2. 河南大学 药学院药物研究所, 河南 开封 475004
2. Institute of Pharmacy, Pharmaceutical College, Henan University Kaifeng 475004, China
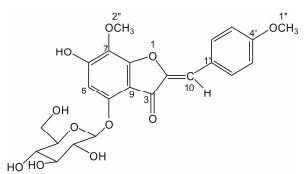
Veratrum dahuricum (Turcz.) Loes. f.,the important species of the genus Veratrum,and its folk name is Xingan Lilu in Chinese. Veratrum dahuricum (Turcz.) Loes. f. distributes mainly in Liaoning,Jilin,Heilongjiang,Neimonggu,and Xinjiang provinces in China. It was originally used as emetic prescription for treatment of hypertension,inflammation of throat disease,excessive phlegm,epilepsy,etc. Modern pharmacological studies have demonstrated that Veratrum dahuricum (Turcz.) Loes. f. possesses antifungus,antihypertension,antiplatelet aggregation,antitumor,and inhibition of multidrug resistance[1, 2, 3, 4]. Extensive chemical studies of this plant led to the isolation of steroidal alkaloids,flavonoids,dipeptides,and so on[5]. In this paper,we report the structure elucidation of a new aurone glycoside,named as (Z)-7,4'-dimethoxy-6-hydroxyl-aurone-4-O- β-glucopyranoside,which was isolated from the 95% ethanol extract of Veratrum dahuricum (Turcz.) Loes. f. (Figure 1),as well as the cytotoxicities of the compoundagainst human tumor cell lines ofHepG-2,MCF7 and A549 cells.
|
Figure 1 The structure of compound 1 |
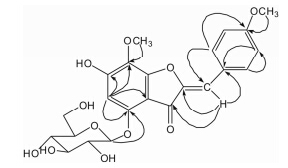
Compound 1 was obtained as a orange-red amorphous powder. The molecular formula C23H24O11 was deduced from HR-ESI-MS (positive) m/z : 477.139 4 [M+H]+ (calcd. for C23H25O11,477.139 7),499.121 3 [M+Na]+ (calcd. for C23H24O11Na,499.121 6),which was supported by the 13C NMR spectrum data. The 1H NMR spectrum (Table 1) of compound 1 exhibited two OMe signals at δ 3.80 (3H,s,H-1'') and 3.58 (3H,s,H-2''),six signals of olefinic H-atoms at δ 5.81 (1H,s,H-5),7.77 (2H,d,J = 8.8 Hz,H-2',6'),7.02 (2H,d,J = 8.8 Hz,H-3',5'),and 6.30 (1H,s,H-10),and signals of one glucose group at δ 4.89 (1H,d,J = 7.6 Hz,H-Glc-1),3.25 (1H,m,H-Glc-2),3.31 (1H,m,H-Glc-3),3.16 (1H,t,J = 8.8 Hz,H-Glc-4),3.28 (1H,m,H-Glc-5),3.70 (1H,d,J = 11.2 Hz,H-Glc-6),and 3.49 (1H,dd,J = 11.2,5.2 Hz,H-Glc-6). By analyses of the 1H,13C NMR and 2D NMR (HMBC,HSQC,NOESY) spectra of 1,it was revealed that 1 should be an aurone glucoside[6, 7]. The 13C NMR spectrum displayed 23 carbon signals,including fourteen olefinic C-atoms,one carbonyl C-atom,two methoxy C-atoms and six O-substituted C-atoms. The HMBC correlations were observed between δ 3.58 (H-2'') and δ 136.3 (C-7),δ 3.80 (H-1'') and δ 159.3 (C-4'),δ 5.81 (H-5) and δ 161.7 (C-6),107.3 (C-9),157.1 (C-4),136.3 (C-7),δ 6.30 (H-10) and δ 148.8 (C-2),131.6 (C-2',6'),180.7 (C-3),δ 7.02 (H-3',5') and δ 125.9 (C-1'),131.6 (C-2',6'),159.3 (C-4'),δ 7.77 (H-2',6') and δ 159.3 (C-4'),125.9 (C-1'),114.4 (C-3',5'),104.8 (C-10),suggesting that the structure of its aglycone was elucidated as 7,4'- dimethoxy-4,6-dihydroxyl-aurone by analysis of HMBC and HSQC spectra. The β configuration of glucose was substantiated by the coupling constant of the anomeric proton δ< /i> 4.89 (1H,d,J = 7.6 Hz,H-Glc-1). The position of the sugar was confirmed by the HMBC spectrum (Figure 2),which showed a correlation between δ 4.89 (1H,d,J = 7.6 Hz,H-Glc-1) and δ 157.1 (C-4),indicating the glucose group was attached to C-4. These assignments were further confirmed by the NOESY spectrum which showed correlations between δ 6.30 (H-10) and δ 7.77 (2H,d,J = 8.8 Hz,H-2',6'),δ 5.81 (1H,s,H-5) and δ 4.89 (1H,d,J = 7.6 Hz,H-Glc-1). The Z-stereochemistry of the double bond at C-10 in 1 was determined by the chemical shift of C-10 (δ 104.8)[8]. Based on these data,the structure of compound 1 was identified,and named as (Z)-7, 4'- dimethoxy-6-hydroxyl-aurone-4-O-β-glucopyranoside.
|
|
Table 1 1H NMR (400 MHz) and 13C NMR (100 MHz) data for compound 1 (in DMSO-d6) |
|
Figure 2 Key HMBC correlations of compound 1 |
UV data was determined by UV-VIS spectrophotometer (Shimadzu,Japan). NMR Spectra was recorded by Bruker ARX- 400 spectrometer in DMSO-d6 with TMS as an internal standard. The chemical shifts were quoted relative to TMS and the coupling constants were in Hz. HR- ESI-MS data were obtained on a MDS Sciex ESI-Q- TOF mass spectrometer. Column chromatography was performed on silica gel G (SiO2; 200-300 mesh,Qingdao Marine Chemical Factory,P. R. China). TLC was carried out on precoated silica gel GF-254 plates (Merck,Germany),and spots were detected with 10% H2SO4 in alcohol followed by heating. The reagents were analytical grade.
Plant materialThe rhizomes and roots of Veratrum dahuricum (Turcz.) Loes. f. were collected in July 2008 at Liaoning province,China and authenticated by Assoc. Prof. Wangjun Yuan from Henan University. The voucher specimen was deposited at the Institute of Pharmacy,Pharmaceutical College,Henan University.
Extraction and isolationThe air-dried rhizomes and roots of Veratrum dahuricum (Turcz.) Loes. f. (10 kg) was extracted with 5-fold 95% EtOH under reflux for 3 times. The extract was concentrated under reduced pressure to yield a residue (387 g),which was subjected to CC (SiO2; CH2Cl2/MeOH 100∶1 to 0∶100) to yield 15 combined fractions. Fr. 6 (0.8 g) was further subjected to CC (SiO2; petroleum ether/Me2CO 1∶1) to yield combined Frs. A-D. Frs. D (0.1 g) was further subjected to CC (SiO2; CH2Cl2/MeOH 10∶1 to 0∶10) and followed by recrystallization in MeOH to afford 1 (11 mg).
(Z)-7,4'-dimethoxy-6-hydroxyl-aurone-4-O-β- glucopyranoside (1)Orange-red amorphous powder; UV (MeOH) λmax (log ε) 257 (3.8),328 (4.6) (nm); 1H and 13C NMR spectral data see Table 1. ESI-MS (positive) m/z 499.1 [M+Na]+,477.1 [M+H]+; HR-ESI- MS (positive) m/z: 477.139 4 [M+H]+ (calcd. for C23H25O11,477.139 7),499.121 3 [M+Na]+ (calcd. for C23H24O11Na,499.121 6).
Cytotoxic assayThe in vitro cytotoxicity assay of compound 1 was evaluated by the MTT method using the HepG-2,MCF7,and A549 cell lines as previously described[9]. Compound 1 exhibited no cytotoxicities against HepG-2,MCF7 and A549 cell
lines with the IC50 value of 121.9,110.5 and 146.3 μmol·L-1,respectively.
| [1] | Mao XF, Shi ZC, Wang YZ. The advances in studies of Veratrums from China [J]. Animal Toxicol (动物毒物学), 2003, 18: 17-21. |
| [2] | Yaakov L, Tovi HO, William G, et al. Inhibitory effect of steroidal alkaloids on drug transport and multidrug resistance in human cancer cells [J]. Anticancer Res, 2001, 21: 1189- 1194. |
| [3] | Tang J, Li HL, Shen YH, et al. Antitumor activity of extracts and compounds from the rhizomes of Veratrum dahuricum [J]. Phytother Res, 2008, 22: 1093 -1096. |
| [4] | Zhao GJ, Peng RX, Ji ZD, et al. Hypotensive effect and its mechanism of three kinds of domestic veratrine (Xingshi veratrine, Maoye veratrine, Mashi veratrine) [J]. Acta Pharm Sin (药学学报), 1962, 9: 591-598. |
| [5] | Tang J, Li HL, Huang HQ, et al. Progress in the research on chemical constituents of Veratrum plants [J]. Prog Pharm Sci (药学进展), 2006, 30: 206-212. |
| [6] | Yu DQ, Yang JS. Analytic Chemical Handbook: Vol 7 (分 析化学手册: 第七卷) [M]. 2nd ed. Beijing: Chemical Industry Press, 2005: 831. |
| [7] | Huang HQ, Li HL, Tang J, et al. A new aurone and other phenolic constituents from Veratrum schindleri Loes. f. [J]. Biochem Syst Ecol, 2008, 36: 590-592. |
| [8] | Pelter A, Ward RS, Heller HG. Carbon-13 nuclear magnetic resonance spectra of (Z)- and (E)-aurones [J]. J Chem Soc, Perkin Trans 1, 1979: 328-329. |
| [9] | Liu MH, Qin C, Liu JK, et al. Study on the antitumor activity of albaconol and its effect on DNA topoisomerase Ⅱ [J]. Chin Pharmacol Bull (中国药理学通报), 2004, 20: 1224- 1228. |
 2015, Vol. 50
2015, Vol. 50


