heated to 70 ℃ for 4 h. The reaction mixture was cooled and poured into water (100 mL). The insoluble substance was filtered. The sodium carbonate solution (10%) was added into the solution to adjust the pH to 7. After 30 min,the precipitate was filtered and dried. 5.61 g of yellow solid 9 was obtained with 83% yield,which was pure enough for the next reaction.
3 Procedure for the synthesis of compound 11A mixture of compound 9 (2.18 g,0.01 mol) and 3-chloro-4-fluoro-benzenamin (1.47 g,0.01 mol) in acetic acid (15 mL) was heated to reflux for 3 h. The mixture was poured into the ice water. After 20 min,the precipitate was filtered and washed three times with water. The target compound was obtained in 91.2% yield,yellow solid (2.90 g). mp 279-281 ℃; IR (KBr): 3 307.68,3 096.44,1 625.21,1 587.82,1 573.43,1 595.99,1 538.13,1 435.48,1 340.91,875.17,848.54,809.04 cm-1; UV-VIS (CH3OH),λ/nm: 218,240,359; 1H NMR (400 MHz,CD3OD): δ 9.51 (d,J = 2.2 Hz,1H),8.70 (s,1H),8.63 (dd,J = 9.2,2.3 Hz,1H),8.09 (dd,J = 6.6,2.5 Hz,1H),7.95 (d,J = 9.2 Hz,1H),7.74 (dd,J = 7.9,3.8 Hz,1H),7.30 (t,J = 8.9 Hz,1H); ESI-MS m/z [M+H]+ 319.08; EA Calcd. for C14H8N4O2ClF: C,52.76; H,2.53; N,17.58. Found: C,52.01; H,2.61; N,17.48.
4 Procedure for the synthesis of compound 12
A mixture of 11 (1.1 g,0.0034 mol) and SnCl2 (2.6 g,0.014 mol) was suspended in 35 mL of ethyl acetate. The mixture was heated to reflux for 2 h. After completion of the reaction,the mixture was cooled to room temperature. The insoluble substance was removed by filtration through Celite,and the filtrate was evaporated under reduce pressure. The resulting solid was recrystallized to give a white solid. 0.97 g,(89%); mp 252-255 ℃; IR (KBr): 3 387.34,3 143.05,1 627.35,1 613.65,1 578.01,1 495.33,1 454.76,1 366.18,877.34,849.19,593.03 cm-1; UV-VIS (CH3OH),λ/nm: 211,237,299,366; 1H NMR (400 MHz,CD3OD): δ 8.32 (s,1H),8.01 (dd,J = 6.6,2.3 Hz,1H),7.66 (dd,J = 7.8,4.0 Hz,1H),7.57 (d,J = 8.8 Hz,1H),7.32- 7.22 (m,3H); ESI-MS m/z [M+H]+ 289.08; EA Calcd. for C14H10N4ClF: C,58.24; H,3.49; N,19.41. Found: C,58.52; H,3.68; N,19.43.
5 General procedure for the synthesis of compound 13a-13m
A mixture of compound 12 (1.0 equiv),CH3CO2H and derivatives of benzaldehyde (1.0 equiv) in absolute ethanol was heated to reflux for 5 h. The reaction mixture was cooled to room temperature and the solid was precipitated. The precipitation was filtered,recrystallized from ethanol and dried to produce the target compound.
5.1N6-(3-nitrobenzylidene)-N4-(3-chloro-4-fluorophenyl)quinazoline-4,6-diamine (13a)The title compound was obtained in 75.5% yield,by condensing 12 (100 mg,0.35 mmol) with 3-nitrobenzaldehyde (53 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol gave 13a as a yellow solid (111 mg). mp 256-257 ℃.
5.2 4-((4-(3-chloro-4-fluorophenylamino)quinazolin- 6-ylimino)methyl)phenol (13b)The title compound was obtained in 81.6% yield,by condensing 12 (100 mg,0.35 mmol) with 4-hydroxybenzaldehyde (43 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol afforded 13b as a yellow solid (113mg). mp 295-298 ℃.
5.3 3-((4-(3-chloro-4-fluorophenylamino)quinazolin- 6-ylimino)methyl)phenol (13c)The title compound was obtained in 78.6% yield,by condensing 12 (100 mg,0.35 mmol) with 3-hydroxybenzaldehyde (43 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol afforded 13c as a yellow solid (107 mg). mp 255-258 ℃.
5.4 4-((4-(3-chloro-4-fluorophenylamino)quinazolin- 6-ylimino)methyl)benzene-1,3-diol (13d)The title compound was obtained in 85.4% yield,by condensing 12 (100 mg,0.35 mmol) with 2,4-dihydroxybenzaldehyde (48 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol gave 13d as a yellow solid (122 mg). mp 262-266 ℃.
5.5 2-((4-(3-chloro-4-fluorophenylamino)quinazolin- 6-ylimino)methyl)phenol (13e)The title compound was obtained in 90.8% yield,by condensing 12 (100 mg,0.35 mmol) with 2-hydroxybenzaldehyde (43 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol afforded 13e as a yellow solid (125 mg). mp 231-232 ℃.
5.6 N6-(4-chlorobenzylidene)-N4-(3-chloro-4-fluorophenyl)quinazoline-4,6-diamine (13f)The title compound was obtained in 94.1% yield,by condensing 12 (100 mg,0.35 mmol) with 4-chlorobenzaldehyde (49 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol gave 13f as a yellow solid (135 mg). mp 234-237 ℃.
5.7 N6-(2,4-dichlorobenzylidene)-N4-(3-chloro-4-fluo rophenyl)quinazoline-4,6-diamine (13g)The title compound was obtained in 96.7% yield,by condensing 12 (100 mg,0.35 mmol) with 2,4-dichlorobenzaldehyde (61 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol afforded 13g as a yellow solid (151 mg). mp 216-219 ℃.
5.8 N6-(2-chlorobenzylidene)-N4-(3-chloro-4-fluorophenyl)quinazoline-4,6-diamine (13h)The title compound was obtained in 92.8% yield,by condensing 12 (100 mg,0.35 mmol) with 2-chlorobenzaldehyde (49 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol gave 13h as a yellow solid (133 mg). mp 254-255 ℃.
5.9 5-((4-(3-chloro-4-fluorophenylamino)quinazolin- 6-ylimino)methyl)-2-methoxyphenol (13i)The title compound was obtained in 81.1% yield,by condensing 12 (100 mg,0.35 mmol) with 3-hydroxy-4-methoxybenzaldehyde (53 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol afforded 13i as a yellow solid (120 mg). mp 257- 261 ℃.
5.10 4-((4-(3-chloro-4-fluorophenylamino)quinazolin- 6-ylimino)methyl)-2-methoxyphenol (13j)The title compound was obtained in 83.2% yield,by condensing 12 (100 mg,0.35 mmol) with 4-hydroxy-3-methoxybenzaldehyde (53 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol gave 13j as a yellow solid (122 mg). mp 272-275 ℃.
5.11 N6-(3,4,5-trimethoxybenzylidene)-N4-(3-chloro- 4-fluorophenyl)quinazoline-4,6-diamine (13k)The title compound was obtained in 87.6% yield,by condensing 12 (100 mg,0.35 mmol) with 3,4,5-trimethoxybenzaldehyde (69 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol afforded 13k as a yellow solid (143 mg). mp 107-110 ℃.
5.12 N6-(4-methylbenzene)-N4-(3-chloro-4-fluorophenyl)quinazoline-4,6-diamine (13l)The title compound was obtained in 67.4% yield,by condensing 12 (100 mg,0.35 mmol) with 4-methylbenzaldehyde (42 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol gave 13l as a yellow solid (92 mg). mp 263-265 ℃.
5.13 N6-benzylidene-N4-(3-chloro-4-fluorophenyl) quinazoline-4,6-diamine (13m)The title compound was obtained in 85.1% yield,by condensing 12 (100 mg,0.35 mmol) with benzaldehyde (37 mg,0.35 mmol) using 5 drops of CH3CO2H as a catalyst. Recrystallization from ethanol gave 13m as a yellow solid (112 mg). mp 279-283 ℃.
6 Biological activity evaluationHyclone DMEM/HIGH Glucose Medium was purchased from Thermo Fisher Scientific,and fetal bovine serum (FBS) was purchased from Gibco-BRL (Grand Island,NY). Monoclonal anti-EGFR (EGF Receptor (D38B1) XP® Rabbit mAb #4267s),polyclonal anti-phospho-EGFR antibodies (Phospho-EGF Receptor (Tyr1068) Antibody #2234) and EGF were purchased from Cell Signaling Technology (Beverly,MA). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from CapitalBio Corporation.
6.1 Inhibition of cell proliferation by MTT assayThe A549 cells (5×107/L) were seeded in 96-well plates and incubated for 24 h. Then the synthesized compounds were incubated with six concentrations (1,2,5,10,20,50,100 µg·mL-1) for 48 h. Controls consisted of wells without drugs. The medium was removed and the cells were incubated for 4 h in the presence of 5 mg·mL-1 of MTT at 37 ℃. The MTT solution was removed and 150 µL of DMSO per well was added. After thorough mixing,absorbance of the wells was read in a SpectraMax 190 Spectrophotometer at test and at reference wavelengths of 490 nm. The data were analyzed and the IC50 values were calaculated by SPSS 10.0.
6.2 Inhibition of autophosphorylation of EGFRTo prepare the whole-cell extract,cells were washed with PBS once and harvested by scraping them in 1 mL lysis buffer (50 mmol·L-1 Tris-HCl pH 7.4,150 mmol·L-1 NaCl,0.2 mmol·L-1 EDTA,0.2% NP-40,10% Glycerin,1 mol·L-1 b-Me,1 µg·mL-1 Aprotin,0.5 µg·mL-1 Leupetin,0.1 mmol·L-1 Na3VO4,0.5 mmol·L-1 4-NPP,0.5 mmol·L-1 NaF,and protease inhibitors). Cellular lysates were centrifuged at 13 200×g for 5 min at 4 ℃. Protein content was determined by the Bradford assay (Bio-Rad Laboratories,Hercules,CA). The extracted proteins were separated in a 10%-12% SDS- polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Amersham Bioscience). The membranes were first blocked with 5% (w/v) nonfat dry milk in PBST and then probed with the prim ary antibodies (EGF Receptor (D38B1) XP® Rabbit mAb #4267s and Phospho-EGF Receptor (Tyr1068) Antibody #2234) with gentle shaking at 4 ℃ overnight. After washing the membranes four times,the membranes were incubated with the appropriate peroxidase conjugated secondary antibodies for 1 h. The signals were detected using an enhanced chemiluminescence kit (Amersham Biosciences).
| [1] | Mastalerz H, Chang M, Gavai A, et al. Novel C-5 aminomethyl pyrrolotriazine dual inhibitors of EGFR and HER2 protein tyrosine kinases[J]. Bioorg Med Chem Lett, 2007, 17:2828-2833. |
| [2] | Suzuki N, Shiota T, Watanabe F, et al. Discovery of novel 5-alkynyl-4-anilinopyrimidines as potent, orally active dual inhibitors of EGFR and Her-2 tyrosine kinases[J]. Bioorg Med Chem Lett, 2012, 22:456-460. |
| [3] | Pierce KL, Luttrell LM, Lefkowitz RJ. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades[J]. Oncogene, 2001, 20:1532-1539. |
| [4] | Graness A, Hanke S, Boehmer FD, et al. Protein-tyrosine-phosphatase-mediated epidermal growth factor(EGF) receptor transinactivation and EGF receptor-independent stimulation of mitogen-activated protein kinase by bradykinin in A431 cells[J]. Biochem J, 2000, 347:441-447. |
| [5] | Lin SY, Makino K, Xia W, et al. Nuclear localization of EGF receptor and its potential new role as transcription factor[J]. Nat Cell Biol, 2001, 3:802-808. |
| [6] | Liu Z, Jiang G, Peter BJ, et al. Epidermal growth factor-induced tumor cell invasion and metastasis intiatede by de-phosphorylation and downregulation of focal adhesion kinase[J]. Mol Cell Biol, 2001, 21:4016-4031. |
| [7] | Johannessen LE, Ringerike T, Molnes J, et al. Epidermal growth factor efficiently actives mitogen-activated protein kinase in Hela cells and Hep2 cells conditionally defective in clathrin-dependent endocytosis[J]. Exp Cell Res, 2000, 260:136-145. |
| [8] | Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy[J]. Pharmacol Ther, 1999, 82:241-250. |
| [9] | Baselga J, Averbuch SD. ZD1839(Ireasa) as an anticancer agent[J]. Drugs, 2000, 60:33-40. |
| [10] | Ryan AJ, Wedge SR. ZD34742 a novel inhibitor of VEGFR and EGFR tyrosine kinase activity[J]. Br J Cancer, 2005, 92:S6-S13. |
| [11] | Mcclure M, Mitchell M, Salaun MC, et al. Synthesis of lapatinib via direct regioselective arylation of furfural[J]. Tetrahedron Lett, 2014, 43:6007-6010. |
| [12] | Janne PA, Vonpawel J, Cohen RB, et al. Multicenter, ran-domized, phase II trial of CI-1033, an irreversible pan-ERBB inhibitor, for previously treated advanced non small-cell lung cancer[J]. J Clin Oncol, 2007, 25:3936-3944. |
| [13] | Deng W, Guo Z, Guo Y, et al. Acry-loyamino-salicylanilides as EGFR PTK inhibitors[J]. Bioorg Med Chem Lett, 2006, 16:469-472. |
| [14] | Potter DW, Hinson JA. Mechanisms of acetaminophen oxidation to N-acetyl-p-benzoquinone imine by horseradish peroxidase and cytochrome P-450*[J]. J Biol Chem, 1987, 262:966-973. |
| [15] | Wang Z, Wang CL, Sun YN, et al. A novel strategy to the synthesis of 4-anilinoquinazoline derivatives[J]. Tetrahedron, 2014, 70:906-913. |
| [16] | Marvania B, Lee PC, Chaniyara R, et al. Design, synthesis and antitumor evaluation of phenyl N-mustard-quinazoline conjugates[J]. Bioorg Med Chem, 2011, 19:1987-1998. |
| [17] | Albuschat R, Lowe W, Weber M. 4-Anilinoquinazolines with lavendustin A subunit as inhibitors of epidermal growth factor receptor tyrosine kinase:syntheses, chemical and pharmacological properties[J]. Eur J Med Chem, 2004, 39:1001-1011. |
| [18] | Chen H, Sun XL, Liu ZH, et al. Preparation of six Schiff bases and their inhibitory effect on HHCC and Bcap-37 cells[J]. J Med Coll PLA, 2003, 18:246-250. |
 2015, Vol. 50
2015, Vol. 50