2. 中国中医科学院医学实验中心, 北京 100700
2. The Experimental Research Center, China Academy of Chinese Medical Sciences, Beijing 100700, China
中医药学的“辨证施治”表明,在机体不同病理状态下,中药可能体现不同的药效甚或毒副作用。因此,研究中药在机体特定病理状态下的药代动力学特征,对于解释中药发挥不同药效或导致毒性的原因,进而揭示中药药效成分和作用机制具有重要意义。黄芩汤 (HQT) 是伤寒论著名方剂,由黄芩、白芍、炙甘草和大枣4味药配伍而成,具有解热、镇痛和抗炎作用[1,2]。前期研究结果显示,在不同配伍和不同剂量下,黄芩汤中黄芩苷、汉黄芩苷、黄芩素、汉黄芩素、千层纸素A、 甘草酸和甘草次酸等成分在正常机体内均具有合适的药代动力学特征[3,4]。为进一步探讨黄芩汤在病理状态下的差异性,本文在多成分LC-MS定量[4]基础上,研究黄芩汤在发热状态下的药效学和药代动力学特征。
材料与方法 仪器Agilent 1200-6130液相色谱-串联质谱联用仪 (美国Agilent公司),数字显示电子体温计 (深圳华路电子有限公司),Sn-69513型免疫计数器 (上海核所日环光电仪器有限公司)。
药品与试剂芍药苷 (paeoniflorin,批号: ZL090328),黄芩苷 (baicalin,批号: ZL20100805),黄芩素 (baicalein,批号: ZL20111009),汉黄芩苷 (wogonoside,批号: ZL20101228),汉黄芩素 (wogonin,批号: ZL20110205),千层纸素A (oroxylin A,批 号: ZL20110125),甘草酸 (glycyrrhizic acid,批号: ZL20110508) 和甘草次酸 (glycyrrhetinic acid,批号: ZL20110625) 等对照品购自南京泽朗医药有限公司,纯度均大于98%; 黄芩汤按本实验室方法[5]制备 (批号: HQT20110529),各成分含量分别为芍药苷0.29%、黄芩苷2.72%、汉黄芩苷0.68%、黄芩素1.07%、汉黄芩素0.09%、千层纸素A 0.07% 和甘草酸0.17%,未检测到甘草次酸; 7.5% EDTA·Na2 (批号: 20100201,北京普尔伟业生物科技有限公司),高活性干酵母 (安琪酵母股份有限公司)。
动物清洁级Wistar大鼠,雄性,110只,体重(213± 23) g,由军事医学科学院实验动物中心提供,合格证号为SCXK-(军) 2007-004。实验前禁食12~16 h,自由饮水。
大鼠发热模型的制备及体温的测定采用文献[6]报道的方法,大鼠皮下注射酵母菌制备发热模型。测定直肠温度,记录大鼠体温。实验前大鼠首先适应实验室环境和体温测定时的刺激,保持实验室温度 (25 ± 0.5) ℃,湿度 (50 ± 5) %,温度计对大鼠直肠进行刺激连续1周,实验前一天,筛选出早中晚体温波动小于0.3 ℃的动物用于实验。给药前测定各组大鼠基础体温 (T1),取血前测定各组大鼠体温 (T2),体温变化值 (change in rectal temperature) = T2 - T1。
大鼠的分组与给药及样品采集大鼠随机分为正常组、正常给药组、模型组和模型给药组。正常给药组和模型给药组给予HQT (20 g·kg-1),正常组和模型组给予相应体积蒸馏水,造模后立即灌胃给药。于给药0.5、1、2、3、4、6、8、10、12、16、20和24 h后眼眶取血,分别置于干净、EDTA·Na2抗凝和肝素抗凝的试管中,4 ℃条件下3 000 r·min-1离心 10 min,分离血清和血浆,-80 ℃冰箱中保存备用。取血时间点至少平行5只动物。
生物样品的测定血浆样品中前列腺素PGE2,血清样品中白细胞介素IL-1β和肿瘤坏死因子TNF-α均使用放射免疫分析法进行测定 (由北京康源瑞得生物技术有限公司检测); 血浆样品中黄芩汤各成分采用已建立的LC-MS方法进行测定[4]。
数据处理药效数据以± s表示,采用t检验进行显著性差异分析; 药时数据采用Phoenix WinNonlin (Version 6.2,Pharsight Corporation,USA) 药代动力学软件处理,非房室方法计算药代动力学参数。
结果 1 LC-MS方法学确证实验结果显示血浆中内源性杂质、代谢产物及 黄芩汤中其他成分不干扰样品测定,8种对照品线 性关系的相关系数均大于0.990,最低定量限均小于20 ng∙mL-1,提取回收率、基质效应、精密度、准确度和稳定性也符合相关要求[4]。
2 药效学研究 2.1 对体温影响正常组、正常给药组、模型组和模型给药组各时间点直肠温度变化结果如图 1所 示。注射酵母菌后,模型组大鼠初始温度有所降低,但3 h后开始升高,10 h达峰,之后趋于基础值,整个发热时间持续20 h左右,这说明本实验酵母菌致热大鼠模型成功。单次给予HQT,大鼠在4、6、8、10和12 h等连续时间点的平均温度均低于模型组,其中在4、10和12 h时间点具有统计学意义 (P < 0.05); 单次给予HQT后,与正常大鼠相比温度相差不大,各时间点均无显著性差异。实验表明黄芩汤能降低酵母菌致发热大鼠的体温,并且不影响正常大鼠的体温。
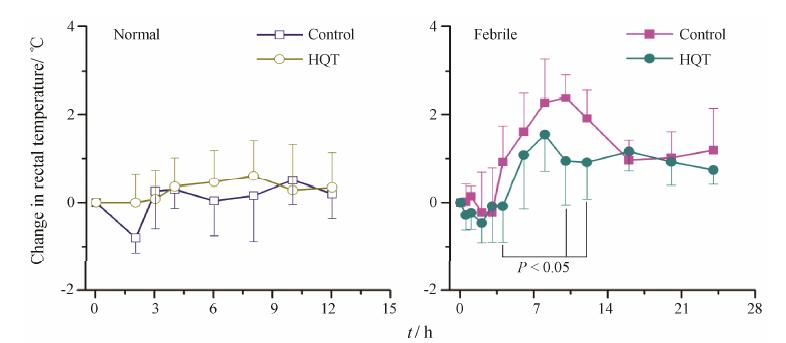 |
Figure 1 Effect of Huangqin Tang (HQT) on the rectal temperature of rats after single oral administration at 20 g∙kg-1. n = 5,± s. P < 0.05 vs control group |
模型组与给药组各时间点介质含量结果如表 1所示,给药后体内促炎性介质PGE2和促炎性细胞因子1L-1β、TNF-α的含量均开始升高,在6 h左右达到峰值,而后又有所降低,这和给药后大鼠直肠温度的变化趋势基本一致; 与模型组相比,给药后PGE2和TNF-α的含量均降低,其中PGE2在4和8 h时有显著性差异 (P < 0.01),TNF-α在8 h时有显著性差异 (P < 0.01); 1L-1β各时间点的含量和模型组相比,有一定的下降趋势,但无统计学意义。
|
|
Table 1 Effects of Huangqin Tang (HQT) on concentrations of PGE2,1L-1β and TNF-α in yeast-induced febrile rats after single oral administration at 20 g∙kg-1. n = 5,± s. **P < 0.01 vs control group |
给予黄芩汤后,按设计的时间点取血,实验过程中大鼠耐受性良好,未见明显的毒副作用。应用建立的LC-MS方法测定发热大鼠各时间点血药浓度,与正常大鼠相比药时曲线和药代参数见图 2和表 2。结果显示,黄芩苷和甘草酸在正常和发热大鼠体内均具有多峰现象; 汉黄芩苷、黄芩素、汉黄芩素和千层纸素A在发热大鼠体内有多峰现象,而在正常大鼠体内没有; 黄芩苷、黄芩素、汉黄芩素、千层纸素A、甘草酸和甘草次酸在发热大鼠体内的达峰时间均晚于正常大鼠2 h以上; 芍药苷、黄芩苷、黄芩素、千层纸素A、甘草酸和甘草次酸在发热大鼠体内的达峰浓度均大于正常大鼠,其中甘草次酸的浓度高2倍,最为明显。8个成分的药时曲线下面积 (AUC) 和体内滞留时间 (MRT) 在发热大鼠体内均大于正常大鼠,其中甘草酸和甘草次酸的AUC均大于3倍。与正常大鼠相比,8个成分的清除率 (CL/F) 在发热大鼠体内均有所降低。
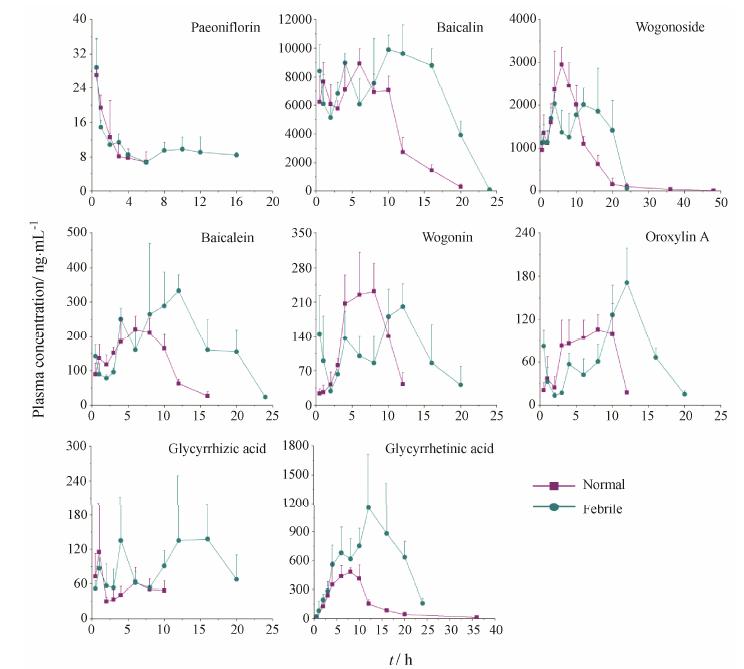 |
Figure 2 Mean plasma concentration-time profiles of eight constituents after oral administration of Huangqin Tang (HQT) at doses 20 g∙kg-1 innormal rats and yeast-induced febrile rats (n = 5) |
|
|
Table 2 Pharmacokinetic parameters of eight constituents of Huangqin Tang (HQT) in normal rats (left) and yeast-induced febrile rats (right) (n = 5). HLλz: Terminal half-life. λz is the first order rate constant associated with the terminal (log-linear) portion of the curve,estimated by linear regression of time vs log concentration; Tmax: Time to Cmax; Cmax: Maximum plasma concentration; AUClast: Area under the curve; Tlast: Time of last measurable mean concentration; Vz/F: Dose/(λz∙AUCinf),where AUCinf is AUC from dosing time extrapolated to infinity,based on the last observed concentration; CL/F: Dose/AUCinf; MRTlast: Residence time from the time of dosing to the time of the last measurable concentration |
将黄酮类成分不同时间点血药浓度用相应成分的给药量标准化后 (Ct/Dose) 对时间 (t) 作图,结果见图 3。黄酮苷和黄酮苷元类成分在体内均具有多个吸收峰值; 它们在体内各时间点的吸收程度不同,黄酮苷 > 黄酮苷元,黄芩苷≥汉黄芩苷,汉黄芩素≥千层纸素A > 黄芩 素; 并且黄芩苷和汉黄芩苷的体内药代动力学参数,消除半衰期 (HLλz) 为1.2和1.7 h,第1次达峰时间同为4.0 h,体内滞留时间 (MRT) 为10.6和11.3 h; 黄芩素和汉黄芩素、千层纸素A的HLλz在2.4~3.5 h之间,达峰时间 (Tmax) 在10.0~12.0 h之间,MRT在9.0~11.3 h之间。黄芩汤中同一类成分在发热大鼠体内具有类似的体内过程,同属于中速吸收、中速消除类成分。
 |
Figure 3 Plasma concentration/dose-time profiles of the flavonoids after oral administration of Huangqin Tang at doses 20 g∙kg-1 inyeast-induced febrile rats. |
将大鼠血浆中各成分药时浓度设为X值,模型给药组与模型组解热效应的体温变化的差值设为Y值,计算浓度和效应之间的Pearson相关系数。为更好拟合各时间点血药浓度和解热效应,各成分血药浓度达峰后低于检测限时间点的血药浓度计为0,如芍药苷12和24 h,汉黄芩素、千层纸素A和甘草酸在 24 h的血药浓度。结果见图 4,血药浓度和解热效应的相关系数,黄芩素为0.71 (P = 0.01),汉黄芩素0.66 (P = 0.02),具有明显的正相关性; 黄芩苷、汉黄芩苷、甘草酸和甘草次酸的血药浓度和解热效应的相关系数在0.25~0.39之间,具有一定的正相关,但不具有统计学意义; 芍药苷的相关系数为 -0.04 (P = 0.91),与解热效应基本不相关。
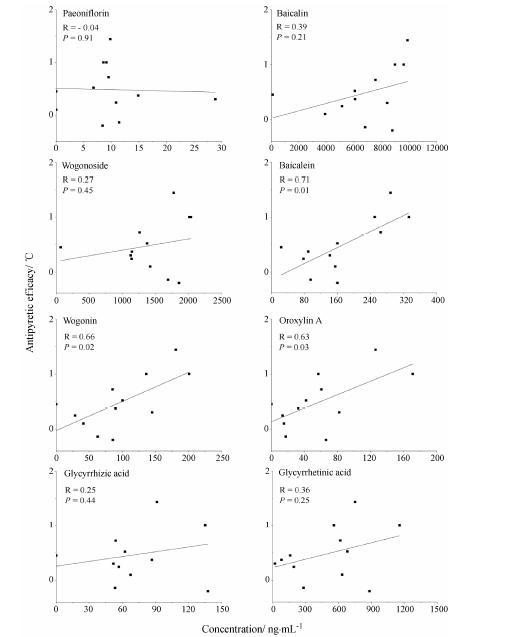 |
Figure 4 The Pearson correlation coefficient (R) and significance (P) between the plasma concentrations of eight constituents and the antifebrile efficacyin yeast-induced febrile rats |
中药药代动力学在我国已开展多年,发展迅速,有学者提出“中药证治药动学”假说,极具中医药特色[7]。黄芩汤为历代清热止利、和中止痛经典方剂,善清泄里热以止下痢。已表明动物皮下注射酵母菌可以引起发热,其全身表现与临床常见炎症的里热证类似[6]。并且黄芩汤中黄芩苷、汉黄芩苷和汉黄芩素等多个成分在正常大鼠体内有合适的药代动力学特征,具有一定的类药属性[4]。因此,本文在酵母菌致热模型的基础上,以多成分代表黄芩汤,研究了发热
大鼠给予黄芩汤后不同时间直肠温度的变化,黄芩汤多个成分血药浓度的变化,并且还同步研究了血液中炎性介质含量的变化,以期揭示黄芩汤在体内的药效动力学和药代动力学特征。
药效学结果显示,黄芩汤可以降低酵母菌致热大鼠的体温,对正常大鼠则无影响,其解热机制可能与非甾体抗炎药类似。已知可溶性介质PGE2、1L-1β与TNF-α在机体炎症和发热过程中有关键作用[8,9]。本实验血液中炎性介质的变化结果显示,与模型组相比,给药后血液中促炎性介质如PGE2和TNF-α的含量能显著降低,黄芩汤的解热作用可能和抑制促炎性介质的释放有关。所以本实验黄芩汤药代动力学特征研究是基于发热模型和单次给药为阳性结果的前提下进行的,其药代动力学结果更具有实际意义。
灌胃黄芩汤后结果显示,与正常大鼠相比,发热大鼠的药代动力学行为有明显差异。发热状态下,大多成分的吸收速度减缓,但吸收程度增加,体内的清除率降低,体内滞留时间延长,最终导致Cmax和AUC增大。甘草酸和甘草次酸AUC的增加最为显著 (均大于3倍)。病理状态影响黄芩苷、甘草酸等单体成分的药代动力学有很多报道[10,11,12,13],研究表明黄芩苷、黄芩素、汉黄芩素、甘草酸等具有抑制促炎性介质释放的作用[14,15,16,17,18],这些成分体内药代动力学行为的改变可能更有利于黄芩汤抗炎解热作用的发挥,因此它们有可能是黄芩汤发挥药效的关键成分。至于发热状态为什么能影响药代动力学行为的改变,这可能和发热状态下机体肝代谢、肾排泄或胃肠转运状态的改变等多种机制有关[10,19,20,21],需要进一步探讨。
在发热状态下,大部分时间点的吸收程度黄芩苷≥汉黄芩苷,这与正常大鼠[4]体内汉黄芩苷 > 黄芩苷的情况相反,说明机体对黄芩苷和汉黄芩苷的吸收或处置方式有所不同,而发热能影响这一过程。与正常状态下相比,发热状态下黄芩苷和汉黄芩苷在体内的清除半衰期和清除率下降均相似; 对比药时曲线的吸收相,在发热状态下黄芩苷和汉黄芩苷的吸收均增加,如黄芩苷在0.5 h处血液浓度是正常状态1.35倍,汉黄芩苷约为1.18倍; 并且药时曲线显示,发热状态下黄芩苷和汉黄芩苷均具有双吸收峰,黄芩苷在10 h处峰值较大,而汉黄芩苷12 h时的峰值较小。因此推断,虽然黄芩苷和汉黄芩苷结构相似,但它们在体内的吸收、转运过程不同。文献[10,22,23]表明,黄芩苷和汉黄芩苷的吸收和处置受P-糖蛋白、多药耐药蛋白及苷元转化的影响,两者间的差异有待进一步研究。
PK-PD相关性研究显示,黄芩素和汉黄芩素的血药浓度和黄芩汤解热效应具有明显的正相关,芍药苷和解热效应之间基本不相关。有报道黄芩素和汉黄芩素均具有较强抗炎解热作用[24,25,26],从解热方面看,黄芩素和汉黄芩素可能为黄芩汤起效的关键成分。黄芩汤成分众多,且黄酮类成分在体内外能相互转化,势必出现作用机制的多样性,所以不能排除黄芩汤中其他成分在解热作用上的贡献。起效的关键 成分可能是中药的有效成分,但更可能是中药中多个成分相互作用在体内的具体表现形式。针对同一病症,单味中药或复方起效关键成分可能只有1种,也可能有多种; 针对不同病症,同一中药或复方的起效关键成分类别也可能不同。本实验暂不考虑作用机制,直接采用Pearson相关系数简单探讨PK-PD相 关,对初步揭示黄芩汤起效关键成分和药代动力学模型的拟合,进而指导临床具有一定意义。
以多成分药代动力学研究中药药代动力学是一个摸索的过程,可能需要以下几个过程: ① 中药给药后体内具有类药属性成分的筛选; ② 机体特定病理状态下中药起效关键成分的鉴别; ③ 体内起效关键成分药代动力学模型的拟合等。目前,关于中药特别是中药复方药代动力学文献的涌现,为体内具类药属性成分的筛选积累了大量数据。然而起效关键 成分筛选较难,针对不同病理状态,起效成分可能不同,并且要求病理模型要符合中医临床,还要求药效指标具有客观性、稳定性和灵敏性等特点。总之,本实验研究黄芩汤的解热药效学和药代动力学,初步探讨了药代和药效的相关性,这为黄芩汤起效关键成分筛选和后续进一步研究奠定了基础。
| [1] | Huang L, Ye W, Cai B, et al. A preliminary study on the pharmacology of the compound prescription Huangqin Tang and its component drugs [J]. China J Chin Mater Med (中国中药杂志), 1990, 15: 51-53. |
| [2] | Lam W, Bussom S, Guan F, et al. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity [J]. Sci Trans Med, 2010, 45: 45-59. |
| [3] | Zuo F, Zhou Z, Zhang Q, et al. Pharmacokinetic study on the multi-constituents of Huangqin Tang decoction in rats [J]. Biol Pharm Bull, 2003, 26: 911-919. |
| [4] | Li T, Wang YW, Wang YL, et al. LC-MS quantification and pharmacokinetics of the multi-constituents of Huangqin Tang in rat plasma after different single oral doses [J]. Acta Pharm Sin (药学学报), 2013, 48: 917-924. |
| [5] | Li T. Study on the Chemical Basis and Pharmacokinetics of Huangqin Tang (黄芩汤物质基础与药代动力学特征研究) [D]. Beijing: China Academy of Chinese Medical Sciences, 2013. |
| [6] | Chen Q. Reaearch Methods in Pharmacology of Chinese Materia Medica (中药药理研究方法学) [M]. Beijing: People's Medical Publishing House, 1993: 295-302. |
| [7] | Chen KJ. March of Integration of TCM and WM Towards 21st Century (迈向21世纪的中西医结合) [M]. Beijing: China Medical Science Press, 1991: 208. |
| [8] | Dinarello CA. Proinflammatory cytokines [J]. Chest J, 2000, 118: 503-508. |
| [9] | Li CH, Huo HR, Jiang TL. Progress in studying the mechanism of fever using knockout mice [J]. Chin J Pathophysiol (中国病理生理杂志), 2002, 18: 1151-1156. |
| [10] | Liu L, Deng YX, Liang Y, et al. Increased oral AUC of baicalin in streptozotocin-induced diabetic rats due to the increased activity of intestinal β-glucuronidase [J]. Planta Med, 2010, 76: 70-75. |
| [11] | Ma SW, Zhao M, Liu HX, et al. Pharmacokinetic effects of baicalin on cerebral ischemia-reperfusion after iv administration in rats [J]. Chin Herb Med, 2012, 4: 53-57. |
| [12] | Li XZ, Chen X, Yang YJ, et al. Pharmacokinetic study of baicalin in rabbits with inflammatory brain edema [J]. Chin Pharm J (中国药学杂志), 1999, 34: 107-109. |
| [13] | Yamamura Y, Tanaka N, Santa T, et al. The relationship between pharmacokinetic behaviour of glycyrrhizin and he-patic function in patients with acute hepatitis and liver cirrhosis [J]. Biopharm Drug Dispos, 1995, 16: 13-21. |
| [14] | Li Q, Ge X. Effect of baicalin on antipyresis and influence on cytokine [J]. China J Chin Mater Med, 2010, 35: 1068-1072. |
| [15] | Hsieh C, Hall K, Ha T, et al. Baicalein inhibits IL-1β and TNF-α-induced inflammatory cytokine production from human mast cells via regulation of the NF-κB pathway [J]. Clin Mol Allergy, 2007, 5: 5. |
| [16] | Piao HZ, Jin SA, Chun HS, et al. Neuroprotective effect of wogonin: potential roles of inflammatory cytokines [J]. Arch Pharm Res, 2004, 27: 930-936. |
| [17] | Wakabayashi I, Yasui K. Wogonin inhibits inducible prostaglandin E2 production in macrophages [J]. Eur J Pharmacol, 2000, 406: 477-481. |
| [18] | Gong G, Yuan LB, Hu L, et al. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1 [J]. Acta Pharmacol Sin, 2012, 33: 11-18. |
| [19] | Zuo F, Zhou ZM, Yan MZ, et al. The metabolism of constituents in Huangqin-Tang, a traditional Chinese preparation in conventional, germ-free and gnotobiote mice [J]. Asian J Drug Metab Pharm, 2001, 3: 181. |
| [20] | Makino T, Ohtake N, Watanabe A, et al. Down-regulation of a hepatic transporter multidrug resistance-associated protein 2 is involved in alteration of pharmacokinetics of glycyrrhizin and its metabolites in a rat model of chronic liver injury [J]. Drug Metab Dispos, 2008, 36: 1438-1443. |
| [21] | Wang Z, Okamoto M, Kurosaki Y, et al. Pharmacokinetics of glycyrrhizin in rats with D-galactosamine-induced hepatic disease [J]. Biol Pharm Bull, 1996, 19: 901-904. |
| [22] | Zhang L, Lin G, Kovács B, et al. Mechanistic study on the intestinal absorption and disposition of baicalein [J]. Eur J Pharm Sci, 2007, 31: 221-231. |
| [23] | Xia B, Zhou Q, Zheng Z, et al. A novel local recycling mechanism that enhances enteric bioavailability of flavonoids and prolongs their residence time in the gut [J]. Mol Pharmacol, 2012, 9: 3246-3258. |
| [24] | Chi Y, Lim H, Park H, et al. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: regulation of inflammation-associated gene expression [J]. Biochem Pharmacol, 2003, 66:1271-1278. |
| [25] | Wakabayashi I. Inhibitory effects of baicalein and wogonin on lipopolysaccharide-induced nitric oxide production in macrophages [J]. Pharmacol Toxicol, 1999, 84: 288-291. |
| [26] | Chen YC, Shen SC, Chen LG, et al. Wogonin, baicalin, and baicalein inhibition of inducible nitric oxide synthase and cyclooxygenase-2 gene expressions induced by nitric oxide synthase inhibitors and lipopolysaccharide [J]. Biochem Pharmacol, 2001, 61: 1417-1427. |
 2014, Vol. 49
2014, Vol. 49


