逆流色谱(countercurrent chromatography)是一种连续高效的液-液分配色谱技术,无需任何固态支撑体,其固定相和流动相由互不相溶的两相溶剂体系组成。在分离过程中,固定相通过离心力等作用力保留在分离柱中,流动相以一定的速度通过固定相并与之混合,样品进入体系后在两相中反复进行分配,不同组分依据其在两相中分配系数的不同先后被洗脱出来,从而达到分离的效果。自20世纪60年代逆流色谱发明以来,出现了液滴逆流色谱[1]、高速逆流色谱[2]、双向逆流色谱[3]、正交轴逆流色谱[4]等逆流色谱设备,在洗脱模式上也出现了梯度洗脱模式、双向洗脱模式、多重双向洗脱模式、闭路循环洗脱模式、洗脱-推挤模式、洗脱-反推模式和pH区带精制模式等多种逆流色谱洗脱模式[5]。随着逆流色谱技术的快速发展,目前已广泛应用于天然产物的分离纯化,包括黄酮、多酚、生物碱和萜类化合物等通过逆流色谱得到了分离制备[6-9]。在上述成分分离中,异构体分子的分离由于其性质相近,因此分离难度相对较大,近年来逆流色谱在异构体分子的分离中已经取得了良好的效果。
同分异构现象在有机化学中极为普遍,异构体可分为两大类,即构造异构体和立体异构体。分子式相同,但分子中原子相互连接的次序不同而产生的异构体,称为构造异构体,构造异构体又可以分为碳链异构、官能团位置异构、官能团异构、互变异构;分子的构造式相同,但分子中的原子或原子团在空间的排列或取向不同而引起的异构体,称为立体异构体,立体异构又可分为顺反异构、手性异构、差向异构、构象异构[10]。由于异构体在理化性质上的相似性,通常很难将其有效地分离,因此,研究同分异构体的分离方法就显得十分重要。目前,分离异构体的方法有很多,比如结晶分离法[11]、化学分离法[12]、动力学分离法[13]、膜法[14]等,色谱类有气相色谱法、液相色谱法、超临界流体色谱法、毛细管电泳法等[15-18]。传统色谱方法分离异构体主要以分析为目的,而逆流色谱技术易实现制备性分离,因此在异构体分子分离中具有一定的优势,目前已经分离成功的主要有构造异构中的官能团异构和官能团位置异构,以及立体异构中的顺反异构、手性异构、差向异构。本文以异构体分子类型为分类方法,对近年来逆流色谱在异构体分子分离领域的研究进展进行总结与归纳。
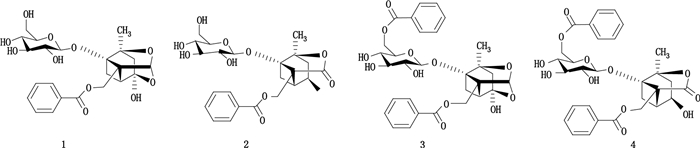
1 构造异构体 1.1 官能团异构因官能团不同而引起的异构现象,称为官能团异构。笔者课题组通过大孔树脂-洗脱-推挤逆流色谱法,开发了一种快速分离白芍2对异构单萜分子的高效分离方法[19]。首先以D101大孔树脂柱富集样品1与2,以正丁醇-乙酸乙酯-水(2:3:5,v/v)为溶剂体系,从200 mg样品1中分离出1对异构体,96 mg芍药内酯苷和48 mg芍药苷纯度为91.1%和96.2%;以正己烷-乙酸乙酯-甲醇-水(0.5:5:1:5,v/v)为溶剂体系,洗脱-推挤逆流色谱和普通逆流色谱从48 mg样品2中分离出另1对异构体14 mg苯甲酰芍药苷和8 mg 6’-O-苯甲酰芍药内酯苷,纯度分别为93.6%和88.9%。洗脱-推挤逆流色谱的分离时间和溶剂消耗明显减少,而分离度仍然良好。2组官能团异构体的结构式见图 1。
|
图 1 官能团异构体芍药苷(1)、芍药内酯苷(2)、苯甲酰芍药苷(3)和6-O-苯甲酰芍药内酯苷(4)的结构 Figure 1 Structures of four functional group isomers paeoniflorin(1), albiflorin(2), benzoylpaeoniflorin(3), and 6'-O-benzoylalbiflorin(4) |
因原子或官能团的位置不同而引起的异构现象,称为官能团位置异构。官能团位置异构在天然产物中大量存在,目前使用逆流色谱已经分离了大量官能团位置异构体,相关文献报道数量最多。譬如,Weisz等[20]报道了3-磺基邻苯二甲酸和4-磺基邻苯二甲酸的分离,分别使用常规的逆流色谱和pH区带精制逆流色谱分离,其中pH区带精制逆流色谱成功实现了克级样品的分离,结构式如图 2。表 1列出了近年来逆流色谱分离官能团位置异构体的文献。
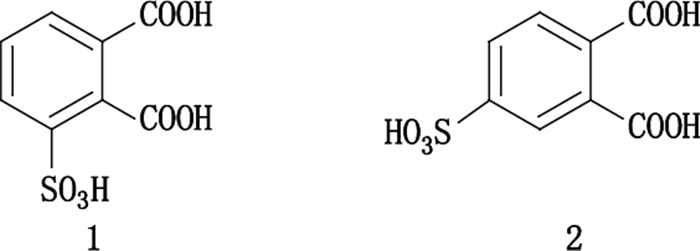
|
图 2 官能团位置异构体3-磺基邻苯二甲酸(1)和4-磺基邻苯二甲酸(2)的结构 Figure 2 Structures of the positional isomers 3-sulfophthalic acid(1)and 4-sulfophthalic acid(2) |
|
|
表 1 逆流色谱分离官能团位置异构体应用 Table 1 Application of countercurrent chromatography in the separation of functional group positional isomers |
在有双键或小环结构(如环丙烷)的分子中,由于分子中双键或环中原子间键的自由旋转受阻碍,存在不同的空间排列方式而产生的立体异构现象,又称为顺反异构。
He等[32]选择由正己烷-乙酸乙酯-甲醇-水(1:2:1:2,v/v/v/v)两相溶剂系统,采用逆流色谱法从爬山虎根中分离多酚顺反异构体。从粗样品中一步分离获得2个顺反异构的白藜芦醇二聚体。500 mg粗样品溶于10 mL上相和10 mL下相,分离得到23.8 mg的Quadrangularin A和25.6 mg的Parthenocissin A,纯度分别为95.4%和97.6%。
Weisz等[33]使用pH区带精制逆流色谱法对一些二羧酸异构体进行了制备性分离研究,包括1-甲基-1,3-环己烷二羧酸和1,3-二甲基-1,3-环己烷二羧酸的顺式和反式混合物。溶剂体系由甲基叔丁基醚-乙腈-水(4:1:5)组成,以三氟乙酸为保留酸,氨水为洗脱碱,从1.3 g混合物分离得到90 mg顺式1-甲基-1,3-环己烷二羧酸(1),128 mg反式1-甲基-1,3-环己烷二羧酸(2),220 mg反式1,3-二甲基-1,3-环己烷二羧酸(3)和470 mg顺式1,3-二甲基-1,3-环己烷二羧酸(4)。并且提出洗脱顺序可能是由空间构型对疏水性和酸性的影响决定的。图 3为顺反异构体化合物1、2、3、4的结构。

|
图 3 顺反异构体化合物1、2、3、4的结构 Figure 3 Structures of the cis-trans-isomers compound 1, 2, 3, 4 |
He等[34]通过逆流色谱从全反式视黄醛异构化反应溶液中分离视黄醛异构体。每次进样200 mg全反式视黄醛异构化反应液,使用正己烷-乙腈(3:1,v/v)为溶剂体系,从反式视黄醛中分离得到63 mg 11-顺-视黄醛,24 mg 13-顺-视黄醛和26 mg 9-顺-视黄醛,纯度均大于95%。该溶剂体系具有简单,可重复使用和多次进样的优点,可用于制备13-顺式-视黄醛,11-顺式-视黄醛和9-顺式-视黄醛以研究光激发下分子在同质异构间发生的结构变化。Li等[35]首次成功应用高速逆流色谱从红花中分离出4个香豆酰亚精胺类似物的顺反异构体。两相溶剂体系为三氯甲烷-甲醇-水(1:1:1,v/v)。单次分离100 mg粗制样品获得1.3 mg的N1,N5,N10-(E)-tri-p-coumaroylspermidine(EEE),4.4 mg的N1(E)-N5-(Z)-N10-(E)-tri-p-coumaroylspermidine(EZE),7.2 mg N1(Z)-N5-(Z)-N10-(E)-tri-p-coumaroylspermidine(ZZE)和11. 5 mg N1,N5,N10-(Z)-tri-p-coumaroylspermidine(ZZZ)。4种组分的纯度分别为95.5%、98.1%、97.5%和96.2%。Han等[21]采用pH区带精制逆流色谱法和制备型高效液相色谱法,从椿叶花椒树皮正丁醇粗提取物中分离得到G-四联体配体。通过传统的逆流色谱和制备型高效液相色谱,以溶剂体系甲基叔丁基醚-正丁醇-乙腈-水(1:5:1:5,v/v)分离得到1个丁香苷和木兰花碱及2个木质素顺反异构体(+)-南烛木树脂酚-3α-O-β-D-葡萄糖苷和(-)-南烛木树脂酚-3α-O-β-D-葡萄糖苷。
2.2 手性异构体手性拆分是色谱研究领域的热点和前沿,目前主要以高效毛细管电泳、毛细管气相色谱、高效液相色谱为主。常规色谱方法分辨率高,但其分离量常在微克级,只能作为分析测试手段。制备液相色谱可以满足一般性制备拆分的需要,但制备液相色谱柱的适应范围有限,且成本很高。逆流色谱相对于高效液相色谱来说,不需要固相载体,可避免固相吸附带来的污染和样品损失等缺点,且一次分离进样量大,仪器简单易维修,运行成本低,对于手性分离来说是一种理想的制备色谱技术[36]。对于建立在单向性流体动力平衡体系的逆流色谱手性分离,一般是把手性选择剂添加到固定相中,通过对映体与手性试剂在互不相溶的两相溶剂系统中具有不同的分配系数将其分离开来[37]。由于逆流色谱具有制备性拆分的特点,进行逆流色谱的手性分离研究具有积极的意义。逆流色谱手性分离通常需要满足以下几个要求[38]:①手性选择剂在溶剂系统中的溶解度要大且只能溶于两相体系中的一相;②手性试剂在溶解相中应有良好的溶解度且能保持良好的手性识别能力,通常分离因子应大于1.4;③手性选择剂要廉价;④产品需要进一步纯化。因此,手性试剂与溶剂体系的选择是逆流色谱手性分离是否能成功的关键。
随着高效手性添加剂的发现和逆流色谱仪器的进一步更新完善,近年来逆流色谱在手性分离方面已经得到了成功的应用。现有逆流色谱手性分离中应用成功的手性选择剂主要有酒石酸类[39-44]、L-脯氨酸衍生物[45-51]、环糊精类[52-61]、大环抗生素类[62]、纤维素与直链淀粉衍生物类[63]、金鸡纳生物碱类[64]、萘普生类[66]和冠醚类[67]等。表 2列举了近年来一些经典的手性拆分实例。
|
|
表 2 逆流色谱手性拆分实例 Table 2 Application of chiral separation by countercurrent chromatography |
分子中具有多个手性碳原子,所有手性碳原子中的手性构型只有1个碳原子不同,其他都相同,这种异构体称之为差向异构体。芦荟素是芦荟叶中主要的蒽醌类成分,通常以芦荟素A和B 2种异构体形式并存,目前已有数篇逆流色谱分离芦荟素A和B差向异构体的报道[68-70]。黄丹凤等[69]采用高速逆流色谱和硅胶柱色谱结合的方法,使用多个溶剂体系包括三氯甲烷-甲醇-水(4:3:2)、乙酸乙酯-甲醇-水(5:1:5)和正丁醇-乙酸乙酯-水(1:3:4),从老芦荟干粉中分离制备了高纯度的芦荟素A(纯度为98%)和芦荟素B(纯度为96%)样品。
Han等[71]使用高速逆流色谱与柱切换阀相结合,建立了一个循环逆流色谱系统,并用该系统首次成功应用于差向异构体的分离制备,以正己烷-甲醇-水(5:4:1,v/v)作为两相溶剂系统,从50 mg植物藤黄提取物中分离制备了28.2 mg藤黄酸和18.4 mg的C-2差向异构体。
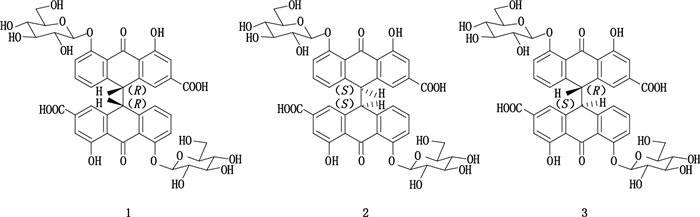
番泻苷A、A1和B是番泻叶主要生物活性成分,是C-10和C-10’位上的(R*R*)、(S*S*)和(R*S*)构象。由于它们结构上的相似之处难以分离。Park等[72]以正丁醇-异丙醇-水(5:1:6,v/v)为逆流溶剂体系,加入不同浓度的甲酸,成功地从番泻叶提取物分离纯化番泻苷A、A1、B。图 4为差向异构体番泻苷A、A1和B的结构。
|
图 4 差向异构体番泻苷A(1)、A1(2)和B(3)的结构 Figure 4 Structures of the epimers sennoside A(1), A1(2)and B(3) |
笔者课题组建立了以羟丙基-β-环糊精为手性拆分剂的逆流色谱法[73],分离了10个芳香酸薄荷醇酯差向异构体。由正己烷-20%~70%甲醇(1:1,v/v)和50 mmol·L-1羟丙基-β-环糊精溶液组成的两相溶剂体系为其中5个薄荷醇酯差向异构体的分离提供了较高的分离因子,成功实现了逆流色谱分离。研究结果表明,芳香酸薄荷醇酯差向异构体的逆流色谱分离必须采用手性拆分剂羟丙基-β-环糊精,否则难以完全分离。而对于液相色谱来说,扁桃酸(-)-薄荷醇酯和对溴扁桃酸(-)-薄荷醇酯差向异构体的分离可以不使用羟丙基-β-环糊精,但其他间氯扁桃酸(-)-薄荷醇酯,3-甲氧基-4-羟基扁桃酸(-)-薄荷醇酯和2-(4-羟基苯基)丙酸(-)-薄荷醇酯差向异构体的分离必须有手性试剂羟丙基-β-环糊精。
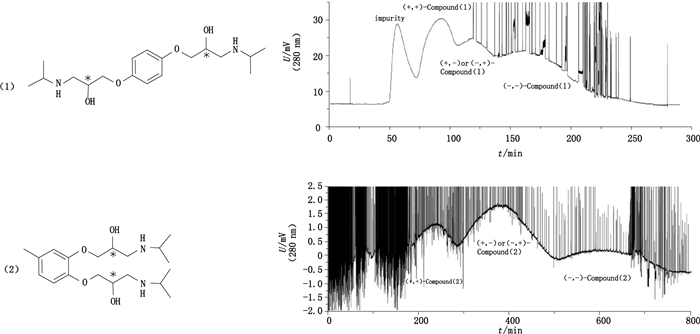
2.4 含2个手性碳原子立体异构体笔者课题组基于酒石酸-硼酸配位在逆流色谱上首次拆分了2个实验室自制的双手性氨基醇类化合物,这是双手性化合物首次在逆流色谱上成功分离对映体[43]。逆流色谱条件为柱温10 ℃,固定相为含0.1 mol·L-1 L-酒石酸正己酯的三氯甲烷溶液,流速2 mL·min-1,进样量为40 mg,保留率60%。化合物(1)由于其对称结构,只有3个异构体;经液相色谱检测,3个异构体纯度约为98%。化合物(2)有4个异构体,在逆流色谱上分离3个异构体,(+,-)和(-,+)构型在同一逆流峰中(图 5)。
|
图 5 双手性中心化合物(1)与(2)结构及其逆流色谱分离色谱图 Figure 5 The structure and chromatogram of the two chiral centers compound (1)and (2) |
逆流色谱技术由于其独特的特点,在异构体分子制备分离中发挥了一定的优势。溶剂体系的筛选依然是异构体分子分离中最为关键的一部分,除了需要满足分层时间短及目标分子分配系数在0.2~5.0之间这些基本要求之外,在手性异构体的分离中还存在手性试剂的筛选问题。然而,在分离异构体分子的溶剂体系筛选中尚未有系统、成熟的理论来指导,选择溶剂体系的方法还是以预测分离物质的极性为主,然后粗选1个溶剂体系,通常都需要多次实验才能筛选出较佳的溶剂系统。以本文中所引用文献为例,强极性体系,如正丁醇-水体系,主要用于分离羧酸、糖苷类异构体;乙酸乙酯-水体系主要用于分离萜类生物碱和含氮化合物,加入酸(盐酸、醋酸等)更有利于生物碱类的分离。中极性体系,如甲基叔丁基醚-水体系主要用于分离羧酸类、含磺酸基或苷类异构体;三氯甲烷-水体系主要用于分离多羟基酯类、醇类,生物碱和β-肾上腺素阻断剂;正己烷-乙酸乙酯-甲醇-水体系应用最为广泛,用于多酚、苯丙素类、苷类、芳香酸类等异构体分子分离。弱极性体系包括极弱极性体系(无水体系)可分离极性非常小的物质,如正己烷-甲醇体系。
逆流色谱作为一种液-液分配色谱,其理论塔板数难以达到高效液相色谱、毛细管电泳等技术的级别,因此分离效率相对较低。为了提高异构体分子间的色谱分离度,可采用逆流色谱多样化洗脱模式弥补其分离效率低的缺陷,譬如闭路循环、多重双向洗脱模式可适当提高异构体分子之间的色谱分离度。另外,可以选择性联用其他技术来分离复杂的异构体,比如联用制备性高效液相色谱在分离异构体的应用中表现出良好的效果。随着新的联用技术逐步开发,在应用中发挥各自优势,达到互补效果,有望实现对复杂异构体的分离。
| [1] |
TANIMURA T, PISANO JJ, ITO Y, et al. Droplet countercurrent chromatography[J]. Science, 1970, 169(3940): 54. DOI:10.1126/science.169.3940.54 |
| [2] |
ITO Y, SANDLIN J, BOWERS WG. High-speed preparative counter-current chromatography with a coil planet centrifuge[J]. J Chromatogr A, 1982, 244(2): 247. DOI:10.1016/S0021-9673(00)85688-5 |
| [3] |
ITO Y. Foam countercurrent chromatography based on dual countercurrent systems[J]. J Liq Chromatogr, 1985, 8(12): 2131. |
| [4] |
ITO Y, KITAZUME E, BHATNAGAR M, et al. Cross-axis synchronous flow-through coil planet centrifuge (type XLL):Ⅰ.Design of the apparatus and studies on retention of stationary phase[J]. J Chromatogr A, 1991, 538(1): 59. |
| [5] |
张虎, 沈芒芒, 颜继忠, 等. 逆流色谱中多种洗脱模式的应用研究进展[J]. 中国现代应用药学, 2014, 31(5): 628. ZHANG H, SHEN MM, YAN JZ, et al. Progress of different elution modes of counter-current chromatography[J]. Chin J Mod Appl Pharm, 2014, 31(5): 628. |
| [6] |
邸多隆, 郑媛媛, 陈小芬, 等. 高速逆流色谱技术分离纯化天然产物中黄酮类化合物的研究进展[J]. 分析化学, 2011, 39(2): 269. DI DL, ZHENG YY, CHEN XF, et al. Advance of application of high speed counter-current chromatography in separation and purification of flavonoids[J]. Chin J Anal Chem, 2011, 39(2): 269. |
| [7] |
王尉, 杜宁, 周晓晶, 等. 高速逆流色谱技术在天然产物研究方面的应用[J]. 现代科学仪器, 2010(4): 123. WANG W, DU N, ZHOU XJ, et al. Application of high-speed countercurrent chromatography in the research of natural botanic products[J]. Mod Sci Instrum, 2010(4): 123. |
| [8] |
陈小芬, 黄新异, 郑媛媛, 等. 高速逆流色谱分离纯化天然产物中生物碱类成分的应用进展[J]. 中草药, 2011, 42(5): 1026. CHEN XF, HUANG XY, ZHENG YY, et al. Application of high-speed counter-current chromatography in separation and purification for alkaloids in natural products[J]. Chin Tradit Herb Drugs, 2011, 42(5): 1026. |
| [9] |
蒋志国, 陈文学, 刘四新. 高速逆流色谱分离制备白胡椒中单萜类化合物[J]. 食品与发酵工业, 2011, 37(3): 202. JIANG ZG, CHEN WX, LIU SX. Study on the separation of monoterpenes from Pipernigrum L.by high-speed counter-current chromatography[J]. Food Ferment Ind, 2011, 37(3): 202. |
| [10] |
裴圣洲. 有机化学中的异构现象[J]. 武汉教育学院学报, 1995(6): 61. PEI SZ. Isomerism in organic chemistry[J]. J Wuhan Inst Educ, 1995(6): 61. |
| [11] |
许辉.二氯硝基苯同分异构体结晶分离的基础研究[D].扬州: 扬州大学, 2014 XU H.Fundamental Research on Crystallization Separation of Dichloronitrobenzene Isomers[D].Yangzhou: Yangzhou University, 2014 |
| [12] |
陈阳生, 李明娟. 骨化三醇的分离及结构测定[J]. 中国医疗前沿月刊, 2009, 4(3): 105. CHEN YS, LI MJ. Determination of chemical constituent and configuration of calcitriol[J]. China Healthcare Front, 2009, 4(3): 105. |
| [13] |
龙安禄, 周元敬, 贾广飞, 等. 动力学拆分方法的研究进展[J]. 贵州师范学院学报, 2015, 31(9): 11. LONG AL, ZHOU YJ, JIA GF, et al. Research status of kinetic resolution method[J]. J Guizhou Educ Univ, 2015, 31(9): 11. DOI:10.3969/j.issn.1674-7798.2015.09.003 |
| [14] |
赵磊, 赵平. 膜分离拆分对映异构体研究进展[J]. 河北工业科技, 2009, 26(1): 53. ZHAO L, ZHAO P. Research development of enantiomer separation by membrane process[J]. Hebei J Ind Sci Technol, 2009, 26(1): 53. |
| [15] |
LAINT KE, MEGURO J, ISO S, et al. Separation of geometric isomers of metal β-diketonates by supercritical fluid chromatography[J]. J Sep Sci, 2015, 16(6): 372. |
| [16] |
JAREMKO M, KASAI Y, BARGINEAR MF, et al. Tamoxifen metabolite isomer separation and quantification by liquid chromatography-tandem mass spectrometry[J]. Anal Chem, 2010, 82(24): 10186. DOI:10.1021/ac102337d |
| [17] |
杨沐, 钟文英, 侯雯. 手性药物分析方法研究进展[J]. 药学进展, 2014(3): 209. YANG M, ZHONG WY, HOU W. Advances in research on analytical methods of chiral drugs[J]. Prog Pharm Sci, 2014(3): 209. |
| [18] |
WAN IW, ARSAD SR, MAAROF H, et al. Chiral separation of four stereoisomers of ketoconazole drugs using capillary electrophoresis[J]. Chirality, 2015, 27(3): 223. |
| [19] |
CHU C, ZHANG SD, TONG SQ, et al. Elution-extrusion counter-current chromatography for the separation of two pairs of isomeric monoterpenes from Paeoniae Alba Radix[J]. J Sep Sci, 2015, 38(17): 3110. DOI:10.1002/jssc.v38.17 |
| [20] |
WEISZ A, MAZZOLA EP, MURPHY CM, et al. Preparative separation of isomeric sulfophthalic acids by conventional and pH-zone-refining counter-current chromatography[J]. J Chromatogr A, 2002, 966(1-2): 111. DOI:10.1016/S0021-9673(02)00695-7 |
| [21] |
HAN T, CAO XL, XU J, et al. Separation of the potential G-quadruplex ligands from the butanol extract of Zanthoxylum ailanthoides Sieb.& Zucc.by countercurrent chromatography and preparative high performance liquid chromatography[J]. J Chromatogr A, 2017, 1507: 104. |
| [22] |
WEISZ A, MAZZOLA EP, MATUSIK JE. Preparative separation of isomeric 2-(2-quinolinyl)-1H-indene-1, 3(2H)-dione monosulfonic acids of the color additive D&C Yellow No.10(quinoline yellow)by pH-zone-refining counter-current chromatography[J]. J Chromatogr A, 2001, 923(1-2): 87. DOI:10.1016/S0021-9673(01)00984-0 |
| [23] |
TONG SQ, YAN JZ, GUAN YX. Preparative separation of isomeric caffeoylquinic acids from Flos Lonicerae by pH-zone-refining counter-current chromatography[J]. J Chromatogr A, 2008, 1212(1): 48. |
| [24] |
LIU WN, LUO JG, KONG LY. Application of complexation high-speed counter-current chromatography in the separation of 5-hydroxyisoflavone isomers from Belamcanda chinensis (L.)DC[J]. J Chromatogr A, 2011, 1218(14): 1842. |
| [25] |
TANG QF, LIU J, XUE J, et al. Preparative isolation and purification of two new isomeric diterpenoid alkaloids from Aconitum coreanum by high-speed counter-current chromatography[J]. J Chromatogr B, 2008, 872(1-2): 181. DOI:10.1016/j.jchromb.2008.07.030 |
| [26] |
GUO W, WANG L, GAO Y, et al. Isolation of isochlorogenic acid isomers in flower buds of Lonicera japonica by high-speed counter-current chromatography and preparative high performance liquid chromatography[J]. J Chromatogr B, 2015, 981-982: 27. DOI:10.1016/j.jchromb.2014.12.020 |
| [27] |
CHEN T, LI HM, ZOU DL, et al. Separation of three anthraquinone glycosides including two isomers by preparative high-performance liquid chromatography and high-speed countercurrent chromatography from Rheum tanguticum Maxim.ex Balf[J]. J Sep Sci, 2016, 39(16): 3105. |
| [28] |
LI HM, CHEN T, SUN J, et al. Separation of six xanthones from Swertia franchetiana by high-speed countercurrent chromatography[J]. J Sep Sci, 2017, 40(11): 2515. DOI:10.1002/jssc.201601134 |
| [29] |
LIU D, SU Z, WANG C, et al. Separation of five isomers of dihydroxybenzoic acid by high-speed counter-current chromatography with dual-rotation elution method[J]. J Chromatogr Sci, 2009, 47(5): 345. |
| [30] |
LI P, WANG YL, GUO ZY, et al. Separation of three bioactive isomers from Bidens pilosa by countercurrent chromatography[J]. J Liq Chromatogr Relat Technol, 2014, 37(18): 2598. DOI:10.1080/10826076.2013.853308 |
| [31] |
LIU Y, FRIESEN JB, KLEIN LL, et al. The Generally Useful Estimate of Solvent Systems (GUESS)method enables the rapid purification of methylpyridoxine regioisomers by countercurrent chromatography[J]. J Chromatogr A, 2015, 1426: 248. |
| [32] |
HE S, LU Y, WU B, et al. Isolation and purification of antioxidative isomeric polyphenols from the roots of Parthenocissus laetevirens by counter-current chromatography[J]. J Chromatogr A, 2007, 1151(1-2): 175. |
| [33] |
WEISZ A, IDINA A, BEN-ARI J, et al. Preparative separation of isomeric and stereoisomeric dicarboxylic acids by pH-zone-refining counter-current chromatography[J]. J Chromatogr A, 2007, 1151(1-2): 82. |
| [34] |
HE MF, DU WK, DU QB, et al. Isolation of the retinal isomers from the isomerization of all-trans-retinal by flash countercurrent chromatography[J]. J Chromatogr A, 2013, 1271(1): 67. DOI:10.1016/j.chroma.2012.11.022 |
| [35] |
LI WC, WANG XY, LIN PC, et al. Preparative separation and purification of four cis-trans isomers of coumaroylspermidine analogs from safflower by high-speed counter-current chromatography[J]. J Chromatogr B, 2013, 938(9): 75. |
| [36] |
郑烨, 颜继忠, 童胜强. 逆流色谱技术在手性分离中的研究进展[J]. 药物分析杂志, 2013, 33(4): 536. ZHENG Y, YAN JZ, TONG SQ. Research progress on countercurrent chromatography in enantioseparations[J]. Chin J Pharm Anal, 2013, 33(4): 536. |
| [37] |
吕迎春, 樊竹青. 逆流色谱技术在手性分离方面的应用[J]. 云南化工, 2014(5): 44. LÜ YC, FAN ZQ. Application of countercurrent chromatography on chiral separation[J]. Yunnan Chem Technol, 2014(5): 44. DOI:10.3969/j.issn.1004-275X.2014.05.011 |
| [38] |
袁黎明. 手性逆流色谱的研究进展[J]. 色谱, 2016, 34(1): 44. YUAN LM. Progress of chiral countercurrent chromatograghy[J]. Chin J Chromatogr, 2016, 34(1): 44. |
| [39] |
DOMON B, HOSTETTMANN K, KOVACEVIC K, et al. Separation of the enantiomers of (±)-norephedrine by rotation locular counter-current chromatography[J]. J Chromatogr A, 1982, 250(1): 149. |
| [40] |
CAI Y, YAN Z, ZI M, et al. Preparative enantioseparation of DL-α-Methylbenzylamine by high-Speed countercurrent chromatography using L-(+)-tartaric acid as chiral selector[J]. J Liq Chromatogr Relat Technol, 2007, 30(9-10): 1489. |
| [41] |
LÜ YC, YAN ZH, MA C, et al. Preparative enantioseparation of ofloxacin by high speed countercurrent chromatography using L-(+)-tartaric acid as chiral selector[J]. J Liq Chromatogr Relat Technol, 2010, 33(13): 1328. |
| [42] |
TONG S, ZHENG Y, YAN J, et al. Preparative enantioseparation of β-blocker drugs by counter-current chromatography using dialkyl L-tartrate as chiral selector based on borate coordination complex[J]. J Chromatogr A, 2012, 1263: 74. |
| [43] |
LÜ L, BU Z, LU M, et al. Stereoselective separation of β-adrenergic blocking agents containing two chiral centers by countercurrent chromatography[J]. J Chromatogr A, 2017, 1513: 235. |
| [44] |
SUN G, TANG K, ZHANG P, et al. Separation of phenylsuccinic acid enantiomers using biphasic chiral recognition high-speed countercurrent chromatography[J]. J Sep Sci, 2014, 37(14): 1736. DOI:10.1002/jssc.v37.14 |
| [45] |
OLIVEROS L, FRANCOPUERTOLAS P, MINGUILLON C, et al. Donor-acceptor chiral centrifugal partition chromatography:complete resolution of two pairs of amino-acid derivatives with a chiral Ⅱ donor selector[J]. J Liq Chromatogr, 1994, 17(11): 2301. DOI:10.1080/10826079408013481 |
| [46] |
MA Y, ITO Y. Chiral separation by high-speed countercurrent chromatography[J]. Anal Chem, 1995, 17(1): 65. |
| [47] |
MA Y, ITO Y, BERTHOD A. A chromatographic method for measuring Kf of enantiomer-chiral selector complexes[J]. J Liq Chromatogr Relat Technol, 1999, 22(19): 2945. |
| [48] |
TONG SQ, SHEN MM, CHENG DP, et al. Chiral ligand exchange high-speed countercurrent chromatography:mechanism and application in enantioseparation of aromatic α-hydroxyl acids[J]. J Chromatogr A, 2014, 1360: 110. |
| [49] |
PEREZ AM, MINGUILLON C. Retention of fluorinated chiral selectors in biphasic fluorinated solvent systems and its application to the separation of enantiomers by countercurrent chromatography[J]. J Chromatogr A, 2010, 1217(7): 1094. DOI:10.1016/j.chroma.2009.09.071 |
| [50] |
DELGADO B, PÉREZ E, SANTANO MC, et al. Enantiomer separation by counter-current chromatography.Optimisation and drawbacks in the use of L-proline derivatives as chiral selectors[J]. J Chromatogr A, 2005, 1092(1): 36. DOI:10.1016/j.chroma.2005.03.034 |
| [51] |
TONG SQ, WANG X, SHEN M, et al. Enantioseparation of 3-phenyllactic acid by chiral ligand exchange countercurrent chromatography[J]. J Sep Sci, 2017, 40(8): 1834. |
| [52] |
BREINHOLT J, LEHMANN SV, VARMING AR. Enantiomer separation of 7-des-methyl-ormeloxifene using sulfated beta-cyclodextrin in countercurrent chromatography[J]. Chirality, 2015, 11(10): 768. |
| [53] |
WEI Y, DU S, ITO Y. Enantioseparation of lomefloxacin hydrochloride by high-speed counter-current chromatography using sulfated-β-cyclodextrin as a chiral selector[J]. J Chromatogr B, 2010, 878(28): 2937. |
| [54] |
YUAN LM, LIU JC, YAN ZH, et al. Enantioseparation of chlorpheniramine by high speed countercurrent chromatography using carboxymethyl-β-cyclodextrin as chiral selector[J]. J Liq Chromatogr Relat Technol, 2005, 28(19): 3057. |
| [55] |
AI P, LIU CJ, ZI M, et al. Enantioseparation of aminoglutethimide by high-speed counter current chromatography using carboxymethly-β-cyclodextrin as chiral selector[J]. Chin Chem Lett, 2006, 17(6): 787. |
| [56] |
TONG SQ, YAN JZ, GUAN YX, et al. Separation of α-cyclohexylmandelic acid enantiomers using biphasic chiral recognition high-speed counter-current chromatography[J]. J Chromatogr A, 2010, 1217(18): 3044. DOI:10.1016/j.chroma.2010.02.077 |
| [57] |
TONG SQ, GUAN Y, YAN JZ, et al. Enantiomeric separation of (R, S)-naproxen by recycling high speed counter-current chromatography with hydroxypropyl-β-cyclodextrin as chiral selector[J]. J Chromatogr A, 2011, 1218(32): 5434. DOI:10.1016/j.chroma.2011.06.015 |
| [58] |
TONG SQ, YAN JZ, GUAN XY, et al. Enantioseparation of phenyl-succinic acid by high speed counte-current chromatography using hydroxypropyl-β-cyclodextrin as chiral selector[J]. J Chromatogr A, 2011, 1218(33): 5602. DOI:10.1016/j.chroma.2011.06.023 |
| [59] |
TONG SQ, ZHENG Y, YAN JZ. Application and comparison of high performance liquid chromatography and high speed counter-current chromatography in enantioseparation of (±)-2-phenylpropionic acid[J]. J Chromatogr A, 2013, 1281(6): 79. |
| [60] |
TONG SQ, ZHENG Y, YAN JZ. Enantioseparation of chiral aromatic acids by multiple dual mode counter-current chromatography using hydroxypropyl-β-cyclodextrin as chiral selector[J]. J Sep Sci, 2013, 36(12): 2035. DOI:10.1002/jssc.201300193 |
| [61] |
CHAO H, XU J, WANG X, et al. Enantioseparation of racemic trans-δ-viniferin using high speed counter-current chromatography based on induced circular dichroism technology[J]. J Chromatogr A, 2014, 1324: 164. |
| [62] |
DURET PH, FOUCAULT A, MARGRAFF R. Vancomycin as a chiral selector in centrifugal partition chromatography[J]. J Liq Chromatogr Relat Technol, 2000, 23(2): 295. DOI:10.1081/JLC-100101453 |
| [63] |
PEREZ E, SANTOS MJ, MINGUILLON C. Application of cellulose and amylose arylcarbamates as chiral selectors in counter-current chromatography[J]. J Chromatogr A, 2006, 1107(1): 165. |
| [64] |
FRANCO P, BLANC J, OBERLEITNER WR, et al. Enantiomer separation by countercurrent chromatography using cinchona alkaloid derivatives as chiral selectors[J]. Anal Chem, 2002, 74(16): 4175. DOI:10.1021/ac020209q |
| [65] |
OYA S, SNYDER JK. Chiral resolution of a carboxylic acid using droplet counter-current chromatography[J]. J Chromatogr A, 1986, 370: 333. DOI:10.1016/S0021-9673(00)94704-6 |
| [66] |
RUBIO N, IGNATOVA S, MINGUILLON C, et al. Multiple dual-mode countercurrent chromatography applied to chiral separations using a (S)-naproxen derivative as chiral selector[J]. J Chromatogr A, 2009, 1216(48): 8505. DOI:10.1016/j.chroma.2009.10.006 |
| [67] |
KIM E, KOOB YM, CHUNG DS. Chiral counter-current chromatography of gemifloxacin guided by capillary electrophoresis using (+)-(18-crown-6)-tetracarboxylic acid as a chiral selector[J]. J Chromatogr A, 2004, 1045(1-2): 119. DOI:10.1016/j.chroma.2004.06.006 |
| [68] |
袁黎明, 谌学先, 刘国祥, 等. 高速逆流色谱对芦荟有效成分的制备性分离研究[J]. 分析化学, 2003, 31(2): 251. YUAN LM, CHEN XX, LIU GX, et al. Study on the preparation of effective components of aloe by high speed counter current chromatography[J]. Chin J Anal Chem, 2003, 31(2): 251. DOI:10.3321/j.issn:0253-3820.2003.02.031 |
| [69] |
黄丹凤, 曹学丽, 赵华, 等. 高速逆流色谱与硅胶柱色谱结合分离制备高纯度芦荟素异构体[J]. 色谱, 2006, 24(1): 42. HUANG DF, CAO XL, ZHAO H, et al. Preparative separation of aloin diastereoisomers by high-speed countercurrent chromatography combined with silica gel column chromatography[J]. Chin J Chromatogr, 2006, 24(1): 42. DOI:10.3321/j.issn:1000-8713.2006.01.011 |
| [70] |
张琦, 李燕, 党培育, 等. 应用高速逆流色谱分离纯化芦荟苷[J]. 食品工业科技, 2011(4): 283. ZHANG Q, LI Y, DANG PY, et al. Separation and purification of aloin from aloe by high-speed counter-current chromatography[J]. Sci Technol Food Ind, 2011(4): 283. |
| [71] |
HAN QB, SONG JZ, QIAO C F, et al. Preparative separation of gambogic acid and its C-2 epimer using recycling high-speed counter-current chromatography[J]. J Chromatogr A, 2006, 1127(1-2): 298. DOI:10.1016/j.chroma.2006.07.044 |
| [72] |
PARK SB, KIM YS. Simultaneous separation of three isomeric sennosides from senna leaf (Cassia acutifolia)using counter-current chromatography[J]. J Sep Sci, 2015, 38(20): 3502. DOI:10.1002/jssc.v38.20 |
| [73] |
WANG X, LÜ L, BU Z, et al. Separation of epimeric aromatic acid (-)-menthol esters by countercurrent chromatography using hydroxypropyl-β-cyclodextrin as an additive[J]. J Sep Sci, 2017, 40(9): 2045. |
 2019, Vol. 39
2019, Vol. 39 
