表观遗传学调控是一个动态可逆的、不涉及DNA序列改变但是可遗传的过程,与细胞增殖、分化和凋亡关系密切。组蛋白的共价修饰可调节染色质的状态,进而影响基因的表达,因而在表观遗传学调控中占有重要地位。组蛋白乙酰化修饰是组蛋白共价修饰的重要方式之一,由组蛋白乙酰转移酶 (histone acetyltransferase,HAT) 和组蛋白去乙酰化酶 (histone deacetylase,HDAC) 协同控制。至今,已有4类18种哺乳动物HDAC得到确认: I类 (HDAC1~3,8)、II类 (II a类: HDAC4,5,7,9; II b类: HDAC6,10)、III类 (SIRT1~7) 和IV类 (HDAC11)。I、II和IV类为Zn2+依赖型蛋白酶; 而III类为NAD+依赖型[1, 2]。
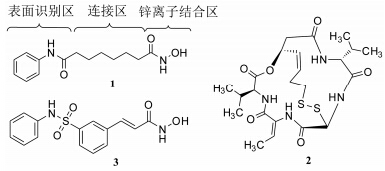
常见的组蛋白去乙酰化酶抑制剂 (HDACi) 包括表面识别区、连接区、锌离子结合区 (ZBG) 3部分。目前,伏立诺他 (vorinostat,1)、罗米地辛(romidepsin,2) 和belinostat (3) (图 1) 已被FDA批 准上市,还有一些抑制剂正在临床研究中。这些抑制剂大多是广谱的或多亚型选择性的,因而可能具有不必要的副作用[3]。HDAC6具有独特的结构和底物特异性,其表达和功能的改变与多种疾病密切相关,因此发展选择性HDAC6抑制剂已引起广泛关注。本文总结了近年来HDAC6及其选择性抑制剂 的研究进展。
|
图 1 FDA批准上市的HDACi |
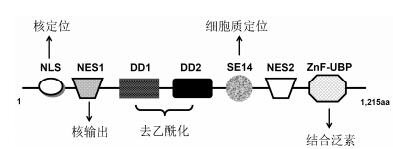
HDAC6基因定位于X染色体p11.22~23区带,约21 923 bp,由28个位于41至677 bp间的外显 子编码[4]。HDAC6蛋白含1 215个氨基酸残基,是HDAC家族中最大的。HDAC6的主要结构域包括核定位信号区 (NLS)、两个保守的富含亮氨酸的核输出信号区 (NES1,NES2)、两个串联的去乙酰化催化区 (DD1,DD2)、含丝氨酸-谷氨酸的十四肽重复区域(SE14) 及锌指结构 (ZnF-UBP)[5, 6, 7, 8, 9]。虽然结构中存在NLS,但是在NES和SE14的联合作用下,HDAC6主要存在于细胞质中。NES的作用是阻止HDAC6与细胞核蛋白结合,有利于其从细胞核转运至细胞质,而SE14则将HDAC6固定在细胞质[5]。两个去乙酰化催化区均有去乙酰化活性,但体外的去乙酰化活性主要由C端的DD2来完成[7, 8]。ZnF-UBP也被称为泛素结合区 (图 2)。
|
图 2 HDAC6的结构示意图 |
与其他亚型不同的是,HDAC6具有独特的非组蛋白底物特异性,其作用的底物主要包括α-微管蛋白、皮动蛋白和热休克蛋白90 (Hsp90)[10, 11, 12, 13, 14]。这些蛋白都是在胞浆中表达的,与HDAC6的亚细胞定位相符,但是某些条件下HDAC6也会出现在细胞核中[15]。
α-微管蛋白是第一个被证实的HDAC6去乙酰化底物,其可逆的乙酰化状态能够显著影响微管的稳定性和功能[10]。例如: HDAC6的过度表达会导致微管蛋白处于低乙酰化水平,并促进趋化性细胞运动; 相反,抑制HDAC6功能导致微管蛋白超乙酰化及黏着斑过度聚积,进而抑制纤维原细胞运动。除了调节微管蛋白依赖的细胞运动,HDAC6还可改变皮动蛋白的乙酰化状态,影响其结合纤维状肌动蛋白的能力,从而调节肌动蛋白依赖的细胞运动[14]。研究人员还发现HDAC6能够调节Hsp90的乙酰化状态[11, 12]。由于Hsp90可增加许多重要信号蛋白的活性和稳定性,因此HDAC6对Hsp90乙酰化状态的调控也是其影 响细胞信号传导的重要因素[16]。
除了去乙酰化酶的活性外,HDAC6还有结合泛素功能[9, 17]。正常情况下错误折叠蛋白可被蛋白酶体有效降解。当蛋白酶体受损时,错误折叠蛋白形成聚合物。此聚合物在去泛素化酶ataxin-3作用下产生HDAC6结合位点。HDAC6与这些聚合物结合后,一方面可与动力蛋白复合物形成三聚体,将错误折叠蛋白沿微管逆向转运到微管组织中心形成聚集体,最终通过自噬消除; 另一方面,可激活热休克转录因子1 (HSF1),诱导Hsp25、Hsp70的表达,指导蛋白质正确折叠,参与错误折叠蛋白的修复降解。
3 HDAC6与疾病HDAC6在某些肿瘤细胞中表达异常[18, 19],例如原发性口腔鳞癌细胞中HDAC6的表达上调,并且 其表达水平与肿瘤分期相关[20]。研究发现HDAC6可激活致癌Ras信号通路和肿瘤细胞存活信号通路,使转化的细胞进行锚定非依赖性的增殖,有利于细胞逃避失巢凋亡现象而存活,从而促进肿瘤的发生和转化[21]。另外,许多致癌蛋白的结构成熟和活性需要HDAC6底物Hsp90的参与,当HDAC6失活时,Hsp90超乙酰化,肿瘤生长受到抑制。
神经退行性疾病中常见轴突运输功能受损、线粒体转运功能紊乱和错误折叠蛋白积聚[22, 23, 24]。HDAC6与相关蛋白相互作用改善并修复这些功能,产生治疗神经退行性疾病的效果。例如,抑制HDAC6可升高α-微管蛋白乙酰化水平,进而改善受损的轴突运输功能,产生神经保护作用; 线粒体泛素化后可以募集HDAC6,从而实现对受损线粒体的自噬。
此外,研究表明HDAC6与自身免疫性疾病相 关[25, 26]。HDAC6可抑制调节性T细胞介导的免疫抑制,而抑制HDAC6可增强调节性T细胞功能并恢复免疫稳态[26]。
4 选择性HDAC6抑制剂HDAC6通过与底物蛋白相互作用,参与并调节众多生理或病理进程,其选择性抑制剂在治疗多种疾病方面具有广阔的前景。在同源模建和分子对接等计算机辅助药物设计技术的帮助下,研究人员设计出了形式多样的选择性HDAC6抑制剂,这些抑制剂具有纳摩尔甚至皮摩尔的HDAC6抑制活性,并表现出明显的HDAC6选择性。
4.1 长链异羟肟酸类第一个选择性HDAC6抑制剂是Haggarty等[27, 28]在2003年报道的tubacin (4)。Tubacin为“T”字型结构,表面识别区结构独特,含五个芳香环及一个三手性中心的二氧六环。研究表明tubacin对HDAC1、HDAC6和HDAC8不同的作用主要来源于I类和II类HDAC催化通道周围蛋白表面的差异[29]。Tubacin可诱导α-微管蛋白的乙酰化,而对组蛋白的乙酰化、基因表达及细胞周期无影响; 此外,研究发现该化合物可抑制HDAC6和动力蛋白dynein之间的相互作用,从而引起泛素化蛋白积聚[30]。
化合物5、6的结构与tubacin类似,仅表面识别区存在差异[31, 32]。研究表明化合物5结构中1,2,3-三氮唑及其取代的变化对HDAC6选择性的影响不 大,取代苯基是产生选择性的结构决定因素; 化合物6连接区的长度及氨基的保护基影响其对HDAC6的活性和选择性。化合物5、6均可抑制胰腺癌细胞生长,且化合物6的抑制效果为SAHA的10倍左右。
Rocilinostat (ACY-1215,7) 是目前唯一一个进入临床研究的选择性HDAC6抑制剂[33],其与bortezomib或lenalidomide合用对多发性骨髓瘤 (multiple myeloma,MM) 可以产生协同治疗作用。该疗法具体的作用机制还未完全明了,其中一种解释为药物同时抑制了泛素-蛋白酶体通路和聚集体降解通路,导致细胞内错误折叠蛋白聚积,最终诱发细胞凋亡。
化合物8为环肽类选择性HDAC6抑制剂,含环α3β四肽骨架,通过珠一化法 (one-bead-one-compound) 获得,可抑制HeLa细胞生长[34]。化合物9为手性大环内酰胺类选择性HDAC6抑制剂,对肺癌和结肠癌细胞 (H460和HCT-116) 表现出优于SAHA的细胞毒活性[35]。
4.2 N-羟基苯甲酰胺类Smil等[36]对apicidin (10) 的结构改造得到了一系列具有手性的选择性HDAC6抑制剂 (如11)。研究表明该类化合物对HDAC6的抑制活性和选择性取决于手性基团的绝对构型,R型化合物优于S型; 大多数化合物可有效诱导α-微管蛋白乙酰化,而对组蛋白H3乙酰化无影响,这进一步确证化合物的HDAC6选择性。
Tubastatin A (12) 及其衍生物是已报道的活性、选择性和类药性最高的HDAC6抑制剂,该类化合物的结构差异在于咔啉环的类型及N-取代[37, 38]。在皮层原代神经元培养中tubastatin A对谷胱甘肽耗竭诱导的氧化应激具有剂量依赖性保护作用。化合物13是tubastatin A的砜类衍生物,研究表明tubastatin A砜类衍生物的活性优于其相应的硫醚类化合物。大多数此类化合物可促进转录因子FOXp3的乙酰化,该转录因子在T细胞免疫应答中发挥重要作用,因而该类化合物具有治疗自身免疫性疾病的潜在价值[39]。
Nexturastat A (14) 和HPOB (15) 为N,N-二取代的“Y”形结构化合物[40, 41]。对nexturastat A及其衍生物的研究表明,脲N-取代,尤其是靠近ZBG的N-取代可增加化合物对HDAC6的抑制活性和选择性。14可有效抑制B16黑色素瘤细胞的生长; 15可增强依托泊苷、多柔比星或SAHA诱导的肿瘤细胞死亡,而且在浓度 ≤ 16 μmol·L-1时15对正常细胞无明显影响。
Blackburn等[42]认为苄基能更有效地与HDAC6 的催化通道结合,因而有利于对HDAC6的选择性,并以4-胺甲基-N-羟基苯甲酰胺的酰化物为先导合成不同大小的杂环衍生物 (如16)。化合物16具有良好的HDAC6抑制作用和选择性,对基质金属蛋白酶几乎没有抑制 (>100 μmol·L-1),而且水溶性好 (pH 7.5时2 mmol·L-1)。
4.3 N-羟基肉桂酸类研究发现大多数含取代苯基和肉桂酸骨架的化合物抗增殖活性优于SAHA[43]。其中ST3595 (17) 具有HDAC6选择性,该化合物与紫杉醇合用可产生协同抗癌作用[44]。2位和3位取代的喹唑啉-4-酮类化合物 (如18) 表现出明显的HDAC6抑制活性和选择性,具有治疗神经退行性疾病的潜力: 对神经元和Vero细胞无毒副作用,在体外可抑制锌离子介导的Aβ聚积,也可诱导轴突生长和突触活动[45]。
Lee等[46]合成一系列含吲哚/氮杂吲哚的N-羟 基肉桂酸类HDAC抑制剂。研究发现含7-氮杂吲哚的化合物19对HDAC6的抑制活性及选择性要优于含吲哚、吲唑、6-氮杂吲哚和7-氮杂吲唑的化合物。该化合物小鼠的口服生物利用度为33%,对结直肠癌HCT116细胞的抑制作用优于SAHA。
4.4 硫醇类巯基具有较好的锌离子亲和力,巯基成酯有利于提高化合物稳定性和亲脂性。研究表明含有氨基甲酸叔丁酯基团及大体积烷基的化合物 (如20) 具有良好的HDAC6抑制活性和选择性[47, 48, 49]。化合物20可抑制雌激素α受体阳性的乳腺癌MCF-7细胞的生长,与紫杉醇合用还可产生协同抗肿瘤作用。该化合物的巯基换为异羟肟酸后仍具有HDAC6选择性,表明巯基或许不是该化合物产生HDAC6选择性的决定因素[50]。
4.5 疏基乙酰胺类研究发现含氨基酸残基修饰的2,4'-二氨基联苯类化合物 (如21) 具有HDAC6选择性,可保护皮层神经元免受氧化应激引起的死亡危害[51],但该类化合物易经氧化二聚形成二硫物而失活。为了解决这个问题,同一课题组通过结构改造并合成了一系列二芳基衍生物 (如22)[52]。这些化合物对胰腺癌细胞具有良好的抑制活性。进一步的研究发现α位取代,尤其是α位R-甲基取代,会提高化合物对HDAC6的选择性[53]。
4.6 其他三氟乙酰噻吩类化合物被发现具有II类HDAC选择性抑制活性[54],该类化合物在细胞中易被羰基还原酶快速代谢而失活。对此改造过程中发现了化合物23,其稳定性提高 (HCT116细胞中t1/2 = 11 h),并具有良好的HDAC6抑制活性和选择性[55]。研究发现线型长链磺胺类化合物 (如24) 具有HDAC6选择性,而具有赖氨酸骨架的磺胺类化合物对HDAC1和HDAC6都均有明显抑制作用[56]。
Inks等[57]通过化合物筛选发现了具有HDAC6选择性的萘醌类化合物 (如25)。虽然结构中含Michael受体,但大多数化合物没有明显毒性。在人AML细胞中,NQN-1 (25) 可诱导微管蛋白和Hsp90的高度乙酰化; 该化合物还能引起Hsp90下游蛋白mutant FLT-3降解及STAT5的成型活化。一些不含表面识别区的异羟肟酸类小分子 (如26) 被证明具有良好的HDAC6抑制活性和选择性[58],研究发现异羟肟酸α位的sp2碳原子有利于化合物的HDAC6抑制活性。
5 小结综上所述,对HDAC6及其选择性抑制剂的研究已取得了丰硕成果。但是,仍有许多制约选择性HDAC6抑制剂发展应用的问题有待解决。例如,HDAC6蛋 白晶体结构未知; HDAC6的底物众多,如何避免脱靶效应; 大多数已知的抑制剂是相对选择性或优先选择性的,如何获得真正选择性的抑制剂等。总之,HDAC6作为新型药物治疗靶点已引起人们广泛关注,其选择性抑制剂在多种疾病的治疗方面前景广阔。
| [1] | Arrowsmith CH, Bountra C, Fish PV, et al. Epigenetic protein families: a new frontier for drug discovery [J]. Nat Rev Drug Discov, 2012, 11: 384-400. |
| [2] | Tan YM, Huang WY, Yu NF. Structure-activity relationships of histone deacetylase inhibitors [J]. Acta Pharm Sin (药学学报), 2009, 44: 1072-1083. |
| [3] | Balasubramanian S, Verner E, Buggy JJ. Isoform-specific histone deacetylase inhibitors: the next step? [J]. Cancer Lett, 2009, 280: 211-221. |
| [4] | Mahlknecht U, Schnittger S, Landgraf F, et al. Assignment of the human histone deacetylase 6 gene (HDAC6) to X chromosome p11.23 by in situ hybridization [J]. Cytogenet Cell Genet, 2001, 93: 135-136. |
| [5] | Bertos NR, Gilquin B, Chan GK, et al. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention [J]. J Biol Chem, 2004, 279: 48246-48254. |
| [6] | Boyault C, Sadoul K, Pabion M, et al. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination [J]. Oncogene, 2007, 26: 5468-5476. |
| [7] | Zhang Y, Gilquin B, Khochbin S, et al. Two catalytic domains are required for protein deacetylation [J]. J Biol Chem, 2006, 281: 2401-2404. |
| [8] | Zou H, Wu Y, Navre M, et al. Characterization of the two catalytic domains in histone deacetylase 6 [J]. Biochem Biophys Res Commun, 2006, 341: 45-50. |
| [9] | Ouyang H, Ali YO, Ravichandran M, et al. Protein aggregates are recruited to aggresome by histone deacetylase 6 via unanchored ubiquitin C termini [J]. J Biol Chem, 2012, 287: 2317-2327. |
| [10] | Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase [J]. Nature, 2002, 417: 455-458. |
| [11] | Bali P, Pranpat M, Bradner J, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors [J]. J Biol Chem, 2005, 280: 26729-26734. |
| [12] | Kovacs JJ, Murphy PJ, Gaillard S, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor [J]. Mol Cell, 2005, 18: 601-607. |
| [13] | Tran AD, Marmo TP, Salam AA, et al. HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions [J]. J Cell Sci, 2007, 120: 1469-1479. |
| [14] | Zhang X, Yuan Z, Zhang Y, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin [J]. Mol Cell, 2007, 27: 197-213. |
| [15] | Palijan A, Fernandes I, Bastien Y, et al. Function of histone deacetylase 6 as a cofactor of nuclear receptor coregulator LCoR [J]. J Biol Chem, 2009, 284: 30264-30274. |
| [16] | Eckl JM, Richter K. Functions of the Hsp90 chaperone system: lifting client proteins to new heights [J]. Int J Biochem Mol Biol, 2013, 4: 157-165. |
| [17] | Boyault C, Zhang Y, Fritah S, et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates [J]. Genes Dev, 2007, 21: 2172-2181. |
| [18] | Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer [J]. J Biomed Biotechnol, 2011, 2011: 875824. |
| [19] | Haakenson J, Zhang X. HDAC6 and ovarian cancer [J]. Int J Mol Sci, 2013, 14: 9514-9535. |
| [20] | Sakuma T, Uzawa K, Onda T, et al. Aberrant expression of histone deacetylase 6 in oral squamous cell carcinoma [J]. Int J Oncol, 2006, 29: 117-124. |
| [21] | Lee YS, Lim KH, Guo X, et al. The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis [J]. Cancer Res, 2008, 68: 7561-7569. |
| [22] | Simoes-Pires C, Zwick V, Nurisso A, et al. HDAC6 as a target for neurodegenerative diseases: what makes it different from the other HDACs? [J]. Mol Neurodegener, 2013, 8:7. |
| [23] | Zhang L, Sheng S, Qin C. The role of HDAC6 in Alzheimer's disease [J]. J Alzheimers Dis, 2013, 33: 283-295. |
| [24] | Gong HC, Wang YL, Wang HW. Epigenetic mechanisms of Alzheimer's disease and related drug research [J]. Acta Pharm Sin (药学学报), 2013, 48: 1005-1013. |
| [25] | Hancock WW, Akimova T, Beier UH, et al. HDAC inhibitor therapy in autoimmunity and transplantation [J]. Ann Rheum Dis, 2012, 71 Suppl 2: i46-54. |
| [26] | de Zoeten EF, Wang L, Butler K, et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells [J]. Mol Cell Biol, 2011, 31: 2066-2078. |
| [27] | Haggarty SJ, Koeller KM, Wong JC, et al. Multidimensional chemical genetic analysis of diversity-oriented synthesis-derived deacetylase inhibitors using cell-based assays [J]. Chem Biol, 2003, 10: 383-396. |
| [28] | Haggarty SJ, Koeller KM, Wong JC, et al. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation [J]. Proc Natl Acad Sci USA, 2003, 100: 4389-4394. |
| [29] | Estiu G, Greenberg E, Harrison CB, et al. Structural origin of selectivity in class II-selective histone deacetylase inhibitors [J]. J Med Chem, 2008, 51: 2898-2906. |
| [30] | Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma [J]. Proc Natl Acad Sci USA, 2005, 102: 8567-8572. |
| [31] | Chen Y, Lopez-Sanchez M, Savoy DN, et al. A series of potent and selective, triazolylphenyl-based histone deacetylases inhibitors with activity against pancreatic cancer cells and Plasmodium falciparum [J]. J Med Chem, 2008, 51: 3437-3448. |
| [32] | Kozikowski AP, Tapadar S, Luchini DN, et al. Use of the nitrile oxide cycloaddition (NOC) reaction for molecular probe generation: a new class of enzyme selective histone deacetylase inhibitors (HDACIs) showing picomolar activity at HDAC6 [J]. J Med Chem, 2008, 51: 4370-4373. |
| [33] | Santo L, Hideshima T, Kung AL, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma [J]. Blood, 2012, 119: 2579-2589. |
| [34] | Olsen CA, Ghadiri MR. Discovery of potent and selective histone deacetylase inhibitors via focused combinatorial libraries of cyclic α3 β-tetrapeptides [J]. J Med Chem, 2009, 52: 7836- 7846. |
| [35] | Auzzas L, Larsson A, Matera R, et al. Non-natural macrocyclic inhibitors of histone deacetylases: design, synthesis, and activity [J]. J Med Chem, 2010, 53: 8387- 8399. |
| [36] | Smil DV, Manku S, Chantigny YA, et al. Novel HDAC6 isoform selective chiral small molecule histone deacetylase inhibitors [J]. Bioorg Med Chem Lett, 2009, 19: 688-692. |
| [37] | Butler KV, Kalin J, Brochier C, et al. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A [J]. J Am Chem Soc, 2010, 132: 10842-10846. |
| [38] | Kalin JH, Butler KV, Akimova T, et al. Second-generation histone deacetylase 6 inhibitors enhance the immunosuppressive effects of Foxp3+ T-regulatory cells [J]. J Med Chem, 2012, 55: 639-651. |
| [39] | De Vreese R, Verhaeghe T, Desmet T, et al. Potent and selective HDAC6 inhibitory activity of N-(4-hydroxycarbamoylbenzyl)-1, 2, 4, 9-tetrahydro-3-thia-9-azafluorenes as novel sulfur analogues of tubastatin A [J]. Chem Commun (Camb), 2013, 49: 3775-3777. |
| [40] | Bergman JA, Woan K, Perez-Villarroel P, et al. Selective histone deacetylase 6 inhibitors bearing substituted urea linkers inhibit melanoma cell growth [J]. J Med Chem, 2012, 55: 9891-9899. |
| [41] | Lee JH, Mahendran A, Yao Y, et al. Development of a histone deacetylase 6 inhibitor and its biological effects [J]. Proc Natl Acad Sci USA, 2013, 110: 15704-15709. |
| [42] | Blackburn C, Barrett C, Chin J, et al. Potent histone deacetylase inhibitors derived from 4-(aminomethyl)-N-hydroxybenzamide with high selectivity for the HDAC6 isoform [J]. J Med Chem, 2013, 56: 7201-7211. |
| [43] | Chen Y, He R, Chen Y, et al. Studies of benzamide-and thiol-based histone deacetylase inhibitors in models of oxidative-stress-induced neuronal death: identification of some HDAC3-selective inhibitors [J]. ChemMedChem, 2009, 4: 842-852. |
| [44] | Zuco V, De Cesare M, Cincinelli R, et al. Synergistic antitumor effects of novel HDAC inhibitors and paclitaxel in vitro and in vivo [J]. PLoS One, 2011, 6: e29085. |
| [45] | Yu CW, Chang PT, Hsin LW, et al. Quinazolin-4-one derivatives as selective histone deacetylase-6 inhibitors for the treatment of Alzheimer's disease [J]. J Med Chem, 2013, 56: 6775-6791. |
| [46] | Lee HY, Tsai AC, Chen MC, et al. Azaindolylsulfonamides, with a more selective inhibitory effect on histone deacetylase 6 activity, exhibit antitumor activity in colorectal cancer HCT116 cells [J]. J Med Chem, 2014, 57: 4009-4022. |
| [47] | Heltweg B, Dequiedt F, Marshall BL, et al. Subtype selective substrates for histone deacetylases [J]. J Med Chem, 2004, 47: 5235-5243. |
| [48] | Suzuki T, Kouketsu A, Itoh Y, et al. Highly potent and selective histone deacetylase 6 inhibitors designed based on a small-molecular substrate [J]. J Med Chem, 2006, 49: 4809- 4812. |
| [49] | Itoh Y, Suzuki T, Kouketsu A, et al. Design, synthesis, structure-selectivity relationship, and effect on human cancer cells of a novel series of histone deacetylase 6-selective inhibitors [J]. J Med Chem, 2007, 50: 5425-5438. |
| [50] | Gupta PK, Reid RC, Liu L, et al. Inhibitors selective for HDAC6 in enzymes and cells [J]. Bioorg Med Chem Lett, 2010, 20: 7067-7070. |
| [51] | Kozikowski AP, Chen Y, Gaysin A, et al. Functional differences in epigenetic modulators-superiority of mercaptoacetamide-based histone deacetylase inhibitors relative to hydroxamates in cortical neuron neuroprotection studie [J]. J Med Chem, 2007, 50: 3054-3061. |
| [52] | Kozikowski AP, Chen Y, Gaysin AM, et al. Chemistry, biology, and QSAR studies of substituted biaryl hydroxamates and mercaptoacetamides as HDAC inhibitors-nanomolar-potency inhibitors of pancreatic cancer cell growth [J]. ChemMedChem, 2008, 3: 487-501. |
| [53] | Kalin JH, Zhang H, Gaudrel-Grosay S, et al. Chiral mercaptoacetamides display enantioselective inhibition of histone deacetylase 6 and exhibit neuroprotection in cortical neuron models of oxidative stress [J]. ChemMedChem, 2012, 7: 425-439. |
| [54] | Jones P, Bottomley MJ, Carfi A, et al. 2-Trifluoroacetylthiophenes, a novel series of potent and selective class II histone deacetylase inhibitors [J]. Bioorg Med Chem Lett, 2008, 18: 3456-3461. |
| [55] | Ontoria JM, Altamura S, Di Marco A, et al. Identification of novel, selective, and stable inhibitors of class II histone deacetylases. Validation studies of the inhibition of the enzymatic activity of HDAC4 by small molecules as a novel approach for cancer therapy [J]. J Med Chem, 2009, 52: 6782-6789. |
| [56] | Wahhab A, Smil D, Ajamian A, et al. Sulfamides as novel histone deacetylase inhibitors [J]. Bioorg Med Chem Lett, 2009, 19: 336-340. |
| [57] | Inks ES, Josey BJ, Jesinkey SR, et al. A novel class of small molecule inhibitors of HDAC6 [J]. ACS Chem Biol, 2012, 7: 331-339. |
| [58] | Wagner FF, Olson DE, Gale JP, et al. Potent and selective inhibition of histone deacetylase 6 (HDAC6) does not require a surface-binding motif [J]. J Med Chem, 2013, 56: 1772- 1776. |
 2015, Vol. 50
2015, Vol. 50


