2. 中国科学院昆明植物研究所, 植物化学与西部植物资源持续利用国家重点实验室, 云南 昆明 650201;
3. 江西农业大学农学院, 江西 南昌 330045
2. State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China;
3. College of Agriculture, Jiangxi Agricultural University, Nanchang 330045, China
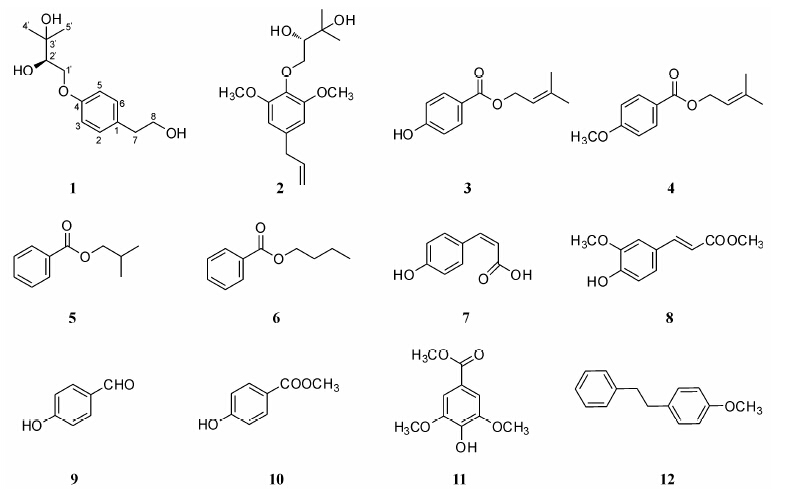
In our previous studies[1, 2]on the chemical constituents of Clausena excavata Burm. f. (Rutaceae),which has been used as a folk medicine for treatment of dysentery,enteritis,and urethra infection[3, 4]. The isolation and structure elucidation of some carbazole alkaloids and coumarins from its roots,leavesand stems were reported. To continue our studies,the roots,stems and leaves of C. excavatawere investigated. Herein,this paper described the isolation and structure elucidation of a new phenethanol,(2'R)-4-(2',3'-dihydroxy-3'-methyl-butanoxy)-phenethanol (1),along with other eleven known benzene derivatives (2-12),and the evaluation of cytotoxic and antimicrobial activities of compound 1. Compounds 3 and 4 are new natural products,and compounds 5-8,10-12 were isolated from C. excavata for the first time.
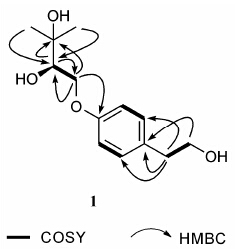
Results and discussion(2'R)-4-(2',3'-dihydroxy-3'-methyl-butanoxy)-phen- ethanol (1),[α] +2.06 (c 0.11,MeOH),was obtained as pale yellow oil. Its molecular formula was determined as C13H20O4 by the HR-EI-MS at m/z 240.136 4 ([M]+,Calcd. 240.136 2). The IR spectrum indicated the presence of OH at 3 406 cm-1. Its 1H NMR spectrum (Table 1) revealed that it contained one 1,4-disubstituted benzene ring (δH 7.12,2H,d,J = 7.5 Hz; 6.86,2H,d,J = 7.5 Hz) and two CH3 (δH 1.25,3H,s; 1.21,3H,s). The 13C NMR spectrum (Table 1) showed a total of thirteen carbon signals corresponding to two CH3,three CH2,five CH and three C. The 1H,1H-COSY (Figure 1) correlations of H-2,6/H-3,5 and H-7/H-8 showed that 1 had a 1,4-disubstituted benzene ring and a [-CH2CH2OH] moiety. The correlations of H-1'/(C-2',C-3'),H-2'/(C-1',C-3'),(H-4',H-5')/C-2' inthe HMBC spectrum (Figure 1) and the correlation of H-1'/H-2' in the 1H,1H-COSY spectrum indicated the presence of a [-CH2CH(OH)C(CH3)2OH] unit. In addition,The correlations of H-1'/C-4 in the HMBC spectrum indicated that [-CH2CH(OH)C(CH3)2OH] is attached to C-4. In the same spectrum,the correlations of H-7/C-1,C-2,C-6 and H-8/C-1 allowed the location of [-CH2CH2OH] at C-1. The optical rotation [α] 2.06 (c 0.11,MeOH) of 1 is opposite to the optical rotations of known compounds lenisin A ([α] -17.09) and lenisin C ([α] -27.41)[5]. It implied that the absolute configurations of 1 at C-2',and lenisin A and C at C-2'are different,because there is only one chiral carbon in them. For the absolute configuration of lenisin A and C at C-2' is S,that of 1 at C-2' should be R. Therefore,the structure of 1 was established to be a (2'R)-4-(2',3'-dihydroxy-3'-methyl-butanoxy)-phenethanol and as shown in Figure 2. 1H,13C NMR data of 1 see Table 1.
|
|
Table 1 1H,13C NMR data of 1 at 400 and 100 MHz,in CD3OD,separately |
|
Figure 1 Key 1H-1H COSY,and HMBC correlations of compound 1 |
|
Figure 2 Structures of compounds 1-12 |
Lenisin A (2): pale yellow oil,[α] -22.19 (c 0.21,MeOH); EI-MS m/z 296 [M]+,C16H24O5. 1H NMR (CDC13,400 MHz): δH 6.59 (2H,s,H-2,6),6.07 (1H,m,H-8),5.18 (2H,m,H-9),4.86 (1H,dd,J = 10.0,3.2 Hz,H-1'a),4.43 (1H,dd,J = 10.0,8.2 Hz,H-1'b),4.28 (1H,dd,J = 8.2,3.2 Hz,H-2'),3.72 (6H,s,2CH3),3.37 (2H,d,J = 6.7 Hz,H-7),1.54 (3H,s,H-5'),1.52 (3H,s,H-4'); 13C NMR (CDC13,100 MHz): δC 152.6 (s,2×C- 3,5),136.9 (s,C-1),136.2 (d,C-8),134.9 (s,C-4),116.2 (t,C-9),105.2 (d,2×C-2,6),75.7 (d,C-2'),75.4 (t,C-1'),71.4 (s,C-3'),55.9 (q,2×OCH3),40.5 (t,C-7),26.7 (q,C-5'),24.9 (q,C-4')[5].
3-Methylbut-2-enyl-4-hydroxybenzoate (3): colorless oil,EI-MS m/z 206 [M]+,C12H14O3. 1H NMR (CDC13,400 MHz): δH 8.05 (2H,d,J = 8.8 Hz,H-2,6),6.94 (2H,d,J = 8.8 Hz,H-3,5),5.49 (1H,m,H-2'),4.57 (2H,d,J = 6.7 Hz,H-1'),1.75 (3H,s,H-4'),1.64 (3H,s,H-5'); 13C NMR (CDC13,100 MHz): δC 171.9 (s,CO),163.3 (s,C-4),138.9 (s,C-3'),132.3 (d,2×C-2,6),121.5 (s,C-1),118.9 (d,C-2'),114.3 (d,2×C-3,5),64.9 (t,C-1'),25.8 (q,C-4'),18.2 (q,C-5')[6].
3-Methylbut-2-enyl 4-methoxybenzoate (4): colorless oil,ESI-MS m/z 243 [M+Na]+,C13H16O3,1H NMR (acetone-d6,400 MHz): δH 7.94 (2H,m,H-2,6),7.01 (2H,m,H-3,5),5.46 (1H,m,H-2'),4.63 (2H,d,J = 6.6 Hz,Hz,H-1'),3.82 (3H,s,4-OCH3),1.75 (3H,s,H-4'),1.73 (3H,s,H-5'); 13C NMR (acetone-d6,100 MHz): δC 166.8 (s,C=O),163.7 (s,C-4),138.5 (s,C-3'),132.1 (d,2×C-2,6),123.2 (s,C-1),120.4 (d,C-2'),115.2 (d,2×C-3,5),65.6 (t,C-1'),51.9 (4-OCH3),25.7 (q,C-4'),18.1 (q,C-5')[7].
Isobutyl benzoate (5): colorless oil,EI-MS m/z 178 [M]+,C11H14O2,1H NMR (CDCl3,500 MHz): δH 8.10 (2H,m,H-2,6),7.91 (2H,m,H-3,5),7.63 (1H,m,H-4),4.45 (2H,d,J = 6.6 Hz,H-1'),2.41 (1H,m,H-2'),1.36 (6H,d,J = 6.7 Hz,2CH3); 13C NMR (CDCl3,125 MHz): δC 167.7 (s,CO),132.3 (s,C-1),130.9 (d,C-4),130.8 (d,2×C-2,6),128.8 (d,2×C-3,5),71.8 (t,C-1'),27.7 (d,C-2'),19.1 (q,2×CH3)[8].
Butyl benzoate (6): colorless oil,EI-MS m/z 178 [M]+,C11H14O2,1H NMR (CDCl3,400 MHz): δH 7.72 (2H,m,H-2,6),7.53 (2H,m,H-3,5),7.67 (1H,m,H-4),4.31 (2H,t,J = 6.7 Hz,H-1'),1.72 (2H,m,H-2'),1.45 (2H,m,H-3'),0.98 (3H,t,J = 7.4 Hz,4'-CH3); 13C NMR (CDCl3,100 MHz): δC 167.7 (s,CO),132.2 (s,C-1),130.9 (d,C-4),130.2 (d,2×C-2,6),128.8 (d,2×C-3,5),65.5 (t,C-1'),30.5 (t,C-2'),19.1 (t,C-3'),13.7 (q,C-4')[9, 10].
(Z)-3-(4'-Hydroxyphenyl)acrylic acid (7): pale yellow oil,ESI-MS m/z 187 [M+Na]+,C9H8O3. 1H NMR (CD3OD,400 MHz): δH 7.59 (1H,d,J = 15.9 Hz,H-3),7.44 (2H,J = 8.5 Hz,H-2',6'),6.80 (2H,J = 8.5 Hz,H-3',5'),6.27 (1H,d,J = 15.9 Hz,H-2); 13C NMR (CD3OD,100 MHz): δC 171.1 (s,C-1),161.2 (s,C-4'),146.7 (d,C-3),131.1 (d,2×C-2',6'),127.2 (s,C-1'),116.8 (d,2×C-3',5'),115.6 (d,C-2)[11].
(E)-Methyl 3-(4-hydroxy-3-methoxyphenyl)acrylate (8): pale yellow oil,ESI-MS m/z 231 [M+Na]+,C11H12O4. 1H NMR (CDCl3,400 MHz): δH 7.61 (1H,d,J = 15.9 Hz,H-1'),7.06 (1H,dd,J = 8.2,1.8 Hz,H-5),7.01 (1H,d,J = 1.8 Hz,H-3),6.91 (1H,d,J = 8.2 Hz,H-6),6.26 (1H,d,J = 15.9 Hz,H-2'),3.91 (3H,s,2-OCH3),3.79 (3H,s,3'-OCH3); 13C NMR (CDCl3,100 MHz): δC 167.7 (s,CO),147.9 (s,C-2),146.7 (s,C-4),144.9 (d,C-1'),126.8 (s,C-1),123.0 (d,C-5),115.1 (d,C-6),114.7 (d,C-2'),109.3 (d,C-3),55.9 (q,2-OCH3),51.6 (q,3'-OCH3)[12].
4-Hydroxybenzaldehyde (9): colorless oil,ESI- MS m/z 145 [M+Na]+,C7H6O2. 1H NMR (CDCl3,400 MHz): δH9.86 (1H,CHO),7.81 (2H,d,J = 8.5 Hz,H-2,6),6.97 (2H,d,J = 8.5 Hz,H-3,5); 13C NMR (CDCl3,100 MHz): δC 191.6 (s,CHO),162.1 (s,C-4),132.9 (d,2×C-2,6),130.2 (s,C-1),116.4 (d,2×C-3,5)[13].
Methyl 4-hydroxybenzoate (10): colorless oil,ESI-MS m/z 175 [M+Na]+,C8H8O3. 1H NMR (CDCl3,400 MHz): δH7.95 (2H,d,J = 8.2 Hz,H-2,6),6.87 (2H,d,J = 8.2 Hz,H-3,5),3.89 (3H,s,OCH3); 13C NMR (CDCl3,100 MHz): δC167.2 (s,CO),160.1 (s,C-4),131.9 (d,2×C-2,6),122.4 (s,C-1),115.2 (d,2×C-3,5),52.1 (q,OCH3)[14].
Methyl syringate (11): colorless oil,ESI-MS m/z 235 [M+Na]+,C10H12O5. 1H NMR (CDCl3,400 MHz): δH 7.32 (2H,s,H-2,6),5.95 (1H,br s,OH-4),3.93 (6H,s,2×MeO-3,5),3.89 (3H,s,COOCH3); 13C NMR (CDCl3,100 MHz) δC: 121. 2 (s,C-1),166.9 (s,CO),146. 7 (s,2×C-3,5) ,139. 3 (s,C-4),106. 8 (d,2×C-2,6),56.5 (q,2×MeO-3,5),52.1 (q,COOCH3)[15].
1-Methoxy-4-phenethylbenzene (12): pale yellow oil,EI-MS m/z 212 [M]+,C15H16O. 1H NMR (CDC13,400 MHz): δH 7.69 (2H,d,J = 7.3 Hz,H-3',5'),7.48 (1H,t,J = 7.3 Hz,H-4'),7.40 (2H,d,J = 7.3 Hz,H-2',6'),7.08 (2H,d,J = 8.4 Hz,H-3,5),6.86 (2H,d,J = 8.4 Hz,H-2,6),3.80 (3H,s,OCH3),3.68 (2H,t,J = 6.5 Hz,H-2''),2.87 (2H,t,J = 6.5 Hz,H-1''); 13C NMR (CDC13,100 MHz): δC 158.3 (s,C-1),143.9 (s,C-1'),134.6 (s,C-4),131.4 (d,2×C-3,5),129.7 (d,2×C-3',5'),128.5 (d,2×C-2',6'),126.8 (d,C-4'),114.1 (d,2×C-2,6),55.2 (q,OCH3),41.3 (t,C-2''),34.7 (t,C-1')[16].
Compound 1 was tested for its cytotoxic activities against A549,Hela and BGC-823 cancer cell lines,and antimicrobial activities against Candida albicans and Staphylococcus aureus[17]. The results showed that 1 did not exhibit cytotoxic and antimicrobial activities.
Experiment section General experiment proceduresOptical rotations were measured with a Horiba SEPA-300 polarimeter. UV spectra were obtained using a Shimadzu UV-2401-A spectrophotometer. IR spectra were recorded with a Tensor 27 FT-IR spectrometer with KBr pellets. The 1H and 13C NMR spectra were acquired with a Bruker AM-400 (1H: 400 MHz,13C: 100 MHz) or DRX-500 (1H: 500 MHz,13C: 125 MHz) spectrometer in acetone-d6 or CD3OD with TMS as the internal standard at room temperature. MS were recorded on an API QSTAR Pular-1 mass spectrometer. Column chromatography (CC) was performed on silica gel (100-200 mesh,200-300 mesh,and 10-40 μm,Qingdao Marine Chemical,Inc.,China) and Lichroprep RP-18 gel (40-63 mm,Merck,Darmstadt,Germany). TLC was carried out on precoated silica gel GF254 glass plates (Qingdao Marine Chemical,Inc.,China) and chromogenic agent (5% H2SO4-dehydrated alcohol). Semi-preparative HPLC was performed on an Agilent 1100 apparatus equipped with a UV detector and an YMC-Pack ODS-A (YMC,1×15 cm) column at a flow rate of 2 mL·min-1.
Plant materialThe roots,stems and leaves of C. excavata were collected at Xishuangbanna,Yunnan Province,P. R. China,in August 2010,which were identified by Prof. Yu-min Shui of Kunming Institute of Botany. A voucher specimen (No.2010813) has been deposited in the State Key Laboratory of Phytochemistry and plant Resources in West China,Kunming Institute of Botany,Chinese Academy of Sciences.
Extraction and isolationThe air-dried and powdered stems and leaves of C. excavata (32 kg) were extracted by refluxing 95% methanol three times (35 L×3). The methanol extract was submitted to the liquid-liquid fractionation with the solvents petroleum ether (PE),AcOEt,and BuOH. The EtOAc soluble fraction (1.1 kg) was applied to silica gel (100-200 mesh) column chromatography,eluting with PE/acetone (10∶1-0∶1) to yield 5 fractions (Fr. 1-Fr. 5). Fr. 2 (33 g) by silica gel (200-300 mesh) column chromatography eluted with PE/acetone (5∶1) gave sub-fractions (Fr. 2.1 to Fr. 2.4). Further separation of Fr. 2.2 (0.9 g) was subjected to a reversed-phase column (RP-18) eluting with MeOH-H2O (30%-90%) to afford 5 subfractions (Fr. 2.2.1-Fr. 2.2.5). Fr. 2.2.2 (81 mg) was subjected to semipreparative reversed-phase HPLC (75% MeOH- H2O) to give 12 (17 mg),10 (11 mg) and 8 (9 mg). Fr. 3 (52 g) was subjected to silica gel (200-300 mesh) column chromatography eluted with PE/acetone (4∶1) gave sub-fractions (Fr. 3.1 to Fr. 3.5). Fr. 3.1 (0.8 g) was subjected to a reversed-phase column (RP-18) eluting with MeOH-H2O (30%-90%) to afford 4 subfractions (Fr. 3.1.1-Fr. 3.1.4). Fr. 3.1.3 (90 mg) was subjected to semipreparative reversed-phase HPLC (65% MeOH-H2O) to give 9 (10 mg),11 (8 mg) and 2 (10 mg). Fr. 5 (21 g) was subjected to silica gel (200-300 mesh) column chromatography eluted with PE/acetone (2∶1) gave sub-fractions (Fr. 5.1 to Fr. 5.3). Fr. 5.3 (120 mg) was subjected to a reversed-phase column (RP-18) eluting with MeOH-H2O (30%-90%) to afford 3 subfractions (Fr. 5.3.1-Fr. 5.3.3). Fr. 5.3.3 (32 mg) was subjected to semipreparative reversed- phase HPLC (50% MeOH-H2O) to give 3 (8 mg),7 (6 mg) and 1 (16 mg).
The air-dried and powdered roots of C. excavata (13 kg) were extracted with 95% EtOH under reflux for three times. The filtrates were combined and evaporated to a small volume,followed by successive partition with petroleum ether (PE),EtOAc and BuOH. The EtOAc soluble fraction (600 g) was applied to silica gel (200-300 mesh) column eluting gradiently with CHCl3-MeOH (10∶0,9∶1,8∶2,7∶3,1∶1,0∶1),to give six fractions,A-F. The separation of fraction C (18 g) over silica gel column was eluted with PE-acetone (10∶1-1∶2) to yield fractions C1-C6. Fraction C2 (1.1 g) was subjected to a silica gel column eluted with PE-acetone (5∶1-1∶1) to give four subfractions (C2-1-C2-4). C2-4 (65 mg) was subjected to semipreparative reversed-phase HPLC (55% MeOH- H2O) to give 6 (9 mg),5 (14 mg) and 4 (20 mg).
(2'R)-4-(2',3'-dihydroxy-3'-methyl-butanoxy)-phen- ethanol (1): pale yellow oil,[α] +2.06 (c 0.11,MeOH); UV (MeOH) λmax (log ε): 201 (4.07),224 (4.12),276 (3.37) nm; IR (KBr): νmax 3 406,2 971,2 936,2 879,1 612,1 513,1 462,1 384,1 299,1 245,1 177,1 096,1 042,827 cm-1; 1H NMR (400 Hz,CD3OD) and 13C NMR (100 Hz,CD3OD):See Table 1; ESI-MS: m/z 263 [M+Na]+; HR-EI-MS: m/z 240.136 4 ([M]+,C13H20O4,Calcd. 240.136 2).
Acknowledgments: The authors are grateful to the members of the analytical group from the State Key Laboratory of Phytochemistry and Plant Resources in West China,Kunming Institute of Botany,Chinese Academy of Sciences,for measuring the [α],IR,UV,NMR and MS spectra.
| [1] | Peng WW, Zheng YQ, Chen YS, et al. Coumarins from the roots of Clausena excavata [J]. J Asian Nat Prod Res, 2013, 15: 215-220. |
| [2] | Peng WW, Zeng GZ, Song WW, et al. A new cytotoxic carbazole alkaloid and two new other alkaloids from Clausena excavata [J]. Chem Biodivers, 2013, 10: 1317-1321. |
| [3] | He HP, Shen YM, Zuo GY, et al. Two new O-terpenoidal coumarins, excavacoumarin A and B from Clausena excavata [J]. Chin Chem Lett, 2000, 11: 539-542. |
| [4] | Institutum Botanicum Kunmingense Academiae Sinicae Edita. Flora Yunnanica (云南植物志) [M]. Beijing: Science Press, 1995: 759. |
| [5] | He HP, Shen YM, Chen ST, et al. Dimeric coumarin and phenylpropanoids from Clausena lenis [J]. Helv Chim Acta, 2006, 89: 2836-2840. |
| [6] | Dey SP, Dey DK. Acid-catalysed rearrangements of allyl 4-hydroxybenzoate and 3-methylbut-2-enyl-4-hydroxybenzoate [J]. J Indian Chem Soc, 2009, 86: 485-487. |
| [7] | Gajare AS, Shaikh NS, Bonde BK, et al. Microwave accelerated selective and facile deprotection of allyl esters catalyzed by montmorillonite K-10 [J]. J Chem Soc, Perkin Trans 1, 2000, (1): 639-640. |
| [8] | You P, Wen R, Deng Z, et al. Synthesis of benzoic esters using sodium bisulfate as a catalyst [J]. Chin J Syn Chem (合成化学), 2003, 11: 544-546. |
| [9] | Lyutikova M, Turov YP. Chemical constituents from wild Oxycoccus palustris fruit from north Tyumen oblast [J]. Chem Nat Compd, 2011, 46: 848-851. |
| [10] | Huang SC, Wu PL, Wu TS. Two coumarins from the root bark of Clausena excavata [J]. Phytochemistry, 1997, 44: 179-181. |
| [11] | Cornelius MTF, Carvalho MG, Silva T, et al. Other chemical constituents isolated from Solanum crinitum Lam. (Solanaceae) [J]. J Brazil Chem Soc, 2010, 21: 2211-2219. |
| [12] | Wang MZ, Cai XH, Luo XD. New phenylphenalene derivatives from water hyacinth (Eichhornia crassipes) [J]. Helv Chim Acta, 2011, 94: 61-66. |
| [13] | Poupot M, Poupot R, Fournie JJ, et al. Phosphorylated dendrimers as antiinflammatory drugs [P]. WO, 2010013086. 2010-01. |
| [14] | Omray A, Choudhary VS, Bhlde YS, et al. Ophthalmic compositions comprising brinzolamide [P]. WO, 2012053011. 2012-05. |
| [15] | Tan J, Jiang S, Zhu D. Studies on the chemical constituents of Pleurospermum lindleyanum [J]. Nat Prod Res Dev (天然产物研究与开发), 2005, 17: 267-271. |
| [16] | Zhang Y, Kong L. Study on constituents of Citrus medica [J]. Modern Chin Med (现代中华医学杂志), 2006, 8: 16- 17, 23. |
| [17] | He WJ, Chu HB, Zhang YM, et al. Antimicrobial, cytotoxic lignans and terpenoids from the twigs of Pseudolarix kaempferi [J]. Planta Med, 2011, 77: 1924-1931. |
 2014, Vol. 49
2014, Vol. 49


