中药益智是姜科 (Zingberaceae) 山姜属植物益智 (Alpinia oxyphylla Miq.) 的干燥成熟果实,别名益智仁,益智子,主产于海南、广东、广西、福建等地,为海南道地药材,也是我国四大南药之一,具有温脾止泻、摄唾涎、暖肾、固精缩尿的功效[1]。现代药理学研究表明: 益智主要具有抗炎、神经保护、抗氧化、强心、舒张血管、提高免疫力等作用,是一种安全性高的药食两用植物资源。目前,从益智中分到的化合物主要为萜类、黄酮类、二苯庚烷类、酚类、甾醇类等成分,其中倍半萜类化合物能抑制脂多糖和IFN-γ诱导的鼠巨噬细胞产生NO[2,3]。本课题组前
期已对益智95% 乙醇提取物的乙酸乙酯萃取部位进行化学成分研究[4],为了进一步阐明其药效物质基础,本文对益智95% 乙醇提取物的正丁醇萃取部位进行研究,分离并鉴定了9个单体化合物 (图 1),包括4个桉叶烷型倍半萜 (化合物1、2、3、4)、1个黄酮 (化合物5)、4个糖苷 (化合物6、7、8、9)。其中化合物1为新倍半萜类化合物,化合物8、9 为新天然产物,化合物2、6为首次从山姜属中分得,化合物7为首次从益智中分得。此前,An等[5]、Ly等[6]从高良姜中分离到一些β-D-吡喃葡萄糖苷类化合物,而本文分离到的4个糖苷类化合物也均为β-D-吡喃葡萄糖苷类。
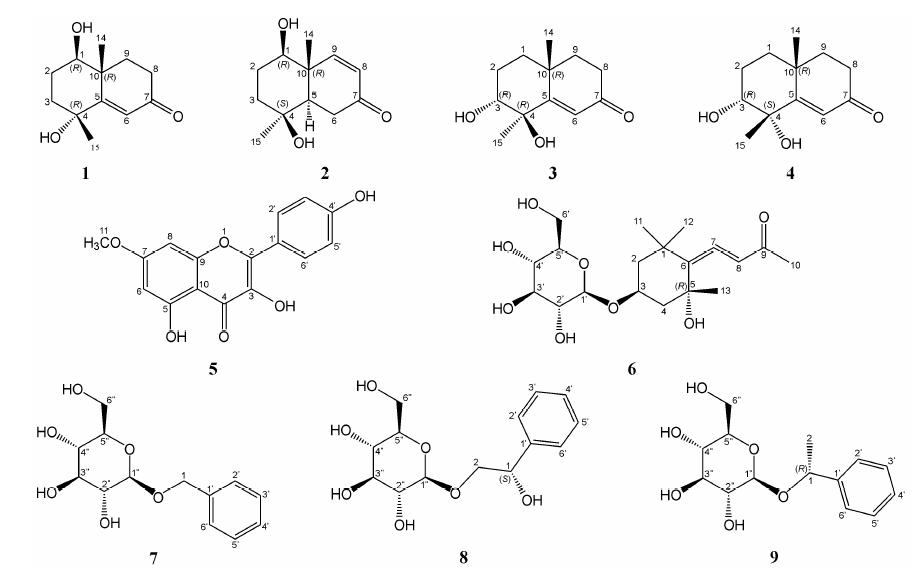 |
Figure 1 The structures of compounds 1-9 |
化合物1 无色油状物。磷钼酸显蓝绿色。HR-ESI-MS (+): m/z 233.114 9 [M+Na]+ (calcd. for C12H18O3Na,233.115 4),并结合NMR数据,确定其分子式为C12H18O3,不饱和度为4。13C NMR (CDCl3,150 MHz) 给出12个碳信号: 其中1个羰基碳 (C=O) 和1个碳碳双键 (C=C) 构成的α,β-不饱和酮结构 [δC 124.5 (C-6)、169.7 (C-5)、201.7 (C-7)]; 此外还有2个甲基碳 (δC 17.7和28.4)、4个亚甲基碳 (δC 26.0、33.7、36.3、37.3)、1个季碳 (δC 41.4) 以及1个连氧的叔碳 (δC 78.3) 和1个连氧的季碳 (δC 70.8)。同时,1H NMR (CDCl3,600 MHz) 显示化合物1有1个烯氢 [δH 6.05 (1H,s)]、2个分别取代在季碳上的甲基氢 [δH 1.32 (3H,s)、1.34 (3H,s)]。由化合物1的NMR数据,结合其不饱和度4,推断结构中含有2个环。根据HSQC图谱数据将对应的碳、氢信号进行了归属 (表 1)。
|
|
Table 1 NMR spectral data of compound 1 in CDCl3 (600 MHz for 1H and 150 MHz for 13C) |
甲基、羟基以及α,β-不饱和酮在双环结构上的取代位置进一步通过HMBC确定,如图 2a所示。在HMBC谱图显示: H-14 (δH 1.34) 与C-10、C-9、C-1有多键相关,推断CH3-14取代在双环C-10位上; H-1 (δH 3.40) 与C-2、C-10、C-9、C-14有多键相关,推断-OH取代在双环C-1位上; H-8α、H-8β (δH 2.42,2.53) 分别与C-9、C-7、C-10,H-9α、H-9β (δH 1.81,2.21) 分别与C-8、C-7、C-10、C-14有多键相关,推断羰基取代在双环C-7位上; H-15 (δH 1.32) 与C-4、C-3有多键相关,推断CH3-15与-OH共取代在双环C-4位上; 此外H-15与C-5、C-6,H-6 (δH 6.05) 与C-5、C-4、C-10、C-8有多键相关,推断碳碳双键形成于双环C-5、6位,与羰基共轭形成α,β-不饱和酮结构。 综上,确定化合物1的平面结构为1,4-二羟基-11,12,13-降碳桉叶烷型倍半萜。
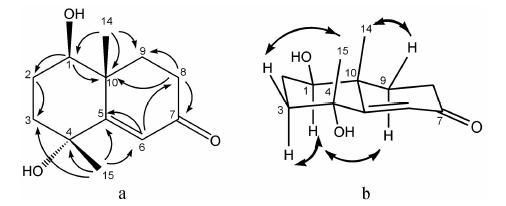 |
Figure 2 Key HMBC correlations (a) and NOESY correlations (b) of compound 1 |
NOESY谱图显示,如图 2b所示: H-3α (δH 1.56) 与H-1,H-3β (δH 1.95) 与H-15有NOESY相关,确定CH3-15与OH-1均处于β位,而4-OH处于α位; H-9α (δH 1.81) 与H-1,H-9β (δH 2.21) 与H-14有NOESY相关,确定CH3-14处于β位,综上,确定化合物1的相对构型为1β,4α-二羟基-11,12,13-降碳桉叶烷型 倍半萜。以上结果与文献[7]报道的Calamusin Ⅰ具有相同的平面结构和相对构型。但化合物1的[α]20D= -28.2 (c 0.02,MeOH),符号与Calamusin Ⅰ相反,推断化合物1与Calamusin Ⅰ的绝对构型存在差异。
化合物1的CD谱 (图 3) 证实以上推测: 在345 nm (Δε -0.16) 处出现由α,β-不饱和环己酮n→π电子跃迁引起的负的Cotton效应,而Calamusin I在340 nm (Δε +0.23) 处出现由α,β-不饱和环己酮n→π电子跃迁引起的正的Cotton效应[7]。综上,确定该化合物的绝对构型为1R,4R,10R。为Calamusin Ⅰ的对映体。
 |
Figure 3 CD spectrum of compound 1 (MeOH) (a); diagram of n→π transition rule of α,β-unsaturated cyclic ketone applied in compound 1 (b) |
综上所述,化合物1的结构为 (1R,4R,10R)-1β,4α-dihydroxy-11,12,13-trinor-5,6-eudesmen-7-one。
实验部分 1 仪器与试剂LTQ-Obitrap XL液质联用仪,Bruker Avance DRX-600型超导核磁共振仪 (美国Bruker公司,TMS为内标),Jasco J-815圆二色谱仪 (日本Jasco公司),Fihser-Johns熔点仪 (温度未校正),Agela CHEETAHTM中压制备液相 (天津博纳艾杰尔科技有限公司),MDS GEL (北京麦迪生新技术开发中心),薄层色谱用硅胶GF254、柱色谱用硅胶H (青岛海洋化工厂),YMC-ODS制备型HPLC色谱柱 (10 mm × 250 mm,5 μm)、中压柱ODS填料 (YMC-ODS,50 μm,北京绿百草科技发展有限公司),所用试剂为色谱纯和分析纯。
中药益智药材购于河北安国药材市场,经中国医学科学院药用植物研究所郭宝林研究员鉴定为姜科植物益智 (Alpinia oxyphylla Miq.) 的干燥成熟果实,凭证样品存放于本所天然药物研究中心。
2提取与分离益智干燥果实89 kg,粉碎后用95% 乙醇渗漉,减压浓缩得醇浸膏10.348 kg,用水混悬后,依次用正己烷 (n-hexane)、乙酸乙酯 (EtOAc)、正丁醇 (n-BuOH) 萃取,n-BuOH萃取液回收溶剂得正丁醇萃取部位0.5 kg。取200 g用MDS中压柱分离,甲醇-水 (0∶10~10∶0) 梯度洗脱,得9个流分。其中,MDS-20%甲醇部分 (11.356 g) 经中压ODS柱分离,甲醇-水 (2∶8~1∶1) 梯度洗脱,合并相似洗脱部分,得到9个流分 (Fr.A~I)。
Fr.A (7.947 g),经中压硅胶柱色谱,氯仿-甲醇 (99∶1~86∶14) 梯度洗脱,得70个流分 (Fr.A-1~70)。Fr. A-(13~18) (616 mg),依次经中压ODS柱色谱、制备HPLC (C18) 分离、制备TLC纯化,得化合物1(10 mg)、2(2 mg)、3(31 mg)、4(20 mg)、5(7 mg); Fr.A-25 (402 mg) 依次经中压ODS柱色谱、制备HPLC (C18) 分离、制备TLC纯化,得化合物7(5 mg); Fr.A-(38~40) (360 mg),依次经中压ODS柱色谱、制备HPLC (C18) 分离,得化合物8 (6 mg); Fr.A-44 (97 mg) 经制备HPLC (C18) 分离得化合物6 (5 mg)。
Fr.B (3.873 g),经中压硅胶柱色谱,氯仿-甲醇 (99∶1~86∶14) 梯度洗脱,得58个流份 (Fr.B1~58)。Fr.B-20 (91 mg) 经制备HPLC (C18) 分离、制备TLC纯化,得化合物9 (16 mg)。
3 结构鉴定化合物1 无色油状物 (甲醇),[α]= -28.2 (c 0.02,MeOH); IR (KBr) νmax: 3 397,2 948,2 961,1 661,1 456,1 374,1 032 cm-1; UV (nm) (logε): 237 (3.94); CD (MeOH) λmax (Δε) 345.5 (-0.16),314.5 (+0.03),291 (-0.07),246 (+0.83),211.5 (-1.41); HR-ESI-MS (+): m/z 233.114 9 [M+Na]+ (calcd. for C12H18O3Na,233.115 4),分子式C12H18O3,1H NMR (600 MHz,CDCl3) 和13C NMR (150 MHz,MeOD) 见表 1。
化合物2 无色油状物。HR-ESI-MS (+): m/z 233.115 0 [M+Na]+ (calcd. for C12H18O3Na,233.115 4),分子式C12H18O3。1H NMR (600 MHz,CD3OD) δ: 7.32 (1H,d,J = 10.0 Hz,H-9),5.81 (1H,d,J = 10.0 Hz,H-8),3.42 (1H,dd,J = 4.4,12.0 Hz,H-1),2.67 (1H,dd,J = 14.2,18.0 Hz,H-6α),2.47 (1H,dd,J = 3.8,18.0 Hz,H-6β),2.01 (1H,m,H-2α),1.80 (1H,dd,J = 3.8,14.2 Hz,H-5),1.75 (1H,ddd,J = 3.2,4.4,13.2 Hz,H-3β),1.65 (1H,m,H-2β),1.57 (1H,dd,J = 4.4,13.2 Hz,H-3α),1.23 (3H,s,H-14),1.15 (3H,s,H-15)。13C NMR (150 MHz,CD3OD) δ: 203.7 (C-7),161.6 (C-9),126.7 (C-8),75.1 (C-1),71.3 (C-4),49.5 (C-5),43.6 (C-10),40.2 (C-3),35.5 (C-6),29.2 (C-15),27.8 (C-2),13.8 (C-14)。以上数据与文献[8]报道一致,故鉴定为1β,4β- dihydroxy-11,12,13-trinor-8,9-eudesmen-7-one。
化合物3 白色蜡状固体,mp 185~186 ℃。HR-ESI-MS (+): m/z 233.114 7 [M+Na]+ (calcd. for C12H18O3Na,233.115 4),分子式C12H18O3。1H NMR (600 MHz,CD3OD) δ: 6.01 (1H,br s,H-6),3.69 (1H,dd,J = 2.8,3.0 Hz,H-3),2.63 (1H,ddd,J = 5.0,13.6,18.0 Hz,H-8β),2.41 (1H,dddd,J = 2.8,4.0,12.0,13.6 Hz,H-2β),2.32 (1H,br d,J = 18.0 Hz,H-8α),1.92 (1H,ddd,J = 4.4,13.6,14.0 Hz,H-9α),1.82 (1H,ddd,J = 4.2,14.0,13.6 Hz,H-1α),1.73 (1H,ddd,J = 2.4,5.0,14.0 Hz,H-9β),1.56 (1H,ddd,J = 3.0,4.2,12.0 Hz,H-2α),1.46 (3H,s,H3-14),1.43 (1H,ddd,J = 3.2,4.0,14.0 Hz,H-1β),1.41 (3H,s,H3-15)。 13C NMR (150 MHz,CD3OD) δ: 203.6 (C-7),172.8 (C-5),126.3 (C-6),76.4 (C-3),74.1 (C-4),41.2 (C-9),36.8 (C-10),35.5 (C-1),35.1 (C-8),25.6 (C-15),25.7 (C-2),25.5 (C-14)。以上数据与文献[9]报道一致,故鉴定为oxyphyllenone A。
化合物4 无色油状物。HR-ESI-MS (+): m/z 233.115 0 [M+Na]+ (calcd. for C12H18O3Na,233.115 4),分子式C12H18O3。1H NMR (600 MHz,CDCl3) δ: 6.28 (1H,s,H-6),3.55 (1H,dd,J = 4.4,12.0 Hz,H-3),2.43 (1H,ddd,J = 4.0,15.0,18.0 Hz,H-8β),2.29 (1H,ddd,J = 2.2,3.4,18.0 Hz,H-8α),1.82 (1H,dddd,J = 4.0,12.0,14.0,15.0 Hz,H-2β),1.79 (1H,ddd,J = 3.4,15.0,13.6 Hz,H-9α),1.72 (1H,ddd,J = 2.2,4.0,13.6 Hz,H-9β),1.68 (1H,dddd,J = 3.6,4.0,4.4,15.0 Hz,H-2α),1.60 (1H,ddd,J = 3.6,4.0,13.8 Hz,H-1β),1.37 (1H,ddd,J = 4.0,14.0,13.8 Hz,H-1α),1.30 (3H,s,H3-15),1.24 (3H,s,H3-14)。13C NMR (150 MHz,MeOD) δ: 200.8 (C-7),174.3 (C-5),123.5 (C-6),77.3 (C-3),76.5 (C-4),40.8 (C-9), 37.9 (C-1),36.1 (C-10),33.7 (C-8),26.7 (C-2),24.8 (C-14),22.9 (C-15)。以上数据与文献[9]报道一致,故鉴定为oxyphyllenone B。
化合物5 黄色针晶,mp 226~228 ℃。HR-ESI- MS (+): m/z 301.070 1 [M+H]+ (calcd. for C16H13O6,301.071 2),分子式C16H12O6。1H NMR (600 MHz,DMSO-d6) δ: 12.43 (1H,s,5-OH),10.13 (1H,s,4'-OH),9.51 (1H,s,3-OH),8.08 (2H,td,H-2',6'),6.93 (2H,td,H-3',5'),6.75 (1H,d,H-8),6.35 (1H,d,H-6),3.90 (3H,s,7-OCH3)。13C NMR (150 MHz,DMSO-d6) δ: 176.0 (C-4),164.9 (C-7),160.3 (C-5),159.3 (C-4'),156.1 (C-9),147.2 (C-2),135.9 (C-3),129.5 (C-2',6'),121.5 (C-1'),115.4 (C-3,5'),104.0 (C-10),97.4 (C-6),92.0 (C-8),56.0 (C-11)。以上数据与文献[10]报道一致,故鉴定为3,5,4'-三羟基-7-甲氧基黄酮 (rhamnocitrin)。
化合物6 无定形粉末。HR-ESI-MS (+): m/z 409.184 8 [M+Na]+ (calcd. for C19H30O8Na,409.183 8),分子式C19H30O8; 1H NMR (600 MHz,CD3OD) δ: 5.85 (1H,s,H-8); 4.46 (1H,d,J = 8.0 Hz,H-1'),4.35 (1H,m,H-3),3.88 (1H,dd,J = 2.0,12.0 Hz,H-6'b),3.69 (1H,dd,J = 5.0,12.0 Hz,H-6'a),3.16 (1H,dd,J = 8.0,9.0 Hz,H-2'),2.38 (1H,ddd,J = 2.0,4.0,13.0 Hz,H-4eq),2.10 (1H,ddd,J = 2.0,4.0,13.0 Hz,H-2eq),2.19 (3H,s,H3-10),1.47 (1H,m,H-2ax),1.46 (1H,m,H-4ax),1.39 (3H,s,H-13),1.38 (3H,s,H-11),1.16 (3H,s,H-12); 13C NMR (150 MHz,CD3OD) δ: 211.7 (C-9),201.1 (C-7),120.3 (C-6),102.9 (C-1'),101.4 (C-8),78.4 (C-3'),78.1 (C-5'),75.3 (C-2'),72.8 (C-3),72.6 (C-5),71.9 (C-4'),62.9 (C-6'),48.3 (C-4),46.8 (C-2),37.2 (C-1),32.5 (C-12),31.0 (C-13),29.6 (C-11),26.7 (C-10)。以上数据与文献[11]报道一致,故鉴定为staphylionoside D。
化合物7 白色针晶状 (甲醇),mp 212~213 ℃。HR-ESI-MS (+): m/z 293.100 3 [M+Na]+ (calcd. for C13H18O6Na,293.100 1),分子式C13H18O6; 1H NMR (600 MHz,CD3OD) δ: 7.42 (2H,d,J = 7.4 Hz,H-2',6'),7.33 (2H,t,J = 7.4 Hz,H-3',5'),7.28 (1H,t,J = 7.4 Hz,H-4'),4.93 (1H,d,J = 11.8 Hz,H-1a),4.67 (1H,d,J = 11.8 Hz,H-1b),4.36 (1H,d,J = 7.8 Hz,H-1''),3.89 (1H,dd,J = 2.0,12.0 Hz,H-6''b),3.69 (1H,dd,J = 6.0,12.0 Hz,H-6''a); 13C NMR (150 MHz,CD3OD) δ: 139.2 (C-1'),129.5 (C-2',6'),129.4 (C-3',5'),128.8 (C-4'),103.4 (C-1''),78.2 (C-3''),78.1 (C-5''),75.3 (C-2''),71.9 (C-1),71.8 (C-4''),62.9 (C-6'')。以上数据与文献[12,13]报道一致,故鉴定为benzyl-1-O-β-D- glucopyranoside。
化合物8 无定形粉末,mp 68~70 ℃。HR-ESI- MS (+): m/z 323.111 3 [M+Na]+ (calcd. for C14H20O7Na,323.110 7),分子式C14H20O7。1H NMR (600 MHz,CD3OD) δ: 7.40 (2H,d,J = 7.2 Hz,H-2',6'),7.34 (2H,t,J = 7.2 Hz,H-3',5'),7.27 (1H,t,J = 7.2 Hz,H-4'),4.89 (1H,dd,J = 3.0,9.0 Hz,H-1),4.34 (1H,d,J = 7.8 Hz,H-1''),4.02 (1H,dd,J = 3.0,10.0 Hz,H-2a),3.86 (1H,dd,J = 2.0,12.0 Hz,H-6''b),3.66 (1H,dd,J = 5.0,12.0 Hz,H-6''a),3.56 (1H,dd,J = 9.0,10.0 Hz,H-2b),3.38 (1H,t,J = 7.8 Hz,H-2'')。13C NMR (150 MHz,CD
化合物9 无色透明状固体。HR-ESI-MS (+): m/z 307.115 4 [M+Na]+ (calcd. for C14H20O6Na,307.115 7),分子式C14H20O6; 1H NMR (600 MHz,CD3OD) δ: 7.42 (2H,d,J = 7.4 Hz,H-2',6'),7.32 (2H,t,J = 7.4 Hz,H-3',5'),7.26 (1H,t,J = 7.4 Hz,H-4'),5.05 (1H,q,J = 6.6 Hz,H-1),4.08 (1H,d,J = 7.8 Hz,H-1''),3.88 (1H,dd,J = 1.8,12.0 Hz,H-6'b),3.68 (1H,dd,J = 6.0,12.0 Hz,H-6'a),3.31~3.33 (4H,m,H-2'',3'',4'',5''),1.46 (3H,d,J = 6.6 Hz,H-2)。13C NMR (150 MHz,CD3OD) δ: 144.3 (C-1'),129.6 (C-3',5'),128.8 (C-4'),128.1 (C-2',6'),101.2 (C-1''),76.2 (C-1),78.2 (C-3''),78.1 (C-5''),75.4 (C-2''),71.9 (C-4''),63.0 (C-6''),24.8 (C-2)。以上数据与文献[15]报道一致,故鉴定为 (S)-1- phenylethyl β-D-glucopyranoside。
| [1] | Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China(中华人民共和国药典)[S]. Vol I. Beijing: China Medical Science Press, 2010: 273-274. |
| [2] | Xu JJ, Ji CJ, Zhang YM, et al. Inhibitory activity of eudesmane sesquiterpenes from Alpina oxyphylla on production of nitric oxide [J]. Bioorg Med Chem Lett, 2012, 22: 1660-1663. |
| [3] | Luo JG, Lv XQ, Wang XB, et al. Sesquiterpenoids from the fruits of Alpina oxyphylla and inhibition of nitric oxide production in lipopolysaccharide-activated macrophages [J]. Phytochem Lett, 2012, 5: 134-138. |
| [4] | Hou L, Lv XX, Xie BB, et al. Studies on the chemical constituents in Alpinia oxyphylla [J]. Nat Prod Res Dev(天然产物研究与开发), 2013, 25: 878-881. |
| [5] | An N, Lin J, Yang SL, et al. A new glycoside from Alpinia officinarum [J]. Acta Pharm Sin(药学学报), 2006, 41: 233-235. |
| [6] | Ly YN, Shimoyamada M, Kato M. Isolation and characterization of some antioxidative compounds from the Rhizomes of smaller Galanga(Alpinia officinarum Hance)[J]. J Agric Food Chem, 2003, 51: 4924-4929. |
| [7] | Hao ZY, Liang D, Luo H, et al. Bioactive sesquiterpenoids from the rhizomes of Acorus calamus [J]. J Nat Prod, 2012, 75: 1083-1089. |
| [8] | Henchiri H, Bodo B, Deville A, et al. Sesquiterpenoids from Teucrium ramosissimum [J]. Phytochemistry, 2009, 70: 1435- 1441. |
| [9] | Muraoka O, Fujimoto M, Tanabe G, et al. Absolute stereostructures of novel norcadinane- and trinoreudesmane- type sesquiterpenes with nitric oxide production inhibitory activity from Alpinia oxyphylla [J]. Bioorg Med Chem Lett, 2001, 11: 2217-2220. |
| [10] | Tu YC, Lian TW, Yen JH, et al. Antiatherogenic effects of kaempferol and rhamnocitrin [J]. Agric Food Chem, 2007, 55: 9969-9976. |
| [11] | Qian YU, Katsuyoshi M, Hideaki O, et al. Staphylionosides A-K: megastigmane glucosides from the leaves of Staphylea bumalda DC [J]. Chem Pharm Bull, 2005, 53: 800-807. |
| [12] | Wen Q, Lin X, Liu Y, et al. Phenolic and lignan glycosides from the butanol extract of Averrhoa carambola L. root [J]. Molecules, 2012, 17: 12330-12340. |
| [13] | Zeng Q, Chang RJ, Qin JJ, et al. New glycosides from Dracocephalum tanguticum Maxim [J]. Arch Pharm Res, 2011, 34: 2015-2020. |
| [14] | Ooi Y, Mitsuo N, Satoh T. Enzymic synthesis of glycosides of racemic alcohols using β-galactosidase and separation of the diastereomers by high-performance liquid chromatography using a conventional column [J]. Chem Pharm Bull, 1985, 33: 5547-5550. |
| [15] | Shimoda K, Katsuragi H. Enzymatic resolution of(RS)-1- phenylalkyl β-D-glucosides to(R)-1-phenylalkyl β-primeverosides and(S)-1-phenylalkyl β-D-glucosides via plant xylosyltransferase [J]. Tetrahedron: Asymmetry, 2010, 21: 2060-2065. |
 2014, Vol. 49
2014, Vol. 49


