2. 中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室, 云南 昆明 650201;
3. 河南省科学院天然产物重点实验室, 河南 郑州 450002
2. State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China;
3. Key Laboratory of Natural Products, Henan Academy of Sciences, Zhengzhou 450002, China
Micromelum integerrimum (Buch.-Ham. ex DC.) Wight & Arn. ex M. Roem. (Rutaceae) is distributed mainly in China[1]. The leaves and barks have been used for the treatment of cold and trauma,and the roots for stomach pain[1]. Previous chemical investigations on this genus afforded a number of structurally interesting coumarins,alkaloids and other compounds[2,3,4,5,6,7]. Although coumarins,alkaloids and phenylpropanoidshave also been isolated from M. integerrimum,some coumarins showed cytotoxicity[3,4,5,8]. As part of our continuous investigation on the chemical and biological constituents of M. integerrimum,chemical investigation on the stems and leaves of M. integerrimum leads to the isolation of two new compounds (1,2) and six known compounds (3-8).
Results and discussionTwo new compounds,a benzene derivative (1) and a norsesquiterpenoid (2),together with six known compounds (3-8)were isolated and determined as microintegerrin C (1),microintegerrin D (2),2-methoxy- 4-(2-propenyl)-phenyl-β-D-glucoside (3)[9],erigesideⅡ (4)[10],acantrifoside E (5)[11],benzyl-β-D-glucoside (6)[12],5,7-dihydroxyl-3,8,4'-trimethoxylflavone (7)[13] and kaempferol 3-O-β-D-glucoside (8)[14] (Figure 1),based on the spectral data analysis such as 1D-,2D- NMR and HR-EI-MS.
Compound 1 was isolated as yellow oil. The molecular formula of C20H26O9 was determined on the basis of its HR-EI-MS molecular ion peak (m/z 410.156 7 [M]+),in combination with an analysis of the 13C NMR spectrum (DEPT),corresponding to eight degrees of unsaturation. The IR spectrum showed absorption bands of hydroxyl (3405 cm-1) and carbonyl groups (1 680 cm-1). The 13C NMR spectrum (Table 1) displayed 20 carbon signals: two CH3 (δC 18.5,14.5),three CH2 (δC 74.6,62.8,62.5),eleven CH (δC 134.4,130.6,130.6,129.7,129.7,122.3,103.3,78.2,78.1,75.2,71.7) and four C (δC 170.0,168.1,139.6,131.6).
|
|
Table 1 1H,13C NMR data of 1 and 2 at 600 and 150 MHz,in CD3OD,respectively (J in Hz) |
The 1H NMR,COSY and HMBC spectra (Table 1,Figure 2) displayed the following moiety signals: one mono-substituted benzoyl [δC 168.1,134.4,131.6,130.6,130.6,129.7,129.7; δH 8.01 (2H,dd,J = 7.8,1.2 Hz),7.60 (1H,m),7.48 (2H,t,J = 7.8 Hz)],one glucosyl [δC 103.3,78.2,78.1,75.2,71.7,62.8; δH 4.28 (1H,d,J = 7.8 Hz),3.86 (1H,dd,J = 12.0,1.8 Hz),3.66 (1H,dd,J = 12.0,5.4 Hz),3.35 (1H,t,J = 9.0 Hz),3.25 (3H,overlapped)],one acetyl [δC 170.0,18.5; δH 2.21 (3H,s)],and one [-OCH2C(CH3)=CHCH2O-] unit [δC 139.6,122.3,74.6,62.5,14.5; δH 5.83 (1H,dt,J = 7.2,1.2 Hz),4.90 (2H,d,J = 7.2 Hz),4.31 (1H,d,J = 12.6 Hz),4.11 (1H,d,J = 12.6 Hz),1.84 (3H,s)]. The β-configuration of the glucose was determined from the coupling constant (7.8 Hz) of the anomeric proton signal in the 1H NMR spectrum[15]. Further analysis of the HMBC spectrum (Figure 2) revealed the following connections: the correlation of H-6''/C-7'' indicated that the acetyl was linked to C-6'' of the β-D-glucopyranosyl unit; the correlation of H-1/C-1'' indicated that the β-D- glucopyranosyl moiety was linked to C-1; the correlation of H-4/C-7' indicated that the [-OCH2C(CH3)=CHCH2O-] unit was linked to C-7'. Thus,the structure of 1 is elucidated and named as microintegerrin C (Figure 1).
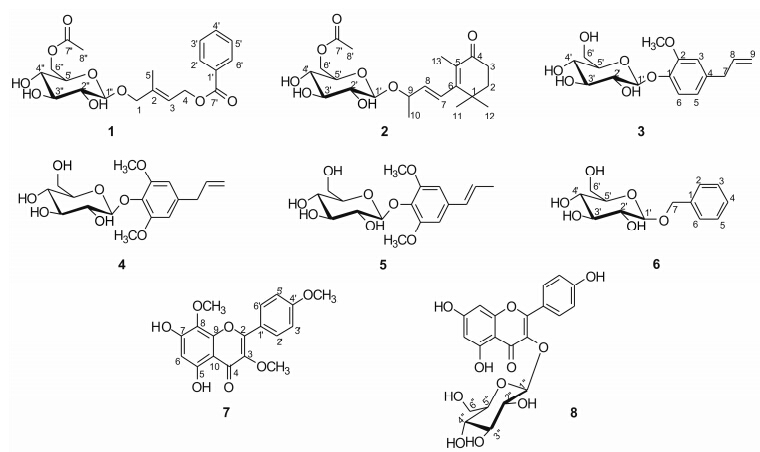
|
Figure 1 Structures of compounds 1-8 |
Compound 2 was obtained as yellow oil. The HR-EI-MS revealed an ion peak at m/z 412.207 0 [M]+,corresponding to the molecular formula C21H32O8 (Calcd. 412.209 7). Comparison of the 1D- and 2D- NMR data of 2 with those of 4-oxo-β-ionol β-D- glucopyranoside[15] suggested their structures were closely related,except for an additional acetyl group [δC 170.0,18.5; δH 2.21 (3H,s)]. In the HMBC spectrum (Figure 2),the correlation of H-6'a/C-7' indicated the acetyl was connected with C-6' of the β-D-glucopyranose. Therefore,compound 2 was elucidated and named as microintegerrin D (Figure 1).

|
Figure 2 Key HMBC correlations of compounds 1 and 2 |
Optical rotations were measured with a Horiba SEPA-300 polarimeter. UV spectra were recorded using a Shimadzu UV-2401A spectrophometer. CD spectra were recorded on a Chirascan Circular Dichroism spectrometer. IR spectra were obtained on a Tensor 27 spectrometer with KBr pellets. 1D and 2D NMR spectra were performed on the Bruker AV-400 (1H: 400 MHz,13C: 100 MHz),Bruker AVANCE III-500 (1H: 500 MHz,13C: 125 MHz) or AV-600 (1H: 600 MHz,13C: 150 MHz) spectrometer with TMS as the internal standard. Mass spectra were measured on a VG Auto Spec-3000 or API-Qstar-Pulsar instrument. Column chromatography was performed using silica gel (100-200 and 200-300 mesh,Qingdao marine Chemical Inc.,China),Sephadex LH-20 (Amersham Biosciences,Sweden),MCI (CHP-20P,Mitsubishi,Japan) or Lichroprep RP-18 (40-63 mm,Merck,Darmstadt,Germany). Fractions were monitored by TLC (GF254,Qingdao Marine Chemical Inc.,China). Spots were first visualized under UV light (254 and 365 nm),followed by spraying with 5% H2SO4 in EtOH and then heating. Analytical or semi-preparative HPLC was performed on Agilent 1100 with Eclipse XDB-C18 (Agilnent,9.4 mm × 250 mm). Preparative HPLC was performed on an Agilent 1100 apparatus with a diode- array detector and a Sun FireTM Pre C18 OBDTM (Waters,19 mm × 250 mm,5 μm) column.
Plant materialThe stems and leaves of M. integerrimum were collected in Xishuangbanna,Yunnan Province,China,in September 2011,and authenticated by Prof. Hua Peng,Kunming Institute of Botany,Chinese Academyof Sciences,where a voucher specimen (KUN No. 0182256) was deposited.
Extraction and isolationAir-dried stems and leaves of M. integerrimum (29.0 kg) were extracted with methanol at 70 ℃ under reflux for four times. The extract was concentrated in vacuum to give a residue (4.5 kg),which was suspended in water,and then partitioned successively with petroleum ether,EtOAc,and n-BuOH. The EtOAc extract (530.0 g) and n-BuOH extract (600.0 g) were subjected separately to silica gel (100-200 mesh) column,and eluted with CHCl3- MeOH gradient (1∶0,30∶1,15∶1,9∶1,8∶2,7∶3,1∶1,0∶1,v/v) to give 26 fractions (Fr. A1 to Fr. A26) and 19 fractions (Fr. B1 to Fr. B19) separately,monitored by TLC. Fr. A18 (21.0 g) was further separated to obtain 2 (20.6 mg),3 (64.8 mg) and 6 (49.4 mg) by MPLC and preparative HPLC with the eluent of MeOH/H2O and CH3CN/H2O. Fr. A24 (10.0 g) was subjected to silica gel column (200-300 mesh) and eluted with a CH3Cl/MeOH gradient to afford 6 fractions (Fr. A24-1 to Fr. A24-6). Fr. A24-3 and Fr. A24-6 were chromatographed through sephadex LH-20 eluted with MeOH and semi-preparative HPLC with the eluent of CH3CN/H2O to yield 1 (5.9 mg) and 8 (16.0 mg). Fr. B3 (30.0 g) was further purified by means of MPLC on RP-18 eluting with MeOH/H2O,followed by semi-preparative HPLC with the eluent of CH3CN/H2O to give the pure 7 (696.1 mg). Fr. B7 was further chromatographed through MCI column with the eluent of MeOH/H2O,semi-preparative and preparative HPLC with the eluent of CH3CN/H2O to yield 4 (0.6 mg) and 5 (183.6 mg).
Microintegerrin C (1)Yellow oil; C20H26O9; [α]17.9 D-22.4 (c 0.16,MeOH); positive ESI-MS m/z : 449 [M+K]+; HR-EI-MS: m/z 410.156 7 [M]+ (Cacld. For C20H26O9: 410.157 7); IR (KBr): 3 405,2 924,1 680,1 452,1 430,1 384,1 278,1 205,1 137,1 074,1 027,839,802 and 720 cm-1; UV (MeOH) λmax (log ε): 201 (4.2),226 (4.0),and 273 (3.4) nm; CD (MeOH): 218 (Δε -0.01) nm; 1H and 13C NMR spectral data,see Table 1.
Microintegerrin D (2)Yellow oil; C21H32O8; [α]23.3 D-30.8 (c 0.21,MeOH); EI-MS m/z: 412 [M]+; HR-EI-MS m/z 412.207 0 [M]+ (Calcd. for C21H32O8: 412.209 7); IR (KBr): 3 383,2 969,2 934,1 677,1 514,1 429,1 383,1 204,1 187,1 138,1 076,1 037,840,802 and 723 cm-1; UV (MeOH) λmax (log ε): 203 (3.9),261 (3.5) nm; CD (MeOH): 265 (Δε -0.50) nm; 1H and 13C NMR spectral data,see Table 1.
2-Methoxy-4-(2-propenyl)-phenyl-β-D-glucopyra- noside (3)White solid; C16H22O7; positive ESI-MS m/z 349 [M+Na]+; 1H NMR (CD3OD,600 MHz) δ: 7.08 (1H,d,J = 7.8 Hz,H-6),6.83 (1H,d,J = 1.8 Hz,H-3),6.72 (1H,dd,J = 7.8,1.8 Hz,H-5),5.95 (1H,m,H-8),5.06 (1H,dd,J = 16.8,1.8 Hz,H-9b),5.03 (1H,dd,J = 9.6,1.8 Hz,H-9a),4.86 (1H,d,J = 7.8 Hz,H-1'),3.87 (1H,d,J = 10.8 Hz,H-6'a),3.84 (3H,s,2-OCH3),3.69 (1H,m,H-6'b),3.31-3.50 (6H,m,H-2'-5',7); 13C NMR (CD3OD,150 MHz) δ: 150.8 (C,C-2),146.4 (C,C-1),139.2 (CH,C-8),136.5 (C,C-4),122.2 (CH,C-5),118.2 (CH,C-6),116.0 (CH2,C-9),114.1 (CH,C-3),103.1 (CH,C-1'),78.3 (CH,C-3'),77.9 (CH,C-5'),75.0 (CH,C-2'),71.4 (CH,C-4'),62.6 (CH2,C-6'),56.8 (3-OCH3),40.9 (CH2,C-7).
ErigesideⅡ (4)Needle (CH3CN-H2O); C17H24O8; positive ESI-MS m/z 379 [M+Na]+,735 [2M+Na]+; 1H NMR (CDCl3, 500 MHz) δ: 6.41 (2H,s,H-3,5),5.89 (1H,m,H-8),5.08 (2H,m,H-9),4.53 (1H,d,J = 7.7 Hz,H-1'),3.81 (6H,s,OCH3 × 2),3.25-3.74 (6H,m,H-2'-6'); 13C NMR (CDCl3,100 MHz) δ: 152.4 (C,C-2,6),137.5 (C,C-1),136.7 (CH,C-8),130.4 (C,C-4),116.3 (CH2,C-9),105.7 (CH,C-3,5),103.0 (CH,C-1'),76.2 (CH,C-3'),76.1 (CH,C-5'),74.1 (CH,C-2') ,69.5 (CH,C-4'),61.6 (CH2,C-6'),56.2 (CH3,OCH3×2),40.4 (CH2,C-7).
Acantrifoside E (5)White powder; C17H24O8; positive ESI-MS m/z 379 [M+Na]+,735 [2M+Na]+; 1H NMR (DMSO-d6,500 MHz) δ: 6.66 (2H,s,H-3,5),6.31 (1H,d,J = 16.1 Hz,H-7),6.23 (1H,m,H-8),4.87 (1H,d,J = 7.1 Hz,H-1'),3.74 (6H,s,2-OCH3,6-OCH3),3.00-3.56 (6H,m,H-2'-6'),1.81 (3H,d,J = 6.2 Hz,H-9); 13C NMR (DMSO-d6, 100 MHz) δ: 152.7 (C,C-2,6),133.5 (C,C-1),133.2 (C,C-4),130.7 (CH,C-7),125.0 (CH,C-8),104.0 (CH,C-3,5),102.6 (CH,C-1'),77.2 (CH,C-3'),76.5 (CH,C-5'),74.2 (CH,C-2'),69.9 (CH,H-4'),60.9 (CH2,C-6'),56.3 (CH3,3,5-OCH3),18.2 (CH3,C-9).
Benzyl β-D-glucopyranoside (6)White solid; C13H18O6; positive ESI-MS m/z 293 [M+Na]+,563 [2M+Na]+; 1H NMR (CD3OD,400 MHz) δ: 7.44 (2H,d,J = 7.2 Hz,H-2,6),7.35 (2H,t,J = 7.2 Hz,H-3,5),7.29 (1H,t,J = 7.2 Hz,H-4),4.95 (1H,d,J = 12.0 Hz,H-7b),4.69 (1H,d,J = 12.0 Hz,H-7a),4.38 (1H,d,J = 7.7 Hz,H-1'),3.92 (1H,d,J = 11.9 Hz,H-6'a),3.72 (1H,dd,J = 11.9,5.3 Hz,H-6'b),3.26-3.38 (4H,m,H-2'-5'); 13C NMR (CD3OD,100 MHz) δ: 139.1 (C,C-1),129.3 (CH,C-3,5),129.2 (CH,C-2,6),128.7 (CH,C-4),103.3 (CH,C-1'),78.1 (CH,C-3') ,78.0 (CH,C-5'),75.1 (CH,C-2'),71.7 (CH2,C-7),71.7 (CH,C-4'),62.8 (CH2,C-6').
5,7-Dihydroxyl-3,8,4'-trimethoxylflavone (7)Yellow needle (CHCl3-MeOH); C18H16O7; positive ESI- MS m/z 711 [2M+Na]+; 1H NMR (CDCl3,400 MHz) δ: 12.45 (1H,s,OH),8.13 (2H,d,J = 8.8 Hz,H-2',6'),7.06 (2H,d,J = 8.8 Hz,H-3',5'),6.43 (1H,s,H-6),4.10 (3H,s,OCH3),3.92 (3H,s,OCH3),3.87 (3H,s,OCH3); 13C NMR (CDCl3,100 MHz) δ: 178.9 (C,C-4),161.7 (C,C-4'),157.3 (C,C-7),155.6 (C,C-5),155.1 (C,C-3),148.0 (C,C-9),138.7 (C,C-2),130.0 (CH,C-2',6'),126.8 (C,C-8),122.7 (C,C-1'),114.2 (CH,C-3',5'),105.5 (C,C-10),98.5 (CH,C-6),61.9 (C,3-OCH3),60.2 (C,8-OCH3),55.4 (C,4'-OCH3),
Kaempferol 3-O-β-D-glucopyranoside (8)Yellow needle (MeOH); C21H20O11: negative ESI-MS m/z 447 [M-H]+,895[2M-H]+; 1H NMR (DMSO-d6,500 MHz) δ: 12.62 (1H,s,OH),10.97 (1H,s,OH),10.26 (1H,s,OH),8.04 (2H,d,J = 8.7 Hz,H-2',H-6'),6.88 (2H,d,J = 8.7 Hz,H-3',H-5'),6.44 (1H,s,H-8),6.21 (1H,s,H-6),5.46 (1H,d,J = 7.5 Hz,H-1''),3.08-3.57 (6H,m,H-2''-6''); 13C NMR (DMSO-d6,150 MHz) δ: 177.5 (C,C-4),164.1 (C,C-7),161.2 (C,C-5),160.0 (C,C-4'),156.4 (C,C-2),156.2 (C,C-9),133.1 (C,C-3),130.9 (CH,C-2',6'),120.9 (C,C-1'),115.1 (CH,C-3',5'),104.0 (C,C-10),100.8 (CH,C-1''),98.7 (CH,C-6),93.6 (CH,C-8),77.5 (CH,C-3''),76.4 (CH,C-5''),74.2 (CH,C-2''),69.9 (CH,C-4''),60.8 (CH2,C-6').
Acknowledgments: The authors thank the analytical group from the State Key Laboratory of Phytochemistry and Plant Resources in West China,Kunming Institute of Botany,Chinese Academy of Sciences,for measuring the IR,UV,CD,NMR,[α] and mass spectra.
| [1] | Huang CJ. Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita. Flora Reipublicae Popularis Sinicae (中国植物志) [M]. Vol. 43. Part 2. Beijing: Science Press, 1997: 115-117. |
| [2] | Huang S, Wang JH, Luo XM, et al. Research process on chemical constituents and pharmacological activities of Micromelum [J]. J Chin Med Mater (中药材), 2011, 34: 1635-1638. |
| [3] | Cassady JM, Ojima N, Chang CJ, et al. An investigation of the antitumor activity of Micromelum integerrmum (Rutaceae) [J]. J Nat Prod, 1979, 42: 274-278. |
| [4] | He HP, Zou Y, Shen YM, et al. Three new coumarins from Micromelum integerrimum [J]. Chin Chem Lett, 2001, 12: 603-606. |
| [5] | Yang XL, Xie ZH, Jiang XJ, et al. A new acridone alkaloid from Micromelum integerrimum [J]. Chem Pharm Bull, 2009, 57: 734-735. |
| [6] | Susidarti RA, Rahmani M, Ismail H, et al. A new coumarin and triterpenes from Malaysian Micromelum minutum [J]. Nat Prod Res, 2006, 20: 145-151. |
| [7] | Susidarti RA, Rahmani M, Ali AM, et al. 8-Methoxycapnolactone and stigmasterol from Micromelum minutum [J]. Ind J Pharm, 2007, 18: 105-109. |
| [8] | Wang ZY, He WJ, Zhou WB, et al. Two new phenylpropanoids from Micromelum integerrimum [J]. Chin J Nat Med, 2014, 12: 1-4. |
| [9] | Zheng XK, Yan H, Li DD, et al. Chemical constituents of Caryopteris terniflora Maxim [J]. Chin Pharm J (中国药学杂志), 2013, 48: 1997-2001. |
| [10] | Meng ZX, Dong HL, Wang CL, et al. Chemical constituents of Dendrobium devonianum [J]. Chin Pharm J (中国药学杂志), 2013, 48: 855-859. |
| [11] | Kiem P, Minn C, Dat N, et al. Two new phenypropanoid glycosides from the stem bark of Acanthopanax trifoliatus [J]. Arch Pharm Res, 2003, 26: 1014-1017. |
| [12] | Nan ZD, Zhao MB, Jiang Y, et al. Chemical constituents from stems of Cistanche deserticola cultured in Tarim desert [J]. China J Chin Mater Med (中国中药杂志), 2013, 38: 2665-2670. |
| [13] | Wang JR, Duan JN, Zhou RH. Chemical constituents from the bark of Cercidiphyllum japonicum [J]. Acta Bot Sin (植物学报), 1999, 41: 209-212. |
| [14] | Zhou RG, Yang ZX, Wang J, et al. Chemical constituents from the leaves of Mangifera persiciformis [J]. Nat Prod Res Dev (天然产物研究与开发), 2012, 24: 1217-1219. |
| [15] | Pabst A, Barron D, Semon E, et al. 4-Oxo-β-ionol and linalool glycosides from Raspberry fruits [J]. Phytochemistry, 1992, 31: 4187-4190. |
 2015, Vol. 50
2015, Vol. 50


