雌性动物初情期是指其初次表现发情特征并发生排卵的时期[1]。研究表明,初情期启动主要由下丘脑-垂体-性腺轴(hypothalamic-pituitary-gonadal axis,HPGA)活化引起[2], HPGA活化引起促性腺激素释放激素(gonadotropin-releasing hormone,GnRH)以脉冲分泌的方式释放到下丘脑-垂体门静脉系统,到达垂体前叶,导致促黄体素(luteinizing hormone, LH)和促卵泡素(follicle-stimulate hormone, FSH)的合成和分泌[3],FSH和LH作用于性腺,促进性腺及第二性征的快速发育与成熟、产生配子和分泌性腺激素,初情期启动[4-5]。由此可见,在动物初情期启动的调控中,GnRH神经元是神经生殖内分泌体系的最终共同通路,GnRH高频率脉冲式分泌的严密调控决定了初情期启动的时间[6]。
初情期启动机制非常复杂,与遗传、神经内分泌、代谢、营养和环境等因素有关,但这些因素中遗传是基础[7-8]。Lomniczi等[9]发现,使用DNA甲基转移酶(DNA methyltransferase,DNMT)抑制剂抑制了雌性大鼠体内相关基因的甲基化,导致初情期启动失败,从而确定了表观遗传沉默是雌性动物初情期神经内分泌调控的一种新机制。近年来,表观遗传机制被认为是调节初情期启动过程的重要协调者。因此,本文总结了DNA甲基化、组蛋白修饰、非编码RNA、染色质重塑、基因组印记、转录因子结合和X染色体失活等表观遗传机制对初情期启动的调控进展(图 1),并展望了未来的研究方向。
1 DNA甲基化与初情期DNA甲基化是DNA甲基转移酶(DNA methyltransferase,DNMT)将一个甲基(-CH3)共价添加到胞嘧啶残基的5′位置,产生5-甲基胞嘧啶(5-methylcytosine, 5mC),这种连接在二核苷酸序列胞嘧啶-鸟嘌呤二核苷酸(CpG)中尤其丰富,CpG序列广泛存在于基因启动子中,其修饰直接关系到基因表达的调控,基因启动子中CpG甲基化导致染色质处于封闭状态进而阻断基因表达[10-13]。
研究发现,体外培养的猕猴GnRH神经元在成熟期间,GnRH mRNA水平增加与其5′启动子区中CpG岛的显著去甲基化一致[14]。在体外培养GnRH神经元第0、14和20天,通过对GnRH进行亚硫酸氢盐测序(bisulfite sequencing PCR, BSP)发现,在0~20天之间其5′端14个CpG位点中有8个位点甲基化水平显著下降,并且GnRH mRNA水平显著增加[15]。Kurian等[16]发现在小鼠发育过程中,其下丘脑视前区(preoptic area,POA)中DNA去甲基化酶Tet2表达量随着小鼠年龄的增长而增加,且成熟的GnRH细胞系(GT1-7)中Tet2表达量高于未成熟的GnRH细胞系(GN11)。这些结果表明,DNA甲基化可以影响GnRH神经元的成熟和GnRH表达,进而影响初情期启动。
另外,有研究报道被认为是动物初情期启动“看门人”[17-18]的Kiss1,其启动子的甲基化水平与初情期有关[19]。如丁赫等[20]报道临近小尾寒羊初情期启动时,其下丘脑Kiss1启动子区域甲基化水平呈下降趋势,但Kiss1转录水平逐渐上升,这提示下丘脑Kiss1甲基化状态与初情期启动密切相关。Yang等[21]通过BSP测序绘制了山羊和大鼠初情期前和初情期下丘脑DNA甲基化谱,发现两物种整体DNA甲基化模式相似,进一步分析差异甲基化基因的结果表明,部分基因在大鼠中上调,在山羊中却下调。这表明不同物种的初情期可能展现出相似的整体甲基化模式,但同一基因在不同物种初情期中可能具有不同作用。总之,DNA甲基化通过影响初情期相关基因的表达来调控初情期启动,但是其调控初情期启动的具体机制需要进一步研究。
2 组蛋白修饰与初情期组蛋白是构成核小体的核心,可以被翻译后修饰(post-translational modifications,PTMs),以多种方式重组染色质[22]。PTMs发生在组蛋白(H2A、H2B、H3和H4)的N端尾部,主要包括乙酰化、甲基化、磷酸化、泛素化和SUMO化等,组蛋白尾部赖氨酸乙酰化和甲基化是两种最常见的PTMs,通常乙酰化与基因转录激活有关,去乙酰化则与基因的转录抑制相关;组蛋白甲基化对基因转录的影响取决于甲基化发生的位置以及甲基化程度,如H3的赖氨酸9和27的甲基化(H3K9me和H3K27me)与转录静止相关,而H3赖氨酸4的三甲基化(H3K4me3)主要存在于活性启动子和增强子区域[23]。
研究表明,成熟GnRH神经元乙酰化H3和H3K4me水平高于未成熟GnRH神经元[24]。Novaira等[25]发现,kisspeptin能够提高GnRH启动子kisspeptin响应元件(KsRE)周围H3acK14、H3acK27和H3K4me3水平,促进GnRH基因转录。初情期启动前,下丘脑弓状核(arcuate nucleus,ARC)中Kiss1的启动子与一系列表观抑制因子(包括EED和CDX7等PcG组分,以及GATAD1和KDM1A等组分)结合[9, 26],而这些抑制因子在初情期启动时却被一些活性组蛋白修饰因子(如组成TrxG的MLL1和MLL3)替代[27]。若干扰动物ARC中这些组蛋白修饰因子则会下调Kiss1基因的转录水平,扰乱初情期启动,进而导致初情期启动后的生殖周期紊乱,影响生殖能力[9, 27]。综上,组蛋白修饰通过影响GnRH神经元成熟、GnRH和Kiss1基因表达来调控初情期启动。抑制去组蛋白甲基化酶KDM6B的表达可降低Kiss1、Nell2和Grm7等控制初情期启动基因的表达[28],增强ARC中的去乙酰化酶SIRT1丰度则延迟雌性大鼠和小鼠的初情期启动[29]。这进一步证明组蛋白修饰参与调控初情期启动,但其调控初情期启动的研究主要集中于组蛋白甲基化和乙酰化,组蛋白的其它翻译后修饰是否参与初情期启动未见报道。
3 非编码RNA与初情期非编码RNA根据长度主要分为长链非编码RNA(long noncoding RNA, lncRNA)和短链非编码RNA(small non-coding RNA,sncRNA)[30]两类。而sncRNA中的miRNA研究最多[31]。近年来,越来越多的证据显示lncRNA和miRNA在初情期启动中扮演着重要角色。
3.1 miRNA关于miRNA参与初情期启动的第一个证据来自独立的全基因组关联研究,发现基因Lin28B位点附近的遗传变异与女性初潮的年龄有关[32-33]。进一步的研究发现Lin28B及其同源物Lin28A的主要功能是抑制miRNA let-7的成熟[34],Lin28A过表达可导致小鼠初情期启动延迟[35]。雌性大鼠从出生至初情期期间,下丘脑中Lin28B mRNA水平显著下降,而let-7家族成员以及miR-132和miR-145的表达显著增加[36],这表明下丘脑中的Lin28/let-7系统参与调控初情期启动。于兰兰[37]对山羊卵巢miRNA进行了测序和比较, 筛选出了可能参与调控初情期启动的miRNA, 发现差异表达miRNA靶基因参与了多个与初情期启动相关的通路。因此miRNA可能在初情期启动中发挥重要作用。
GnRH神经元中缺乏RNAse Ⅲ内切酶Dicer的雌性小鼠[38]和以及Kiss1神经元中缺乏Dicre的雌性大鼠,均表现出促性腺功能减退和不孕症[39],而Dicer是最终生成成熟miRNA所必需的元件,这表明miRNA可影响GnRH和Kiss1神经元来调控生殖功能。最近的研究表明,在雌性大鼠出生后,下丘脑中miR-30b表达量增加,抑制了E3泛素连接酶(makorin ring finger protein 3,MKRN3)表达,进而促进初情期启动[40]。因此,miRNA在动物初情期启动中发挥重要作用,但调控初情期启动的miRNA种类有待进一步挖掘,其具体作用机制仍需探究。
3.2 lncRNAlncRNA在体内参与X染色质失活、基因印记等多种重要生理过程,主要通过转录水平、转录后水平、表观遗传水平、翻译及翻译后水平发挥其调控作用[41]。有研究表明lncRNA参与初情期启动。
Gao等[42]发现,初情期山羊下丘脑存在特异的lncRNA表达谱,提示lncRNA在山羊初情期中可能发挥重要调节作用。在体外培养的小鼠下丘脑GnRH神经元中,发现lncRNA GnRH-E1RNA的表达与GnRH mRNA的高水平表达相关,进一步的研究表明,GnRH-E1RNA具有诱导GnRH启动子活性的功能,可能在GnRH神经元成熟和发育过程中激活或抑制GnRH基因转录[43]。lncRNA RMST第二个内含子中的SNP rs76369685与女性初潮年龄有关,它能够直接与转录调节因子SOX2结合,影响早期GnRH神经元的发育,此外,RMST的缺失可影响MKRN3基因的表达,进而影响GnRH产生[44]。这些结果均表明,lncRNA与初情期启动密切相关,但其具体调控作用和机制需要进一步阐明。
4 染色质重塑与初情期染色质重塑是指染色质重塑酶作用于核小体,将它们从染色质纤维(由DNA分子和蛋白质形成的有效聚合物)中置换和移除的过程,这种染色质纤维结构的局部修饰可以影响转录过程[45]。
色域解旋酶DNA结合蛋白7 (variants of chromodomain helicase DNA binding protein 7,CHD7)是第一个被发现参与人类青春期的染色质重塑蛋白,低形态或显性CHD7等位基因可影响HPGA,CHD7功能失调可影响GnRH神经元的发生[46]。Kim等[47]证明,CHD7 mRNA在迁移和迁移后的GnRH神经元细胞系中表达。最近的一项研究发现,色域解旋酶DNA结合蛋白3(variants of chromodomain helicase DNA binding protein 3,CHD3)的突变导致中枢性性早熟(central precocious puberty,CPP),提示CHD3变异可能影响HPGA功能[48]。DNA甲基化组和基因表达变化的联合分析显示,在垂体-卵巢轴成熟开始时,染色质重塑基因在卵巢中显著高甲基化和上调[49]。总之,这些研究表明染色质重塑与初情期启动息息相关,但迄今为止,有关染色质重塑在初情期启动方面的研究报道较少,具体机制尚不清楚。
5 基因组印记与初情期基因组印记是沉默或印记一个等位基因,而不修改DNA序列,导致常染色体基因从单一父母染色体中表达[50]。研究发现,印记基因在生长和发育中扮演重要角色,如贝克威综合症(beckwith-weidemann,BWS)和罗素银综合征(russell-silver syndrome,RSS)等疾病相关的生长变化均与印记基因有关[51]。MKRN3属于母系印记基因,位于15号染色体上的普拉德-威利综合征(prader-willi syndrome,PWS)区域,主要在下丘脑区域表达,其功能缺失突变与CPP相关,是目前已知CPP最常见的遗传基因[52]。小鼠下丘脑中Mkrn3基因的表达在初情期启动前下降,给新生雌性小鼠双侧脑室内注射表达Mkrn3的重组腺病毒,导致初情期延迟[53]。而在雌性小鼠Mkrn3缺失则导致初情期提前[54]。这表明印记基因Mkrn3调控初情期启动。最近报道,母系印记基因δ样非典型Notch配体1 (delta like non-canonical notch ligand 1,DLK1)的突变也与CPP有关[55]。尽管目前只报道了2个母系印记基因参与初情期启动,但提示其他印记基因也可能与初情期启动有关。
6 转录因子结合与初情期转录因子(transcription factor)也称为反式作用因子,是指能够与真核基因的顺式作用元件发生特异性相互作用,并对基因的转录有激活或抑制作用的DNA结合蛋白[56]。研究表明,与女性初潮年龄相关的32个基因中有15个是转录因子[57]。转录抑制因子中锌指(zinc finger,ZNF)超家族成员在恒河猴的幼年发育过程中可以控制GnRH脉冲的发生,阻止初情期启动提前[58]。雌性大鼠下丘脑中转录因子甲状腺特异性转录因子1(thyroid-specific transcription factor 1,TTF1)mRNA水平在发育过程中逐渐增加,并在初情期前达到峰值,在雌性大鼠ARC和下丘脑前腹侧室旁核(anteroventral periventricular nucleus,AVPV)中下调TTF1可降低Kiss1和GnRH表达,导致初情期延迟和卵巢功能异常[59]。转录因子新生螺旋环螺旋蛋白2(nescint helix loop helix 2,NHLH2)在Kiss1神经元中富集,敲除雌性小鼠Kiss1神经元中NHLH2可扰乱雌性小鼠的发情周期[60]。这些研究证明,转录因子在初情期启动中扮演着重要的角色,但一些关键的转录因子及其靶点有待发现。
7 X染色体失活与初情期X染色体失活是确保雌性和雄性细胞之间X连锁剂量补偿的表观遗传机制,X染色体失活对于雌性胚胎在发育过程中生存至关重要,需要对许多不同因素进行精确的时空调节,以实现显著的整个染色体范围内的基因沉默[61]。
特纳综合征(turner syndrome,TS)是与一条X染色体的完全或部分丢失有关的一种罕见的女性疾病,具有身材矮小、初情期延迟、卵巢发育不良和不孕等症状[62]。卡尔曼氏综合征(kallmann syndrome,KS)的遗传背景是性染色体的不分离,最为常见的是存在额外的X染色体,但X染色体失活也存在于KS患者中,属于KS中的特殊遗传背景,促性腺激素功能减退和嗅觉减退或缺失为该病主要特征,少数患者也表现初情期延迟[63]。这表明X染色体的失活可能也参与初情期启动,但是相关的报道较少,仍需进一步探索。
8 总结与展望综上所述,DNA甲基化、组蛋白修饰、非编码RNA、染色质重塑、基因组印记、转录因子结合和X染色体失活等表观遗传学机制均参与了初情期启动,但其中lncRNA、染色质重塑和X染色体失活具体的作用途径和机制尚未阐明。Kiss1和GnRH是初情期启动中的两个核心物质,在初情期前和初情期,DNA甲基化、组蛋白修饰、miRNA、基因组印记和转录因子均参与Kiss1和GnRH启动子的调控(图 1)。在初情期启动的表观遗传学机制中,DNA甲基化可能发挥主要作用,且可能与其他表观遗传学机制之间存在交互调控,但是未见报道,因此未来重点关注表观遗传学机制之间是否存在交互调控初情期启动。另外,目前有关表观遗传学调控初情期的研究主要聚焦于GnRH及其上游基因的表型方面,关于其调控机制仍不明确,因此,对这些基因的表观遗传学调控机制的深入研究将有助于我们更深入地理解初情期启动的机制。然而GnRH下游基因在初情期启动中也起着重要的作用,对这些基因表观遗传调控的研究可能会揭示出初情期启动的新机制。此外,大多数的研究都集中在基因的启动子上,而对其他作用元件,如增强子和终止子等的研究却鲜有报道。总之,表观遗传学虽然调控初情期启动,但其具体机制仍然未知,需要进一步研究。这些表观遗传分子机制的解析将有助于控制雌性动物初情期启动时间,为初情期调控提供新靶点。
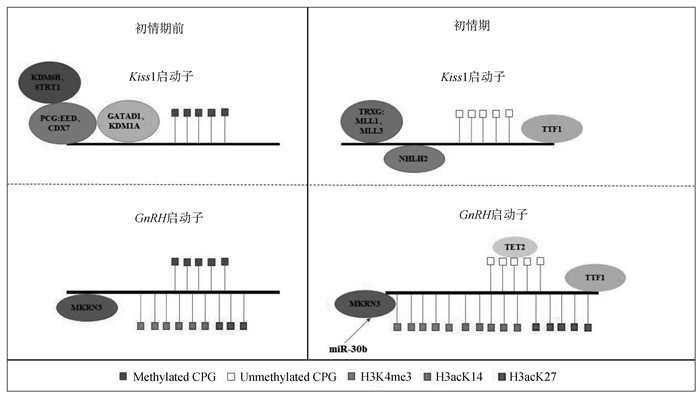
|
图 1描述了Kiss1和GnRH启动子调控的部分表观遗传学机制。从初情期前至初情期,Kiss1启动子结合的抑制性成分PcG成员,如EED和CBX7,和与PcG组分互作的KDM6B和STRT1,以及组蛋白抑制性修饰因子GATAD1、KDM1A,在初情期启动时这些抑制性成分被激活性成分TrxG成员取代,如MLL1和MLL3,此外转录激活因子NHLH2和TTF1与Kiss1启动子结合,同时Kiss1启动子的甲基化水平下降,这些变化共同导致Kiss1的转录增加。对于GnRH启动子,从初情期前至初情期,其甲基化水平在Tet2酶的作用下降低,而激活组蛋白标记的H3Kme3、H3acK14和H3acK27水平升高,miR-30调控与GnRH启动子结合的抑制印记基因MKRN3,GnRH启动子也与转录激活子TTF1结合,这些变化共同导致GnRH的转录增加 The figure 1 describes the regulation of Kiss1 and GnRH promoters by some epigenetic mechanisms. From prepuberty to puberty, inhibitory components bound to the Kiss1 promoter, such as PcG members EED and CBX7, KDM6B and STRT1 that interact with PcG components, and histone inhibitory modification factors GATAD1 and KDM1A, all are replaced by activating components of the TrxG family, such as MLL1 and MLL3, at the onset of puberty. In addition, transcriptional activators NHLH2 and TTF1 bind to the Kiss1 promoter, and the methylation level of the Kiss1 promoter decreases. These changes lead to an increase in Kiss1 transcription together. For the GnRH promoter, from prepuberty to puberty, its methylation level decreases under the action of the Tet2 enzyme, while the levels of activating histone markers H3Kme3, H3acK14, and H3acK27 increase. The inhibitory imprint gene MKRN3, which binds to the GnRH promoter, is regulated by miR-30, and the GnRH promoter also binds to the transcriptional activator TTF1. These changes lead to an increase in GnRH transcription together 图 1 表观遗传学与初情期(改自Manotas等[64]) Fig. 1 Epigenetic and the puberty(change from Manotas et al[64]) |
| [1] |
李孝君, 隋志远, 王晨光, 等. 多浪羊PRLR基因克隆测序及不同组织差异表达分析[J]. 四川农业大学学报, 2023, 41(2): 344-351. LI X J, SUI Z Y, WANG C G, et al. Cloning sequencing of PRLR gene and differential expression analysis of different tissues[J]. Journal of Sichuan Agricultural University, 2023, 41(2): 344-351. (in Chinese) |
| [2] |
ESQUIVEL-ZUNIGA R, ROGOL A D. Functional hypogonadism in adolescence: an overlooked cause of secondary hypogonadism[J]. Endocr Connect, 2023, 12(11): e230190. |
| [3] |
WICKRAMASURIYA N, HAWKINS R, ATWOOD C, et al. The roles of GnRH in the human central nervous system[J]. Horm Behav, 2022, 145: 105230. DOI:10.1016/j.yhbeh.2022.105230 |
| [4] |
VAZQUEZ M J, DAZA-DUEÑAS S, TENA-SEMPERE M. Emerging roles of epigenetics in the control of reproductive function: focus on central neuroendocrine mechanisms[J]. J Endocr Soc, 2021, 5(11): bvab152. DOI:10.1210/jendso/bvab152 |
| [5] |
ARGENTE J, DUNKEL L, KAISER U B, et al. Molecular basis of normal and pathological puberty: from basic mechanisms to clinical implications[J]. Lancet Diabetes Endocrinol, 2023, 11(3): 203-216. DOI:10.1016/S2213-8587(22)00339-4 |
| [6] |
HERBISON A E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons[J]. Nat Rev Endocrinol, 2016, 12(8): 452-466. DOI:10.1038/nrendo.2016.70 |
| [7] |
ABREU A P, MACEDO D B, BRITO V N, et al. A new pathway in the control of the initiation of puberty: the MKRN3 gene[J]. J Mol Endocrinol, 2015, 54(3): R131-R139. DOI:10.1530/JME-14-0315 |
| [8] |
AVENDAÑO M S, VAZQUEZ M J, TENA-SEMPERE M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty[J]. Hum Reprod Update, 2017, 23(6): 737-763. DOI:10.1093/humupd/dmx025 |
| [9] |
LOMNICZI A, LOCHE A, CASTELLANO J M, et al. Epigenetic control of female puberty[J]. Nat Neurosci, 2013, 16(3): 281-289. DOI:10.1038/nn.3319 |
| [10] |
HAMIDI T, SINGH A K, CHEN T P. Genetic alterations of DNA methylation machinery in human diseases[J]. Epigenomics, 2015, 7(2): 247-265. DOI:10.2217/epi.14.80 |
| [11] |
薛倩, 李国辉, 殷建玫, 等. 鸡繁殖性能近交衰退相关CpG岛差异甲基化基因的筛选[J]. 畜牧兽医学报, 2021, 52(4): 943-953. XUE Q, LI G H, YIN J M, et al. Screening of genes with differential methylated CpG island related to inbreeding depression of chicken reproduction[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(4): 943-953. (in Chinese) |
| [12] |
杨小耿, 张慧珠, 李键, 等. DNA甲基化在哺乳动物卵母细胞和早期胚胎发育中的研究进展[J]. 畜牧兽医学报, 2023, 54(2): 443-450. YANG X G, ZHANG H Z, LI J, et al. Research progress of the DNA methylation in mammalian oocyte and early embryo development[J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(2): 443-450. (in Chinese) |
| [13] |
雒瑞瑞, 王彩莲, 郎侠. DNA甲基化在家畜繁殖中的研究进展[J]. 农业生物技术学报, 2023, 31(10): 2190-2199. LUO R R, WANG C L, LANG X. Research progress on DNA methylation in domestic animal reproduction[J]. Journal of Agricultural Biotechnology, 2023, 31(10): 2190-2199. DOI:10.3969/j.issn.1674-7968.2023.10.018 (in Chinese) |
| [14] |
TERASAWA E, KURIAN J R, GUERRIERO K A, et al. Recent discoveries on the control of gonadotrophin-releasing hormone neurones in nonhuman primates[J]. J Neuroendocrinol, 2010, 22(7): 630-638. DOI:10.1111/j.1365-2826.2010.02027.x |
| [15] |
KURIAN J R, KEEN K L, TERASAWA E. Epigenetic changes coincide with in vitro primate GnRH neuronal maturation[J]. Endocrinology, 2010, 151(11): 5359-5368. DOI:10.1210/en.2010-0555 |
| [16] |
KURIAN J R, LOUIS S, KEEN K L, et al. The methylcytosine dioxygenase ten-eleven translocase-2 (tet2) enables elevated GnRH gene expression and maintenance of male reproductive function[J]. Endocrinology, 2016, 157(9): 3588-3603. DOI:10.1210/en.2016-1087 |
| [17] |
SOBRINO V, AVENDAÑO M S, PERDICES-LÓPEZ C, et al. Kisspeptins and the neuroendocrine control of reproduction: recent progress and new frontiers in kisspeptin research[J]. Front Neuroendocrinol, 2022, 65: 100977. DOI:10.1016/j.yfrne.2021.100977 |
| [18] |
SIVALINGAM M, PARHAR I S. Hypothalamic kisspeptin and kisspeptin receptors: species variation in reproduction and reproductive behaviours[J]. Front Neuroendocrinol, 2022, 64: 100951. DOI:10.1016/j.yfrne.2021.100951 |
| [19] |
WYATT A K, ZAVODNA M, VILJOEN J L, et al. Changes in methylation patterns of kiss1 and kiss1r gene promoters across puberty[J]. Genet Epigenet, 2013, 5: 51-62. |
| [20] |
丁赫, 宫永胜, 王军, 等. 初情期启动过程中小尾寒羊下丘脑KiSS-1基因甲基化状态与表达量相互关系[J]. 中国兽医学报, 2018, 38(11): 2201-2204. DING H, GONG Y S, WANG J, et al. Relationship between methylation status and expression of KiSS-1 gene in small tail han sheep during onset of puberty[J]. Chinese Journal of Veterinary Science, 2018, 38(11): 2201-2204. (in Chinese) |
| [21] |
YANG C, YE J, LIU Y, et al. Methylation pattern variation between goats and rats during the onset of puberty[J]. Reprod Domest Anim, 2018, 53(3): 793-800. DOI:10.1111/rda.13172 |
| [22] |
张宸艺博, 余彤, 任斌斌, 等. 动物早期胚胎发育中表观重编程的机制[J]. 畜牧兽医学报, 2023, 54(12): 4898-4909. ZHANG C Y B, YU T, REN B B, et al. Mechanism of epigenetic reprogramming of early animal embryos[J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(12): 4898-4909. (in Chinese) |
| [23] |
TORO C A, AYLWIN C F, LOMNICZI A. Hypothalamic epigenetics driving female puberty[J]. J Neuroendocrinol, 2018, 30(7): e12589. DOI:10.1111/jne.12589 |
| [24] |
IYER A K, BRAYMAN M J, MELLON P L. Dynamic chromatin modifications control GnRH gene expression during neuronal differentiation and protein kinase C signal transduction[J]. Mol Endocrinol, 2011, 25(3): 460-473. DOI:10.1210/me.2010-0403 |
| [25] |
NOVAIRA H J, SONKO M L, RADOVICK S. Kisspeptin induces dynamic chromatin modifications to control GnRH gene expression[J]. Mol Neurobiol, 2016, 53(5): 3315-3325. DOI:10.1007/s12035-015-9269-0 |
| [26] |
LOMNICZI A, WRIGHT H, CASTELLANO J M, et al. Epigenetic regulation of puberty via zinc finger protein-mediated transcriptional repression[J]. Nat Commun, 2015, 6: 10195. DOI:10.1038/ncomms10195 |
| [27] |
TORO C A, WRIGHT H, AYLWIN C F, et al. Trithorax dependent changes in chromatin landscape at enhancer and promoter regions drive female puberty[J]. Nat Commun, 2018, 9(1): 57. DOI:10.1038/s41467-017-02512-1 |
| [28] |
WRIGHT H, AYLWIN C F, TORO C A, et al. Polycomb represses a gene network controlling puberty via modulation of histone demethylase Kdm6b expression[J]. Sci Rep, 2021, 11(1): 1996. DOI:10.1038/s41598-021-81689-4 |
| [29] |
VAZQUEZ M J, TORO C A, CASTELLANO J M, et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression[J]. Nat Commun, 2018, 9(1): 4194. DOI:10.1038/s41467-018-06459-9 |
| [30] |
蔡含芳, 李明勋, 陈宏. 长链非编码RNA及其在家畜中的应用与展望[J]. 中国牛业科学, 2015, 41(6): 65-68. CAI H F, LI M X, CHEN H. The biological function of long non-coding RNA and its application and prospect in domestic animals[J]. China Cattle Science, 2015, 41(6): 65-68. DOI:10.3969/j.issn.1001-9111.2015.06.015 (in Chinese) |
| [31] |
HOMBACH S, KRETZ M. Non-coding RNAs: classification, biology and functioning[M]//SLABY O, CALIN G A. Non-Coding RNAs in Colorectal Cancer. Cham: Springer, 2016: 3-17.
|
| [32] |
PERRY J R B, STOLK L, FRANCESCHINI N, et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche[J]. Nat Genet, 2009, 41(6): 648-650. DOI:10.1038/ng.386 |
| [33] |
HE C Y, KRAFT P, CHEN C, et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause[J]. Nat Genet, 2009, 41(6): 724-728. DOI:10.1038/ng.385 |
| [34] |
邢凤, 高庆华, 祁鑫, 等. 多浪羊Lin28A基因克隆及其在初情期启动过程中的表达研究[J]. 畜牧兽医学报, 2019, 50(1): 78-85. XING F, GAO Q H, QI X, et al. Cloning and expression of Lin28A gene in the onset of puberty in Duolang sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(1): 78-85. (in Chinese) |
| [35] |
ZHU H, SHAH S, SHYH-CHANG N, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies[J]. Nat Genet, 2010, 42(7): 626-630. DOI:10.1038/ng.593 |
| [36] |
SANGIAO-ALVARELLOS S, MANFREDI-LOZANO M, RUIZ-PINO F, et al. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty[J]. Endocrinology, 2013, 154(2): 942-955. DOI:10.1210/en.2012-2006 |
| [37] |
于兰兰. 参与初情期启动的山羊卵巢miRNA筛选与鉴定[D]. 合肥: 安徽农业大学, 2016. YU L L. Screening and identification of ovarian miRNA involved in puberty initiation in goat[D]. Hefei: Anhui Agricultural University, 2016. (in Chinese) |
| [38] |
MESSINA A, LANGLET F, CHACHLAKI K, et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty[J]. Nat Neurosci, 2016, 19(6): 835-844. DOI:10.1038/nn.4298 |
| [39] |
ROA J, RUIZ-CRUZ M, RUIZ-PINO F, et al. Dicer ablation in Kiss1 neurons impairs puberty and fertility preferentially in female mice[J]. Nat Commun, 2022, 13(1): 4663. DOI:10.1038/s41467-022-32347-4 |
| [40] |
HERAS V, SANGIAO-ALVARELLOS S, MANFREDI-LOZANO M, et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3[J]. PLoS Biol, 2019, 17(11): e3000532. DOI:10.1371/journal.pbio.3000532 |
| [41] |
项门们, 史颖超, 赖振雨, 等. 雄性动物生殖相关lncRNA的研究进展[J]. 家畜生态学报, 2019, 40(8): 1-7. XIANG M M, SHI Y C, LAI Z Y, et al. Advances on lncRNA related to male animal reproduction[J]. Acta Ecologae Animalis Domastici, 2019, 40(8): 1-7. DOI:10.3969/j.issn.1673-1182.2019.08.001 (in Chinese) |
| [42] |
GAO X X, YE J, YANG C, et al. Screening and evaluating of long noncoding RNAs in the puberty of goats[J]. BMC Genomics, 2017, 18(1): 164. DOI:10.1186/s12864-017-3578-9 |
| [43] |
HUANG P P, BRUSMAN L E, IYER A K, et al. A novel gonadotropin-releasing hormone 1 (Gnrh1) enhancer-derived noncoding RNA regulates Gnrh1 gene expression in GnRH neuronal cell models[J]. PLoS One, 2016, 11(7): e0158597. DOI:10.1371/journal.pone.0158597 |
| [44] |
STAMOU M, NG S Y, BRAND H, et al. A balanced translocation in Kallmann syndrome implicates a long noncoding RNA, RMST, as a GnRH neuronal regulator[J]. J Clin Endocrinol Metab, 2020, 105(3): e231-e244. DOI:10.1210/clinem/dgz011 |
| [45] |
BLOSSEY R, SCHIESSEL H. The latest twists in chromatin remodeling[J]. Biophys J, 2018, 114(10): 2255-2261. DOI:10.1016/j.bpj.2017.12.008 |
| [46] |
XU W W, ZHOU W B, LIN H Y, et al. A novel heterozygous mutation of CHD7 gene in a Chinese patient with Kallmann syndrome: a case report[J]. BMC Endocr Disord, 2021, 21(1): 193. DOI:10.1186/s12902-021-00836-0 |
| [47] |
KIM H G, KURTH I, LAN F, et al. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome[J]. Am J Hum Genet, 2008, 83(4): 511-519. DOI:10.1016/j.ajhg.2008.09.005 |
| [48] |
LEBRETON L, ALLAIN E P, PARSCAN R C, et al. A novel CHD3 variant in a patient with central precocious puberty: expanded phenotype of Snijders Blok-Campeau syndrome?[J]. Am J Med Genet A, 2023, 191(4): 1065-1069. DOI:10.1002/ajmg.a.63096 |
| [49] |
MOHAMED A R, NAVAL-SANCHEZ M, MENZIES M, et al. Leveraging transcriptome and epigenome landscapes to infer regulatory networks during the onset of sexual maturation[J]. BMC Genomics, 2022, 23(1): 413. DOI:10.1186/s12864-022-08514-8 |
| [50] |
SCAGLIOTTI V, ESSE R, WILLIS T L, et al. Dynamic expression of imprinted genes in the developing and postnatal pituitary gland[J]. Genes (Basel), 2021, 12(4): 509. DOI:10.3390/genes12040509 |
| [51] |
ROBERTS S A, KAISER U B. GENETICS IN ENDOCRINOLOGY: genetic etiologies of central precocious puberty and the role of imprinted genes[J]. Eur J Endocrinol, 2020, 183(4): R107-R117. DOI:10.1530/EJE-20-0103 |
| [52] |
PERRY J R B, DAY F, ELKS C E, et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche[J]. Nature, 2014, 514(7520): 92-97. DOI:10.1038/nature13545 |
| [53] |
ROBERTS S A, NAULÉ L, CHOUMAN S, et al. Hypothalamic overexpression of makorin ring finger protein 3 results in delayed puberty in female mice[J]. Endocrinology, 2022, 163(11): bqac132. DOI:10.1210/endocr/bqac132 |
| [54] |
NAULÉ L, MANCINI A, PEREIRA S A, et al. MKRN3 inhibits puberty onset via interaction with IGF2BP1 and regulation of hypothalamic plasticity[J]. JCI Insight, 2023, 8(8): e164178. DOI:10.1172/jci.insight.164178 |
| [55] |
SHIM Y S, LEE H S, HWANG J S. Genetic factors in precocious puberty[J]. Clin Exp Pediatr, 2022, 65(4): 172-181. DOI:10.3345/cep.2021.00521 |
| [56] |
刘坤, 李国婧, 杨杞. 参与植物非生物逆境响应的DREB/CBF转录因子研究进展[J]. 生物技术通报, 2022, 38(5): 201-214. LIU K, LI G J, YANG Q. Research progress in DREB/CBF transcription factor involved in responses in plant to abiotic stress[J]. Biotechnology Bulletin, 2022, 38(5): 201-214. (in Chinese) |
| [57] |
RZECZKOWSKA P A, HOU H Y, WILSON M D, et al. Epigenetics: a new player in the regulation of mammalian puberty[J]. Neuroendocrinology, 2014, 99(3-4): 139-155. DOI:10.1159/000362559 |
| [58] |
OJEDA S R, LOMNICZI A, LOCHE A, et al. The transcriptional control of female puberty[J]. Brain Res, 2010, 1364: 164-174. DOI:10.1016/j.brainres.2010.09.039 |
| [59] |
ZANG S L, YIN X Q, LI P. Downregulation of TTF1 in the rat hypothalamic ARC or AVPV nucleus inhibits Kiss1 and GnRH expression, leading to puberty delay[J]. Reprod Biol Endocrinol, 2021, 19(1): 30. DOI:10.1186/s12958-021-00710-7 |
| [60] |
LEON S, TALBI R, MCCARTHY E A, et al. Sex-specific pubertal and metabolic regulation of Kiss1 neurons via Nhlh2[J]. Elife, 2021, 10: e69765. DOI:10.7554/eLife.69765 |
| [61] |
LODA A, COLLOMBET S, HEARD E. Gene regulation in time and space during X-chromosome inactivation[J]. Nat Rev Mol Cell Biol, 2022, 23(4): 231-249. |
| [62] |
GRAVHOLT C H, VIUFF M H, BRUN S, et al. Turner syndrome: mechanisms and management[J]. Nat Rev Endocrinol, 2019, 15(10): 601-614. DOI:10.1038/s41574-019-0224-4 |
| [63] |
BONOMI M, ROCHIRA V, PASQUALI D, et al. Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism[J]. J Endocrinol Invest, 2017, 40(2): 123-134. DOI:10.1007/s40618-016-0541-6 |
| [64] |
MANOTAS M C, GONZÁLEZ D M, CÉSPEDES C, et al. Genetic and epigenetic control of puberty[J]. Sex Dev, 2022, 16(1): 1-10. DOI:10.1159/000519039 |
(编辑 郭云雁)



