2. 中国科学院昆明植物研究所, 植物化学与西部植物资源持续利用国家重点实验室, 云南 昆明 650201
2. State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China
The mushroom Tyromyces chioneus belongs to the family Polyporaceae,which has a wide distribution in most parts of China,such as Hebei,Shanxi and Heilongjiang provinces. Previous chemical investigation on this fungus only reported a cadinane sesquiterpenoid,which was demonstrated to exhibit significant anti-HIV-1 activity[1]. In the course of searching for more biologically active compounds[2,3,4,5],two new sesquiterpenoids,tyromol A (1) and tyromol B (2),together with two previously known sesquiterpenoids 3 and 4,were obtained (Figure 1) from cultures of T. chioneus. Herein,we report the isolation and structural elucidation of these compounds.
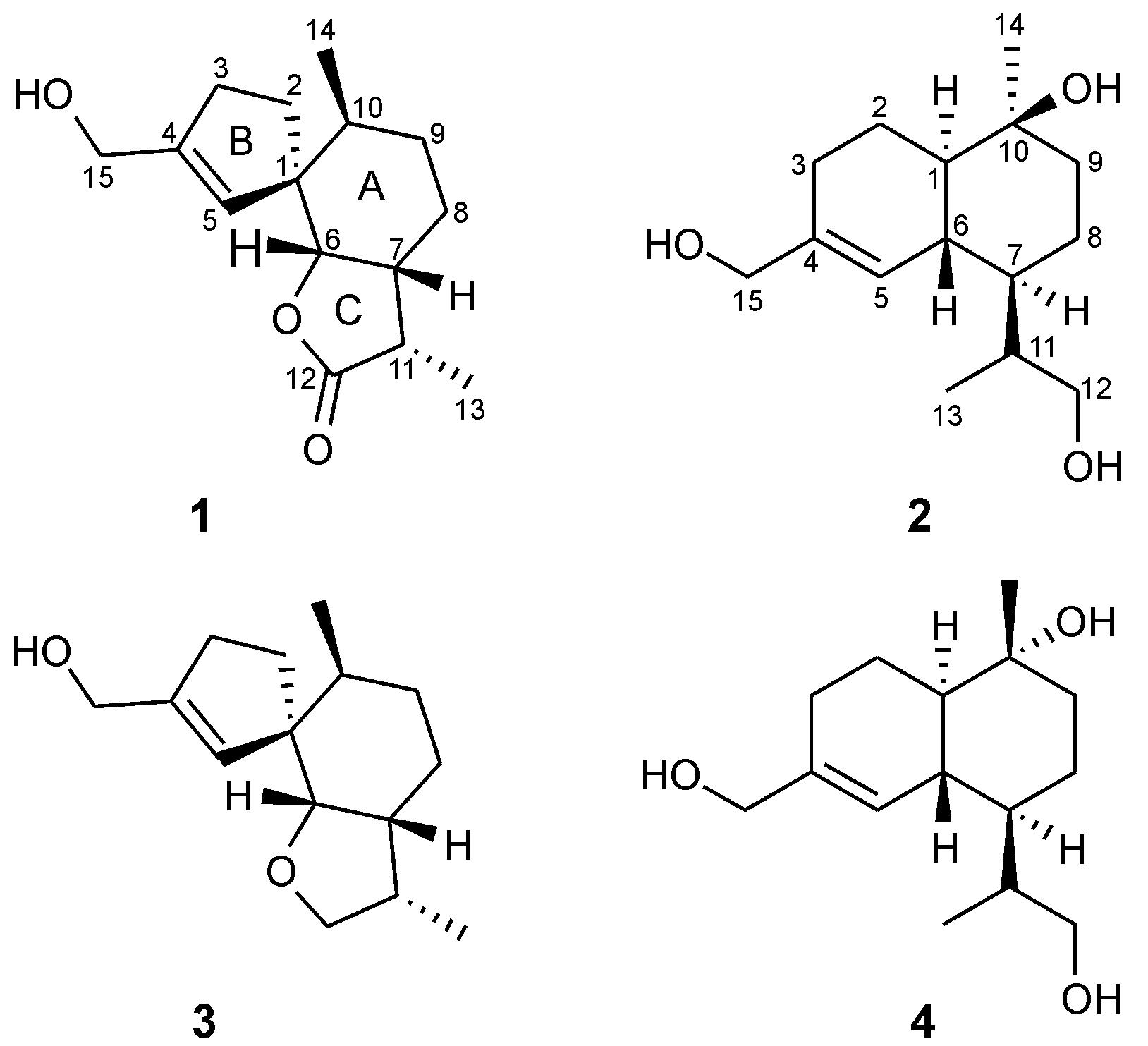 |
Figure 1 Structures of compounds 1-4 |
Compound 1 was isolated as white powder. Its molecular formula C15H22O3 was deduced from HR- ESI-MS at m/z 273.145 9 [M+Na]+ (calcd. 273.146 6),requiring for five degrees of unsaturation. The IR spectrum showed absorption bands for OH (3 428 cm-1),C=O (1 770 cm-1) and C=C (1 632 cm-1) moieties. The 1H NMR spectrum (Table 1) exhibited two secondary methyls (δH 1.14,d,J = 7.2 Hz; δH 0.81,d,J = 6.8 Hz),
|
|
Table 1 1H and 13C NMR spectroscopic data of 1-2 at 600/150 MHz,respectively. Chemical shift values δ in ppm,coupling constants J in Hz (in parentheses) |
three protons attached to two oxygenated carbons separately (δH 4.23,2H,dd,J = 14.5,10.0 Hz; δH 4.04,1H,d,J = 3.4 Hz),and one olefinic proton (δH 5.48,br s). The 13C NMR and DEPT spectra (Table 1) revealed the presence of one double bond (δC 148.7,C-4; δC 123.2,C-5),one oxygenated methine carbon (δC 85.9,C-6),one oxygenated methylene carbon (δC 62.1,C-15) and one carbonyl carbon (δC 179.6,C-12),as well as two methyl groups (δC 16.0,C-14; δC 9.1,
C-13),four methylenes (δC 32.3,C-2; δC 32.2,C-3; δC 29.3,C-9; δC 23.3,C-8),three methines (δC 42.8,C-11; δC 38.5,C-7; δC 34.2,C-10),and one quaternary carbon (δC 55.0,C-1).
The 1H-1H COSY spectrum displayed signals establishing two fragments as shown in Figure 2. The HMBC correlations from H-10 and H-6 to C-1 established a substituted hexatomic ring A,while the HMBC correlations from H-3 and H-15 to C-4 and from H-2 and H-5 to C-1 constructed a five-membered carbon ring B. In addition,the HMBC correlations
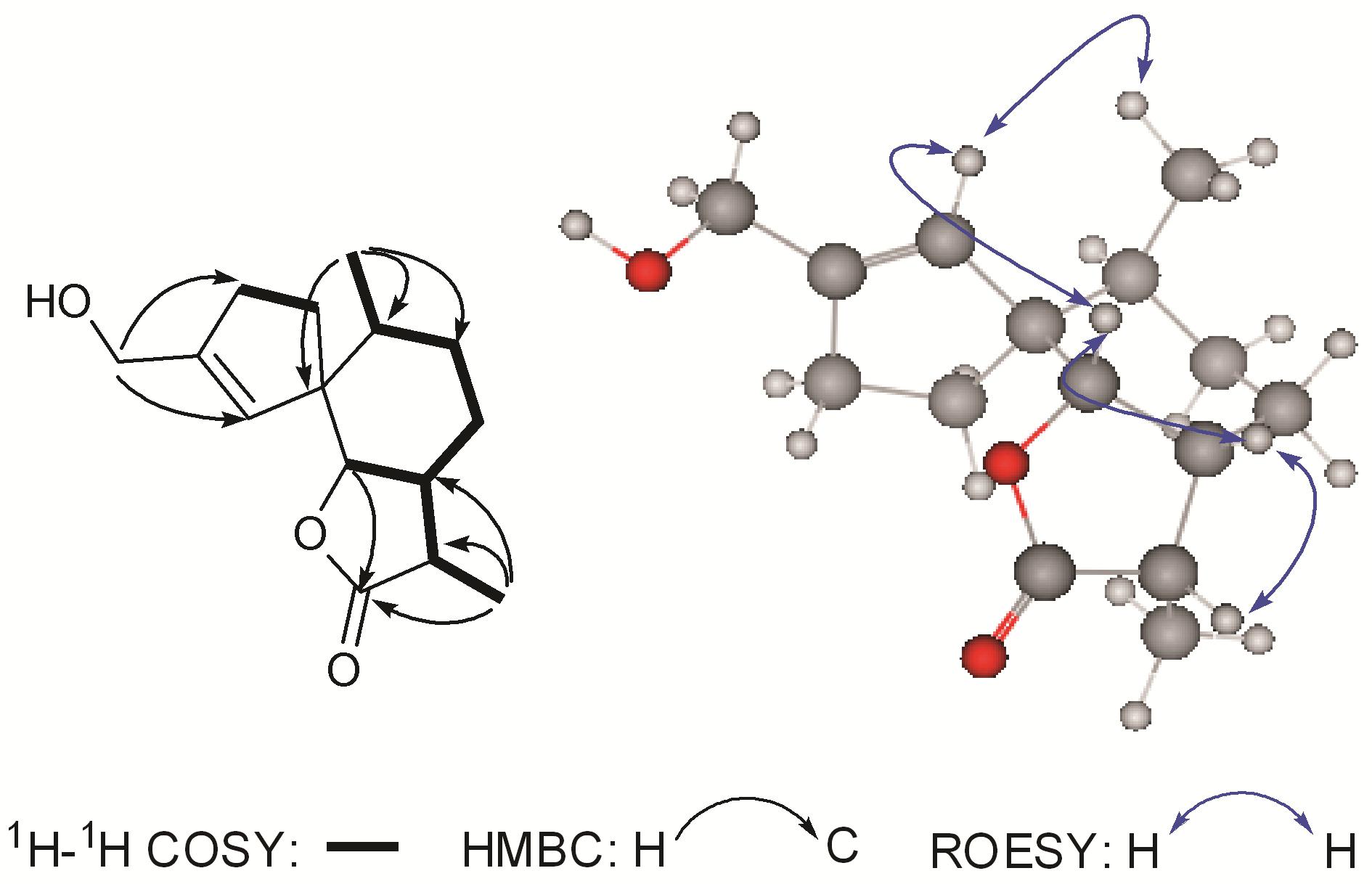 |
Figure 2 Key 1H-1H COSY,HMBC and ROESY correlations of 1 |
from H-6,H-11,and H-13 to C-12 suggested a five- membered lactone ring C. Therefore,the planar structure of 1 was established,which was similar to that of 15-hydroxy-6α,12-epoxy-7β,10αH,11βH-spiroax-4- ene (3)[6],except that C-12 was oxygenated to a carbonyl carbon,as supported by the HMBC correlations from H-6,H-7,and H-13 to δC 179.6 (s,C-12). The relative configuration of 1 was assigned on the basis of an ROESY experiment (Figure 2). ROESY correlations of H-5/H-6,H-5/H-14,and H-6/H-7 suggested that H-5,H-6,H-7,and Me-14 were at the same side,while the ROESY correlations of H-7/H-11 suggested that H-11 should be at the same side with H-7. Thus,the structure of compound 1 was established and named as tyromol A.
Compound 2 was also obtained as white powder. Its molecular formula was determined to be C15H26O3 by HR-ESI-MS,corresponding to three degrees of unsaturation. The IR spectrum showed absorption bands for a hydroxy group (3 427 cm-1) and double bonds (1 634 cm-1). The 13C NMR and DEPT spectra (Table 1) showed 15 carbon signals that attributed to two methyls,six methylenes,five methines,and two quaternary carbons. The 1H and 13C NMR spectra of 2 were similar to those of agripilol C (4)[7]. Careful comparison of their NMR data indicated that they were a pair of isomers,with the only difference assigned to the configuration of H3-14. The HMBC spectrum suggested that its planner structure was identical to that of compound 4. In the ROSEY spectrum,correlations of H3-14/H-1/H-7 and OH-10/H-6 indicated the α- orientation of H3-14 and β-orientation of OH-10. Thus,compound 2 (tyromol B) was established as shown.
The known compounds 3 and 4 were purified as white powders,and characterized as 15-hydroxy-6α,12- epoxy-7β,10αH,11βH-spiroax-4-ene (3)[6],and agripilol C (4)[7] by comparison of their 1H and 13C NMR data with those reported in the literature. Compounds 3 and 4 were isolated from this fungus for the first time. Experimental section General experimental procedures
Optical rotations were measured on a Jasco-P-1020 polarimeter. UV spectra were measured on a Shimadzu UV-2401 PC spectrophotometer. IR spectra were obtained by using a Bruker Tensor 27 FT-IR spectrometer with KBr pellets. NMR spectra were acquired with an Avance III 600 MHz instrument. HR-ESI-MS were measured on an API QSTAR Pulsar mass spectrometer. Silica gel (200-300 mesh and 80-100 mesh,Qingdao Marine Chemical Inc.,China),RP-18 gel (40-75 µm,Fuji Silysia Chemical Ltd.,Japan)and Sephadex LH-20 (Amersham Biosciences,Sweden) were used for column chromatography (CC). Fractions were monitored by TLC (Qingdao Marine Chemical Inc.,China) and spots visualized by heating silica gel plates immersed in vanillin-H2SO4 in EtOH. Fungal material and cultivation conditions
The fungus T. chioneus was collected from the Ailao Mountain in Yunnan Province,People’s Republic of China,in July 2003. The fungus was identified by Prof. Mu Zang at the Kunming Institute of Botany. The voucher specimen was deposited at the Herbarium of Kunming Institute of Botany (No. HFC 20110812X). Culture medium: glucose (5%),pork peptone (0.15%),yeast (0.5%),KH2PO4 (0.05%),MgSO4 (0.05%). The fermentation was carried out on a shaker at 24 ℃ and 150 r·min-1 for 20 d. Extraction and isolation The culture broth (20 L) was extracted three times with EtOAc (3 × 10 L). The combined EtOAc extracts were evaporated in vacuo to give a residue (10.0 g). The residue was subjected to silica gel CC with a gradient elution system of petroleum ether-acetone (30∶1→0∶1) to obtain six fractions (A-F). Fraction C was eluted with petroleum ether-acetone (5∶1). It was then subjected to Sephadex LH-20 CC (CHCl3-MeOH,1∶1) and silica gel CC with petroleum ether-acetone (4∶1) to afford 2 (2.0 mg) and 4 (3.0 mg). Fraction E was separated by repeated silica gel CC (petroleum ether-acetone,10∶1→0∶1) to yield fractions E01-E03. Fraction E02 was chromatographed on a RP-18 column (MeOH− H2O,30%) and then purified by CC on silica gel with petroleum ether-acetone (2∶1) to give 1(1.0 mg) and 3 (2.0 mg).
Tyromol A (1): C15H22O3,white powder; [α] +16.7 (c 0.10,CHCl3); UV (CHCl3) λmax (log ε) 240 (2.1),199 (1.8) nm; IR (KBr) νmax 3 428,2 923,1 770,1 632,1 169 cm-1; 1H NMR (CDCl3,600 MHz) and 13C NMR (CDCl3,150 MHz) data,see Table 1; HR- ESI-MS m/z 273.145 9 [M+Na]+ (calcd. for C15H22O3Na 273.146 6).
Tyromol B (2): C15H26O3,white powder; [α] +8.8 (c 0.10,CHCl3); UV (CHCl3) λmax (log ε) 239 (1.9) nm; IR (KBr) νmax 3 427,2 926,1 634,1 384,1 035 cm-1; 1H NMR (CDCl3 and CD3OD,600 MHz) and 13C NMR (CDCl3,150 MHz) data,see Table 1; ESI-MS (positive) m/z 277 [M+Na]+; HR-ESI-MS m/z 277.177 4 [M+Na]+ (calcd. for C15H26O3Na 277.177 9).
| [1] | Liu DJ, Wang F, Yang LM, et al. A new cadinane sesquiterpene with significant anti-HIV-1 activity from the cultures of the basidiomycete Tyromyces chioneus [J]. J Antibiot, 2007, 60: 332-334. |
| [2] | Liu JK. N-Containing compounds of macromycetes [J]. Chem Rev, 2005, 105: 2723-2744. |
| [3] | Liu JK. Natural terphenyls: developments since 1877 [J]. Chem Rev, 2006, 106: 2209-2223. |
| [4] | Jiang MY, Zhang L, Liu R, et al. Speciosins A-K, oxygenated cyclohexanoids from the basidiomycete Hexagonia speciosa [J]. J Nat Prod, 2009, 72: 1405-1409. |
| [5] | Liu DZ, Wang F, Liao TG, et al. Vibralactone: a lipase inhibitor with an unusual fused β-lactone produced by cultures of the basidiomycete Boreostereum vibrans [J]. Org Lett, 2006, 8: 5749-5752. |
| [6] | Liu DZ, Jia RR, Wang F, et al. A new spiroaxane sesquiterpene from cultures of the basidiomycete Pholiota adipose [J]. Z Naturforsch, 2008, 63: 111-113. |
| [7] | Shan WG, Chen XX, Ying YM, et al. Sesquiterpenoids from Fusarium sp., an endophytic fungus in Agriminia pilosa [J]. HeIv Chim Acta, 2011, 94: 1254-1259. |
 2014, Vol. 49
2014, Vol. 49


