姜黄素 [curcumin,1,7-二 (4-羟基-5-甲氧基) 苯基-1,6-庚二烯3,5-二酮] 是姜黄 (Curcuma longa. L) 主要活性成分,其化学结构见图 1。姜黄素具有广泛的药理活性: 抗氧化、抗炎、清除氧自由基、利胆、降血脂、抗病毒和抗肿瘤等。已注册的临床研究有99个 (http://clinicaltrails.gov/),主要涉及抗炎 (肠炎、胰腺炎、风湿性关节炎、老年痴呆、囊性纤维化等) 和抗肿瘤 (直肠癌、乳腺癌、胰腺癌、多发性骨髓瘤等)[1]。在亚洲传统医学中被广泛用于治疗黄疸及其他肝功能紊乱[2,3,4],近年多项体内外研究显示姜黄素能够从多个环节干预肝脏疾病的病理进程,具有抗肝损伤、抗脂肪变、抗纤维化及抗肝癌的药效。但由于姜黄素水溶性差、生物利用度低,极大地限制其临床应用,因此,围绕姜黄素作为先导化合物进行的剂型改变和结构修饰是目前研究的热点。本文就姜黄素及姜黄素衍生物作用于肝脏疾病的最新进展综述如下。
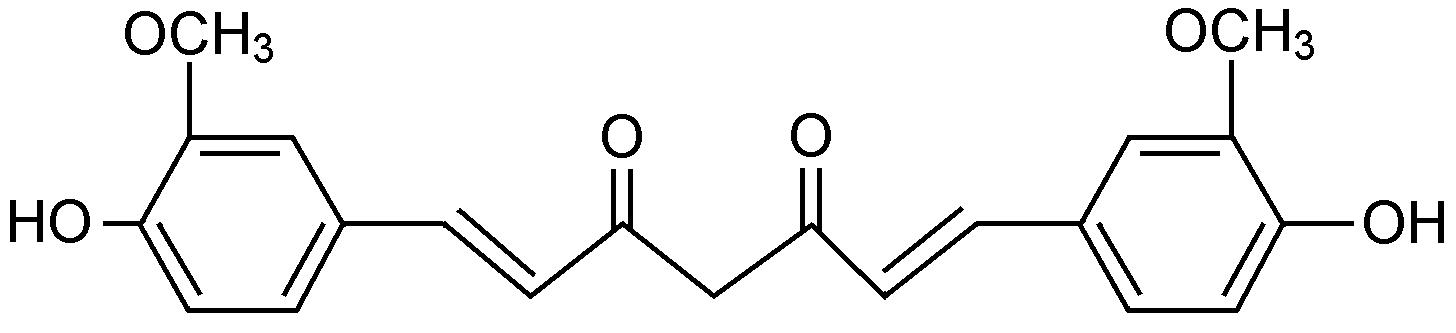 |
Figure 1 Chemical structure of curcumin |
肝炎是指由多种致病因素如病毒、细菌、化学药物、酒精等侵害肝脏,造成肝功能受损。急性肝损伤实验表明,姜黄素通过抗炎、抗氧化应激和保护线粒体等功能抵抗急性肝损伤。
首先,姜黄素通过抑制炎症因子、趋化因子等释放发挥抗炎作用。在伴刀豆球蛋白引起的肝脏损伤 模型中,姜黄素能够抑制肿瘤坏死因子α (TNF-α)、干扰素γ (IFN-γ) 和白介素4 (IL-4) 分泌,刺激抗炎因子IL-10的表达,并抑制肝细胞间黏附分子-1 (ICAM-1)、高迁移率蛋白-1 (HMGB1)、CXCL10、TLR2、TLR4和TLR9的表达,减轻肝脏受损[5,6,7]。在吡喹酮治疗肝吸虫感染仓鼠的模型中,姜黄素激活核转录因子Nrf2和抑制核转录因子NF-κB,发挥抗氧化应激和硝化应激作用,从而抑制环氧酶2 (COX-2)、可诱导性一氧化氮合酶 (iNOS)、白介素1β (IL-1β) 和TNF-α的表达[8]。
其次,姜黄素具有明显抗氧化作用,通过清除过剩自由基,减轻药物对肝细胞膜脂质过氧化作用,使受损的肝细胞得到一定程度的恢复,从而保护肝脏受损。Li等[9]在对乙酰氨基酚 (APAP) 诱导的急性小鼠肝损模型中证实了姜黄素的抗氧化能力,可显著降低APAP诱导的血清谷丙转氨酶 (ALT) 和肝组织丙二醛 (MDA) 水平,提高超氧化物歧化酶 (SOD) 活性,上调细胞凋亡相关基因Bcl-2/Bax比值,抑制肝细胞凋亡。Palma等[10]在糖尿病大鼠模型中也发现姜黄素可增强肝脏中过氧化氢酶 (CAT) 及SOD的活性。Cerny等[11]在脂多糖/半乳糖胺诱导的肝损伤模型中证实,姜黄素预处理可以增加血红素氧化酶-1 (HO-1) 和减少一氧化氮合成酶-2 (NOS-2) mRNA在肝脏组织中的表达。
此外,肝脏线粒体损伤也是肝细胞受损的重要原因之一,肝脏富含线粒体,线粒体呼吸链复合体 利用电子传递生产ATP,是活性氧 (ROS) 的主要来源。ROS的增多会加剧线粒体损伤以及线粒体RNA (mtRNA) 碱基的氧化。García-Niño等[12]证实姜黄素预处理抵抗重铬酸钾引起的大鼠肝脏氧化损伤效应与其保护线粒体功能有关,姜黄素增加线粒体氧消耗量,增强线粒体呼吸链复合物I的活性和膜通透性转换孔的开放。Waseem等[13]也发现姜黄素预处理可以减轻抗癌药物顺铂引起的大鼠肝脏线粒体氧化应激,恢复线粒体谷胱甘肽 (GSH) 水平及谷胱甘肽S-转移酶 (GST) 的活性,降低活性氧自由基 (ROS) 的产生。 2 姜黄素对非酒精性脂肪肝的治疗作用
非酒精性脂肪性肝病 (NAFLD) 是指除酒精和其他明确的肝损伤因素所致的,以弥漫性肝细胞脂肪变为主要特征的临床病理综合征,与肥胖、胰岛素抵抗、2型糖尿病相关,其病理变化进程表现为单纯性脂肪变、脂肪性肝炎 (NASH)、脂肪性肝纤维化和肝硬化。Day和James提出的“二次打击”学说被用于解释NAFLD的发病机制: 第一次打击主要营养过剩和胰岛素抵抗,造成游离脂肪酸 (FAA) 和甘油三酯 (TG) 在肝细胞中的脂质沉积; 第二次打击主要是氧化应激和脂质过氧化,是疾病进展的关键[2]。
姜黄素能够调节甘油三酯和胆固醇 (TC) 代谢起到降脂作用。最早的降脂报告可追溯到1970年,Rao等[14]发现姜黄素和胆固醇共同喂养组大鼠血清和肝脏胆固醇水平明显低于胆固醇喂养组。在高脂饮食动物模型中,姜黄素及其衍生物能够显著降低血清中血糖、TG、TC、低密度脂蛋白 (LDL)、载脂蛋白A1 (APOA1)、载脂蛋白B (APOB) 和血清胎球蛋白A的水平,进一步研究表明姜黄素及其衍生物可以激活AMP依赖的蛋白激酶 (AMPK) 和过氧化物酶体增殖物激活受体α (PPAR-α) 信号通路,抑制固醇调节元件结合蛋白1 (SREBP-1)、乙酰辅酶A羧化酶1 (ACC1) 及脂肪酸合酶 (FAS) 的升高以及降低11β-羟化类固醇脱氢酶1 (11β-HSD1) 的活性调节脂质代谢,减轻肝脏脂肪变[15,16,17,18]。在蛋氨酸胆碱缺乏饲料诱导的小鼠脂肪肝模型中也发现姜黄素能够激活肝组织PPARγ表达,抑制NF-κB活化,降低小鼠血清中炎症因子TNF-α和IL-6的水平[19]。
姜黄素具有预防和治疗NASH的生理活性,其作用机制与姜黄素抗炎、抗氧化、减少脂肪生成以及增加胰岛素敏感性相关。在小鼠腹腔注射TNF-α的实验中观察到姜黄素能够减轻枯否氏细胞和中性粒细胞的肝内浸润,降低髓过氧化物酶的活性、脂质过氧化和亚硝酸盐水平,减轻TNF-α引起肝毒性[20]。在高脂喂养的瘦素缺乏小鼠C57BL/6J ob/ob实验中发现姜黄素通过下调肝组织中SREBP-1c、FAS和ACC表达减少肝内TG堆积和降低血中TG水平; 姜黄素可引起信号转导与转录激活因子3 (STAT3) 磷酸化,抑制细胞因子信号抑制物3 (SOCS3) 表达,从而减少肝脏巨噬细胞浸润以及TNF-α、MCP-1、IL-6 mRNA表达; 姜黄素通过上调线粒体DNA (mtDNA)、细胞核呼吸因子 (NRF1) 和转录因子A (Tfam) 表达恢复线粒体功能; 姜黄素增加脂肪组织中脂联素的水平和肝脏NF-κB活性改善高脂饮食喂养小鼠血糖状态和胰岛素敏感性[21,22]。Shao等[23]也证实姜黄素能上调高脂饮食的C57BL/6J小鼠脂肪组织和肝细胞中PKB/Akt Ser473的磷酸化水平、脂肪组织中GSH/GSSG比值和HO-1的表达水平,抑制脂肪组织中NF-κB和JNK信号通路的活化,降低肝脏脂肪生成转录因子碳水化合物反应元件结合蛋白 (ChREBP)、固醇调节元件结合蛋白1c(SREBP-1c) 以及下游的L-丙酮酸激酶 (L-PK) 的mRNA表达水平,从而改善胰岛素信号通路,降低肝内脂质的含量,减少肥胖及糖尿病的发生。其他体外研究显示,姜黄素能够修复线粒体损伤,抑制ROS、PEPCK及G6Pase的产生,活化Akt信 号通路,从而导致JNK通路的阻断,有利于提高肝 细胞对FFA的敏感性和减轻过量铁对胰岛素信号的损伤[24,25]。
此外,姜黄素延缓NASH肝纤维化进程与其抑制肝星状细胞 (HSC) 活化密切相关。当肝细胞受损时,静息HSC细胞失去脂滴进而活化,产生大量的细胞外基质 (ECM)。研究发现,姜黄素抑制胰岛素诱导的HSC活化与其增加HSC内的脂滴有关,姜黄素通过降低HSC上胰岛素受体 (InsR) 磷酸化水平抑制InsR表达进而干扰HSC上胰岛素信号通路,通过诱导胱氨酸连接酶的表达,合成谷胱甘肽从而减轻胰岛素诱导的氧化应激[26]。在高糖处理HSC时观察到姜黄素能够干扰p38 MAPK信号通路阻止葡萄糖转运子2 (GLUT2) 的膜转位,进而激活PPARγ抑制Glut2基因表达,减轻氧化应激,干扰胰岛素受体底物/IRS-PI3K-Akt信号通路抑制GLUT4膜转位,从而抑制HSC活化[27,28]。 3 姜黄素对肝纤维化的治疗作用
肝纤维化是各种慢性肝病进展成肝硬化的共同病理基础和必经阶段,是肝脏损伤后的一种修复过程,其主要特征是以胶原为主的细胞外基质在肝脏中的过度沉积[29]。多项体内外研究显示,姜黄素可以作用于多个环节,干预纤维化进展。
去除病因是治疗肝纤维化最有效的方法,如针对病毒、代谢、药物、酒精和自身免疫性等病因进行治疗。最近在姜黄素抑制肝炎病毒复制方面取得进 展,Rechtman等[30]报道姜黄素通过下调PPARγ辅助活化因子1α (PGC-1α),抑制乙型肝炎病毒 (HBV) 的表达和复制。Chen等[31]证实姜黄素通过诱导Huh7.5细胞HO-1的表达,调控AKT信号通路从而抑制丙 型肝炎病毒 (HCV) 的复制。Anggakusuma等[32]发现由于姜黄素与HCV病毒颗粒相结合,导致HCV病毒颗粒膜的流动性改变,影响HCV病毒颗粒与肝细胞膜的结合和融合,从而阻断HCV病毒在肝细胞间的感染,突破HCV基因型和耐药屏障,进而大胆提出姜黄素与抗病毒药物联合使用治疗HCV感染的设想。
HSC是肝脏特有的周细胞,位于肝血窦内皮细胞 (SEC) 与肝细胞之间的Disse间隙内,HSC活化并转变为肌成纤维细胞是纤维化的中心环节,抑制HSC活化和诱导其凋亡,调节ECM形成的动态平衡也是抗肝纤维化的有效策略。体外实验证实,姜黄素参与调节α-平滑肌动蛋白 (α-SMA)、胶原蛋白、DLK1、 Bax、Bcl-2等基因表达来调控HSC增殖与凋亡[33,34,35,36],姜黄素还可诱导HSC糖基化终产物受体 (AGE-R1) 的表达,抵抗晚期糖基化终末产物 (AGE) 对HSC的激活[37,38]。多项酒精、四氯化碳 (CCl4) 和硫代乙酰胺等诱导的肝纤维化模型体内实验显示,姜黄素能够抑制炎症因子TNF-α、IL-6、MCP-1、HMGB1、TLR2、TLR4等的分泌表达,抑制肝组织中α-SMA、TGF-β、Collagen I、大麻素受体 (CBR1) 等的表达,减少细胞外基质过度生成[39,40,41,42]。体内外研究证实,姜黄素抗纤维化作用机制与其调控TGF-β/Smad、β-连环蛋白、NF-κB、PPARγ、细胞外调节激酶 (ERK)、Nrf2及瘦素信号通路有关。
此外,HSC对肝脏微血管的结构与功能具有重 要的调节作用,活化的HSC可表达多种促血管生成因子如血管内皮生长因子(VEGF)、血小板衍生生长因子 (PDGF)、瘦素、血管生成素-1等作用于SEC,促进病理性血管生成并加速肝纤维化进程。研究发现,姜黄素可下调肝内缺氧诱导因子 (HIF-1α)、VEGF、血管内皮生长因子受体 (VEGFR-1)、PDGF和环氧合酶因子 (COX2) 的表达,改善肝脏肝窦的毛细血管化,延缓纤维化[43,44]。
随着微小RNA (miRNA) 研究的逐步深入,已发现若干miRNA可参与调控肝纤维化相关因子、HSC活化与凋亡,促进或延缓纤维化进程。关于肝纤维化与miRNA研究已有陆续报道。Hassan等[45]首次报道姜黄素可以下调CCl4诱导的小鼠肝纤维化模型肝脏组织中miR-199和miR-200至正常水平,发挥抗纤维化作用。Zheng等[46]认为miRNA介导的表观遗传调控可能是姜黄素抗肝纤维化的新机制,研究发现姜黄素上调miR-29b表达,导致肝星状细胞中DNA甲基转移酶DNMT3b表达下调,通过表观遗传学调控PTEN低甲基化从而抑制肝星状细胞活化。 4 姜黄素对肝癌的治疗作用
肝癌是发生在肝脏的恶性肿瘤,常指原发性肝癌,由肝细胞或肝内胆管上皮细胞所引发的癌变。由于姜黄素具有抗炎、抗氧化性质,能够改变肿瘤生长的微环境,抑制血管生成拟态 (VM) 形成,发挥抗癌作用[47,48]。新近研究报道,姜黄素可以降低苯并芘喂养小鼠肝脏中脱乙基酶 (EROD)、脱烷基酶 (PROD) 及醌还原酶 (QR) 等致癌生物转化酶的活性,抑制肿瘤生长[49]。姜黄素通过下调AKT和STAT3的磷酸化水平,降低人肝癌细胞SK-Hep-1迁移能力和MMP9表达[48]。姜黄素下调c-myc、COX2、VEGF、细胞周期蛋白CyclinD1、细胞周期蛋白依赖性激酶CDK4的表达,抑制肝癌细胞的增殖与转移,其作用与姜黄素激活ROS/TLR-4/MyD88信号通路,下调β-连环蛋白表达阻断Wnt信号通路有关[50,51,52]。Bae等[53]认为姜黄素下调HepG2缺氧诱导因子-1α (HIF-1α) 的表达致VEGF降低,从而影响了缺氧状态下血管再生和肝癌细胞生长。Cao等[54]发现姜黄素诱导的线粒体超极化和mtDNA损伤是HepG2凋亡的始动因素。Fan等[55]认为姜黄素通过抑制脂肪酸合成,诱导HepG2细胞凋亡。
近年来,蛋白激酶、细胞周期调节因子、细胞凋亡调节因子等都已成为肿瘤治疗的新靶点。多个研 究显示,姜黄素能够抑制微管聚合,阻滞细胞周期于G2/M期,并诱导肝癌细胞的凋亡。Wang等[56]证实姜黄素通过激活细胞周期检测点激酶1 (Chk1) 的表达,引起肝癌细胞株如Huh7、Hep3B、SK-Hep-1及QGY- 7703的G2/M周期阻滞和凋亡。Cheng等[57]报道姜黄素通过降低J5肝癌细胞Cdc2的表达引起G2/M周期阻滞,诱导内质网应激和线粒体功能障碍抑制肿瘤细胞增殖。Jiang等[58]揭示姜黄素通过上调HepG2微管蛋白表达,改变细胞膜结构及细胞骨架从而干扰肿瘤细胞周期。Wang等[59]发现姜黄素通过激活P38上调Huh7细胞凋亡基因Fas和Fas配体的表达诱导细胞凋亡。Yu等[60]报道姜黄素通过上调Bax/Bcl-2比值,促进肝癌细胞SMMC-7721凋亡。 5 姜黄素衍生物的研究进展
姜黄素生物利用度低、体内代谢快,限制其临 床应用。台湾1项临床试验表明口服姜黄素0.5~8 g/天,持续3个月,未观察到明显的毒副作用。药代动力学研究显示患者接受4~8 g/天剂量时,血药浓度峰值出现在服药后1~2 h,4 g/天剂量时最大血药浓度为 (0.5 ± 0.11) μmol·L-1,8 g/天剂量为 (1.77 ± 1.87) μmol·L-1,低剂量时血中检测不到姜黄素[61]。因此,如何提高姜黄素生物利用度是突破姜黄素临床应用受限的瓶颈,国内外学者围绕姜黄素剂型改变、化学结构修饰以及联合用药方面进行了许多尝试。
纳米粒运载系统是一种有效的药物运载方式,姜黄素纳米粒可有效地改善姜黄素水溶性,提高其生物利用度。Bisht等[62]通过N-异丙基丙烯酰胺、乙烯吡咯烷酮、丙烯酸包裹姜黄素形成易溶于水的纳米粒NanoCurc,腹腔注射6和12 h后,肝细胞中仍可检测到姜黄素,NanoCurc显著改善CCl4诱导的肝损伤和炎性因子的释放,诱导肝星状细胞凋亡。Ucisik等[63]将姜黄素包裹在软脂酸甘油酯和磷脂中形成平均直径286 nm的纳米粒CurcuEmulsomes (图 2),溶解度达到11 mg·mL-1。CurcuEmulsomes进入HepG2细胞后缓慢释放出姜黄素,导致更多的细胞滞留在G2/M期。Ghosh等[64]采用聚乳酸-羟基乙酸 (PLGA) 包被姜黄素制备纳米粒Nano Cur,在DMN诱导的肝癌模型中,Nano Cur表现出更好的抗氧化损伤和抗癌作用。
 |
Figure 2 Schematic drawing of CurcuEmulsome. CurcuEmulsome is composed of a solid tripalmitin core surrounded by phospholipid multi-layers. The lipophilic load,i.e. curcumin,can locate itself in the inner core,as well as inside the phospholipid layers of the nanocarrier[63] |
Tang等[65]通过β-硫酯键将双亲分子乙二醇引入到姜黄素的结构中,形成易溶于水的姜黄素衍生物Curc-OEG (图 3),溶解度大于50 mg·mL-1,在水中形成稳定的纳米粒。药代动力学显示,小鼠尾静脉注射Curc-OEG (剂量25 mg·kg-1) 1 h后,血药浓度高达6.8 μg·mL-1,是普通姜黄素的50~500倍,组织分布显示肝脏含量达8~12 μg·g-1。本课题组以实验性非酒精性脂肪肝炎大鼠模型评价Curc-OEG治疗NASH效果,发现Curc-OEG显著降低高脂饮食所致的大鼠血清中ALT、AST、TC水平,降低肝组织TG和TC 水平,明显延缓NASH纤维化进程,这与Curc- OEG下调TNF-α、NF-κB、羟甲戊二单酰辅酶A (HMG- CoA) 还原酶表达密切相关[66]。而且,应用CCl4诱导的肝纤维化大鼠模型和原代HSC考察Curc-OEG抗纤维化效果,体外实验发现,低剂量 (6.25 μg·mL-1) Curc-OEG能抑制原代HSC的活化增殖,高剂量 (50 μg·mL-1) 可诱导活化的HSC凋亡,但对不增殖的 原代大鼠肝细胞没有明显的细胞毒性作用; 体内实验证实,Cur-OEG能够抑制炎症因子TNF-α、IL-1β、IL-6、COX2表达,上调肝组织中基质金属蛋白酶 13 (MMP-13) 和PPAR表达,下调α-SMA、TGF-β、Collagen I、金属蛋白酶抑制因子1 (TIMP-1) 表达,50 mg·kg-1姜黄素衍生物表现出良好的抗纤维化效果[67,68]。
联苯二氟酮EF24 [3,5-(E)-二 (2-氟亚苄基) 哌啶- 4-酮乙酸盐,图 3] 是缩短姜黄素不饱和β-二酮链得到的姜黄素类似物,对多种肿瘤具有抗癌活性[69]。Liu等[70]体外证实EF24能够引起肝癌细胞Hepa1-6和H22的G2/M细胞周期阻滞,下调cyclinB1、Cdc2和Bcl-2基因的表达,上调pp53、p53、p21和Bax基因的表达,诱导肿瘤细胞的凋亡。体内肝癌模型也证实,EF24通过诱导线粒体凋亡、细胞周期阻滞以及抗血管生成,抑制肿瘤的生长。进一步研究表明EF24合并索拉非尼可以下调VHL依赖的HIF-1α表达和抑制NF-κB激活从而克服索拉非尼对肝癌治疗的耐受[71]。Sehgai等[72]发现姜黄素和胡椒粉联用较姜黄素单用能更有效减轻肝脏组织8-羟基-2'-脱氧鸟苷的含量及受损DNA百分比,缓解苯并芘引起的肝脏组织DNA损伤。
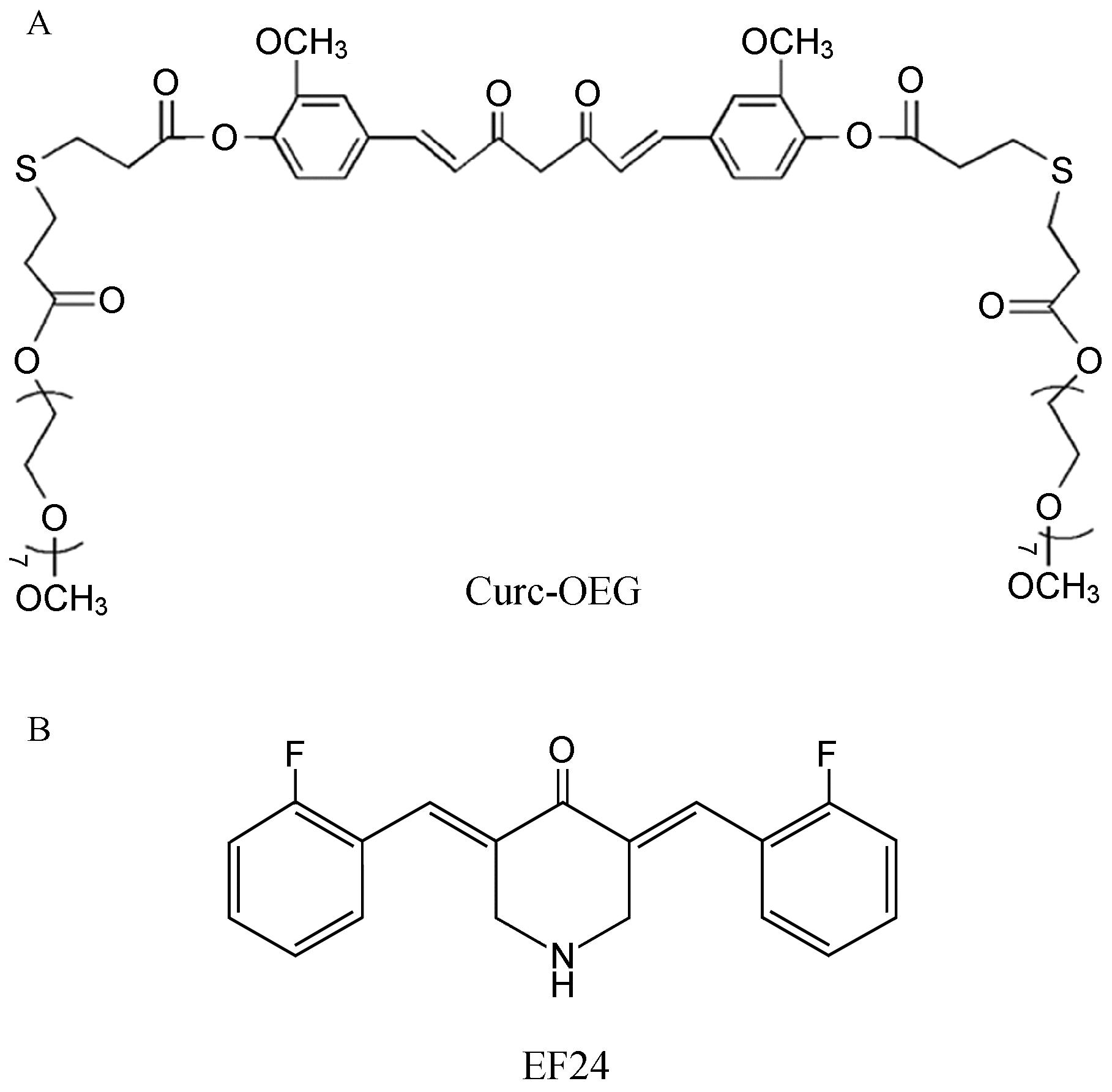 |
Figure 3 Chemical structures of curcumin derivatives. A: Curc-OEG: Curcumin conjugated with two oligo(ethylene glycol) chains[65]; B: EF24: 3,5-bis(2-flurobenzylidene)piperdin-4-one[69] |
由于姜黄素具有广泛的药理活性且安全性好,在肝脏疾病的基础研究中展示出良好的应用前景,相信在药物化学家和临床专家的共同努力下,未来会有新型的姜黄素运载系统或姜黄素衍生物应用于肝脏疾病的临床治疗。
| [1] | Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research [J]. Altern Med Rev, 2009, 14: 141-153. |
| [2] | Vera-Ramirez L, Perez-Lopez P, Varela-Lopez A, et al. Curcumin and liver disease [J]. BioFactors, 2013, 39: 88-100. |
| [3] | Ghosh N, Ghosh R, Mandal V, et al. Recent advances in herbal medicine for treatment of liver diseases [J]. Pharm Biol, 2011, 49: 970-988. |
| [4] | Shapiro H, Bruck R. Therapeutic potential of curcumin in non-alcoholic steatohepatitis [J]. Nutr Res Rev, 2005, 18: 212-221. |
| [5] | Tu CT, Han B, Liu HC, et al. Curcumin protects mice against concanavalin A-induced hepatitis by inhibiting intrahepatic intercellular adhesion molecule-1(ICAM-1)and CXCL10 expression [J]. Moll Cell Biochem, 2011, 358: 53-60. |
| [6] | Tu CT, Han B, Yao QY, et al. Curcumin attenuates concanavalin A-induced liver injury in mice by inhibition of Toll-like receptor(TLR)2, TLR4 and TLR9 expression [J]. Int Immunopharmacol, 2012, 12: 151-157. |
| [7] | Wang C, Nie H, Li K, et al. Curcumin inhibits HMGB1 releasing and attenuates concanavalin A-induced hepatitis in mice [J]. Eur J Pharmacol, 2012, 697: 152-157. |
| [8] | Charoensuk L, Pinlaor P, Prakobwong S, et al. Curcumin induces a nuclear factor-erythroid 2-related factor 2-driven response against oxidative and nitrative stress after praziquantel treatment in liver fluke-infected hamsters [J]. Int J Parasitol, 2011, 41: 615-626. |
| [9] | Li G, Chen JB, Wang C, et al. Curcumin protects against acetaminophen-induced apoptosis in hepatic injury [J]. World J Gastroenterol, 2013, 19: 7440-7446. |
| [10] | Palma HE, Wolkmer P, Gallio M, et al. Oxidative stress parameters in blood, liver, and kidney of diabetic rats treated with curcumin and/or insulin [J]. Mol Cell Biochem, 2014, 386: 199-210. |
| [11] | Cerny D, Lekic N, Vanova K, et al. Hepatoprotective effect of curcumin in lipopolysaccharide/-galactosamine model of liver injury in rats: relationship to HO-1/CO antioxidant system [J]. Fitoterapia, 2011, 82: 786-791. |
| [12] | García-Niño WR, Tapia E, Zazueta C, et al. Curcumin pretreatment prevents potassium dichromate-induced hepatotoxicity, oxidative stress, decreased respiratory complex I activity, and membrane permeability transition pore opening [J]. Evid Based Complement Alternat Med, 2013, doi: 10.1155/2013/424692. |
| [13] | Waseem M, Parvez S. Mitochondrial dysfunction mediated cisplatin induced toxicity: modulatory role of curcumin [J]. Food Chem Toxicol, 2013, 53: 334-342. |
| [14] | Rao DS, Sekhara NC, Satyanarayana MN, et al. Effect of curcumin on serum and liver cholesterol levels in the rat [J]. J Nutr, 1970, 100: 1307-1315. |
| [15] | Hu GX, Lin H, Lian QQ, et al. Curcumin as a potent and selective inhibitor of 11β-hydroxysteroid dehydrogenase 1: improving lipid profiles in high-fat-diet-treated rats [J]. PLoS One, 2013, 8: e49976. doi: 10.1371/journal.pone.0049976. |
| [16] | Um MY, Hwang KH, Ahn J, et al. Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase [J]. Basic Clin Pharmacol Toxicol, 2013, 113: 152-157. |
| [17] | Kang OH, Kim SB, Seo YS, et al. Curcumin decreases oleic acid-induced lipid accumulation via AMPK phosphorylation in hepatocarcinoma cells [J]. Eur Rev Med Pharmacol Sci, 2013, 17: 2578-2586. |
| [18] | Oner-Lyidog Y, Kocak H, Seyidhanoglu M, et al. Curcumin prevents liver fat accumulation and serum fetuin-a increase in rats fed a high-fat diet [J]. J Physiol Biochem, 2013, 69: 677-686. |
| [19] | Wang Y, Li J, Zhuge L, et al. Comparison between the efficacies of curcumin and puerarin in c57bl/6 mice with steatohepatitis induced by a methionine and choline deficient diet [J]. Exp Ther Med, 2014, 7: 663-668. |
| [20] | Mouzaoui S, Rahim I, Djerdjouri B. Aminoguanidine and curcumin attenuated tumor necrosis factor(TNF)-alpha- induced oxidative stress, colitis and hepatotoxicity in mice [J]. Int Immunopharmacol, 2012, 12: 302-311. |
| [21] | Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity [J]. Endocrinology, 2008, 149: 3549-3558. |
| [22] | Kuo JJ, Chang HH, Tsai TH, et al. Positive effect of curcumin on inflammation and mitochondrial dysfunction in obese mice with liver steatosis [J]. Int J Mol Med, 2012, 30: 673-679. |
| [23] | Shao W, Yu Z, Chiang Y, et al. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes [J]. PLoS One, 2012, 7: e28784. doi: 10.1371/journal.pone. 0028784. |
| [24] | Kuo JJ, Chang HH, Tsai TH, et al. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis [J]. Int J Mol Med, 2012, 30: 643-649. |
| [25] | Messner DJ, Rhieu BH, Kowdley KV. Iron overload causes oxidative stress and impaired insulin signaling in AML-12 hepatocytes [J]. Dig Dis Sci, 2013, 58: 1899-1908. |
| [26] | Lin J, Zheng S, Chen A. Curcumin attenuates the effects of insulin on stimulating hepatic stellate cell activation by interrupting insulin signaling and attenuating oxidative stress [J]. Lab Invest, 2009, 89: 1397-1409. |
| [27] | Lin J, Chen A. Curcumin diminishes the impacts of hyperglycemia on the activation of hepatic stellate cells by suppressing membrane translocation and gene expression of glucose transporter-2 [J]. Mol Cell Endocrinol, 2011, 333: 160-171. |
| [28] | Tang Y, Chen A. Curcumin prevents leptin raising glucose levels in hepatic stellate cells by blocking translocation of glucose transporter-4 and increasing glucokinase [J]. Br J Pharmacol, 2010, 161: 1137-1149. |
| [29] | Lee U, Friedman SL. Mechanisms of hepatic fibrogenesis [J]. Best Pract Res Clin Gastroenterol, 2011, 25: 195-206. |
| [30] | Rechtman MM, Har-Noy O, Bar-Yishay I, et al. Curcumin inhibits hepatitis B virus via down-regulation of the metabolic coactivator PGC-1a [J]. FEBS Lett, 2010, 584: 2485-2490. |
| [31] | Chen MH, Lee MY, Chuang JJ, et al. Curcumin inhibits HCV replication by induction of heme oxygenase-1 and suppression of AKT [J]. Int J Mol Med, 2012, 30: 1021-1028. |
| [32] | Anggakusuma, Colpitts CC, Schang LM, et al. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells [J]. Gut, 2014, 63: 1137-1149. |
| [33] | Lin YL, Lin CY, Chi CW, et al. Study on antifibrotic effects of curcumin in rat hepatic stellate cells [J]. Phytother Res, 2009, 23: 927-932. |
| [34] | Qiu J, Zhou Q, Zhai X, et al. Curcumin regulates delta-like homolog1 expression in activated hepatic stellate cell [J]. Eur J Pharmacol, 2014, 728: 9-15. |
| [35] | Zheng S, Chen A. Activation of PPAR-γ is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro [J]. Biochem J, 2004, 384: 149-157. |
| [36] | Chen A, Zheng S. Curcumin inhibits connective tissue growth factor gene expression in activated hepatic stellate cells in vitro by blocking NF-κB and ERK signaling [J]. Br J Pharmacol, 2008, 153: 557-567. |
| [37] | Lin J, Tang Y, Kang Q, et al. Curcumin eliminates the inhibitory effect of advanced glycation end-products(AGEs)on gene expression of AGE receptor-1 in hepatic stellate cells in vitro [J]. Lab Invest, 2012, 92: 827-841. |
| [38] | Tang Y, Chen A. Curcumin eliminates the effect of advanced glycation end-products(AGEs)on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling [J]. Lab Invest, 2014, 94: 503-516. |
| [39] | Reyes-Gordillo K, Segovia J, Shibayama M, et al. Curcumin protects against acute liver damage in the rat by inhibiting NF-κB, proinflammatory cytokines production and oxidative stress [J]. Biochim Biophys Acta, 2007, 1770: 989-996. |
| [40] | Reyes-Gordillo K, Segovia J, Shibayama M, et al. Curcumin prevents and reverses cirrhosis induced by bile duct obstructtion or CCl4 in rats: role of TGF-β modulation and oxidative stress [J]. Fundam Clin Pharmacol, 2008, 22: 417-427. |
| [41] | Zhang Z, Guo Y, Zhang S, et al. Curcumin modulates cannabinoid receptors in liver fibrosis in vivo and inhibits extracellular matrix expression in hepatic stellate cells by suppressing cannabinoid receptortype-1 in vitro [J]. Eur J Pharmacol, 2013, 721: 133-140. |
| [42] | Tu CT, Yao QY, Xu BL, et al. Protective effects of curcumin against hepatic fibrosis induced by carbon tetrachloride: modulation of high-mobility group box 1, Toll-like receptor 4 and 2 expression [J]. Food Chem Toxicol, 2012, 50: 3343- 3351. |
| [43] | Yao Q, Lin Y, Li X, et al. Curcumin ameliorates intrahepatic angiogenesis and capillarization of the sinusoids in carbon tetrachloride-induced rat liver fibrosis [J]. Toxicol Lett, 2013, 222: 72-82. |
| [44] | Zhang F, Zhang Z, Chen L, et al. Curcumin attenuates angiogenesis in liver fibrosis and inhibits angiogenic properties of hepatic stellate cells [J]. J Cell Mol Med, 2014, 18: 1392- 1406. |
| [45] | Hassan ZK, Al-Olayan EM. Curcumin reorganizes miRNA expression in a mouse model of liver fibrosis [J]. Asian Pac J Cancer Prev, 2012, 13: 5405-5408. |
| [46] | Zheng J, Wu C, Lin Z, et al. Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation - a novel mechanism suppressing liver fibrosis [J]. FEBS J, 2014, 281: 88-103. |
| [47] | Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review [J]. Curr Pharm Biotechnol, 2012, 13: 218-228. |
| [48] | Chiablaem K, Lirdprapamongkol K, Keeratichamroen S, et al. Curcumin suppresses vasculogenic mimicry capacity of hepatocellular carcinoma cells through STAT3 and PI3K/AKT inhibition [J]. Anticancer Res, 2014, 34: 1857-1864. |
| [49] | Sehgai A, Kumar M, Jain M, et al. Modulatory effects of curcumin in conjunction with piperine on benzo(a)pyrene- mediated DNA adducts and biotransformation enzymes [J]. Nutr Cancer, 2013, 65: 885-890. |
| [50] | Huang CZ, Huang WZ, Zhang G, et al. In vivo study on the effects of curcumin on the expression profiles of anti-tumour genes(VEGF, CyclinD1 and CDK4)in liver of rats injected with DEN [J]. Mol Biol Rep, 2013, 40: 5825-5831. |
| [51] | Kim HJ, Park SY, Park OJ, et al. Curcumin suppresses migration and proliferation of Hep3B hepatocarcinoma cells through inhibition of the Wnt signaling pathway [J]. Mol Med Rep, 2013, 8: 282-286. |
| [52] | Yoysungnoen P, Wirachwong P, Bhattarakosol P, et al. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice [J]. Clin Hemorheol Microcirc, 2006, 34: 109-115. |
| [53] | Bae MK, Kim SH, Jeong JW, et al. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1 [J]. Oncol Rep, 2006, 15: 1557-1562. |
| [54] | Cao J, Liu Y, Jia L, et al. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells [J]. Free Radic Biol Med, 2007, 43: 968-975. |
| [55] | Fan H, Tian W, Ma X. Curcumin induces apoptosis of HepG2 cells via inhibiting fatty acid synthase [J]. Target Oncol, 2013, doi: 10.1007/s11523-013-0286-5. |
| [56] | Wang WZ, Cheng J, Luo J, et al. Abrogation of G2/M arrest sensitizes curcumin-resistant hepatoma cells to apoptosis [J]. FEBS Lett, 2008, 582: 2689-2695. |
| [57] | Cheng CY, Lin YH, Su CC. Curcumin inhibits the proliferation of human hepatocellular carcinoma J5 cells by inducing endoplasmic reticulum stress and mitochondrial dysfunction [J]. Int J Mol Med, 2010, 26: 673-678. |
| [58] | Jiang J, Jin H, Liu L, et al. Curcumin disturbed cell-cycle distribution of HepG2 cells via cytoskeletal arrangement [J]. Scanning, 2013, 35: 253-260. |
| [59] | Wang WZ, Li L, Liu MY, et al. Curcumin induces FasL-related apoptosis through p38 activation in human hepatocellular carcinoma Huh7 cells [J]. Life Sci, 2013, 92: 352-358. |
| [60] | Yu J, Zhou X, He X, et al. Curcumin induces apoptosis involving bax/bcl-2 in human hepatoma SMMC-7721 cells [J]. Asian Pac J Cancer Prev, 2011, 12: 1925-1929. |
| [61] | Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high- risk or pre-malignant lesions [J]. Anticancer Res, 2001, 21: 2895-2900. |
| [62] | Bisht S, Khan MA, Bekhit M, et al. A polymeric nanoparticle formulation of curcumin(NanoCurcTM)ameliorates CCl4- induced hepatic injury and fibrosis through reduction of pro- inflammatory cytokines and stellate cell activation [J]. Lab Invest, 2011, 91: 1383-1395. |
| [63] | Ucisik MH, Küpcü S, Schuster B, et al. Characterization of curcuEmulsomes: nanoformulation for enhanced solubility and delivery of curcumin [J]. J Nanobiotechnology, 2013, doi: 10.1186/1477-3155-11-37. |
| [64] | Ghosh D, Choudhury ST, Ghosh S, et al. Nanocapsulated curcumin: oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat [J]. Chem Biol Interact, 2012, 195: 206-214. |
| [65] | Tang H, Murphy CJ, Zhang B, et al. Amphiphilic curcumin conjugate-forming nanoparticles as anticancer prodrug and drug carriers: in vitro and in vivo effects [J]. Nanomedicine, 2010, 5: 855-865. |
| [66] | Zeng CH, Zeng P, Deng YH, et al. The effects of curcumin derivative on experimental Steatohepatitis [J]. Chin J Hepatol(中华肝脏病杂志), 2011, 19: 454-459. |
| [67] | Shen N, Deng YH, Ling N, et al. Effects and its mechanisms of Curc-OEG on experimental hepatic fibrosis in rats [J]. Chin J Clin Pharmacol(中国临床药理学杂志), 2012, 28: 358- 360. |
| [68] | Deng YH, Shen N, Ling N, et al. Efficacy of the Curc-OEG on rat liver fibrosis induced carbon tetrachloride [J]. Chin J Clin pharmacol Ther(中国临床药理学与治疗学), 2012, 17: 147-153. |
| [69] | Adams BK, Ferstl EM, Davis MC, et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents [J]. Bioorg Med Chem, 2004, 12: 3871-3883. |
| [70] | Liu H, Liang Y, Wang L, et al. In vivo and in vitro suppression of hepatocellular carcinoma by EF24, acurcumin analog [J]. PLoS One, 2012, 7: e48075. doi: 10.1371/journal.pone.0048075. |
| [71] | Liang Y, Zheng T, Song R, et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in hepatocellular carcinoma [J]. Hepatology, 2013, 57: 1847-1857. |
| [72] | Sehgai A, Kumar M, Jain M, et al. Combined effects of curcumin and piperine in ameliorating benzo(a)pyrene induced DNA damage [J]. Food Chem Toxicol, 2011, 49: 3002-3006. |
 2014, Vol. 49
2014, Vol. 49


