2. 宁夏医科大学 生育力保持教育部重点实验室,银川 750004
2. Key Laboratory of Fertility Maintenance of Ministry of Education, Ningxia Medical University, Yinchuan 750004, China
卵母细胞体外成熟(in vitro maturation,IVM)的关键在于用体外成熟培养基模拟卵泡液微环境,使未成熟的卵母细胞同时达到细胞核和质成熟。但是,卵母细胞体外成熟效率普遍低于体内成熟[1-3],氧化应激是一大重要因素。卵母细胞氧化应激和抗氧化能力在体内能够达到平衡状态,但在IVM过程中,线粒体电子传递链中产生的活性氧(ROS)过度积累,氧化和抗氧化功能紊乱,阻碍卵母细胞的成熟[4],另外还会造成线粒体功能障碍、纺锤体异常、细胞凋亡和发育受损,从而损伤卵母细胞,进一步导致卵母细胞成熟受阻[5-7]。
目前,已有多种抗氧化剂及其他类型的物质被添加到体外成熟培养基中,如白藜芦醇、褪黑素和亚硒酸钠[5, 8-12]等,但因其可能存在细胞毒性作用、成本较高而并不被广泛用于卵母细胞体外成熟培养。研究表明,药用植物中的抗氧化剂能够对多种疾病发挥作用,具有出色的抗氧化能力。Imai等[13]及Zhou等[14]曾从枸杞叶和枸杞根中分离出β-谷甾醇(β-sitosterol, SITO),它是一种常见的植物甾醇,具有广泛的生物学效应,能够治疗多种慢性疾病,如氧化应激、肥胖、糖尿病和癌症等[15-20],SITO可以恢复谷胱甘肽/氧化谷胱甘肽比例的损伤,这表明它可能是ROS清除剂[21]。SITO的抗氧化能力提示其可能减少卵母细胞体外培养过程中的氧化应激,可能有助于促进卵母细胞体外成熟。
目前,尚无任何研究表明SITO对卵母细胞体外成熟和早期胚胎发育有影响。因此,本研究评估了添加SITO能否改善猪卵母细胞IVM效率和早期胚胎发育质量,对提高卵母细胞体外成熟率及胚胎发育具有重要的理论意义和实用价值。
1 材料与方法 1.1 主要试剂除特别说明,本试验所有试剂均购自Sigma公司。活性氧检测试剂盒、细胞凋亡检测试剂盒、线粒体膜电位检测试剂盒购自碧云天生物技术有限公司;反转录试剂盒、荧光定量试剂盒购自TaKaRa公司;引物由上海生工生物工程有限公司合成。
1.2 试验方法1.2.1 猪卵母细胞的收集和体外培养 从屠宰场获得猪卵巢,在双抗生理盐水37 ℃、2 h内运至实验室。注射器抽取卵泡直径3~8 mm的卵泡液,在体视显微镜下挑拣出卵泡液中的卵丘-卵母细胞复合体(cumulus-oocyte complexes, COCs),选择有至少3层致密颗粒细胞且胞质均匀的COCs进行培养。培养条件为5% CO2、38.5 ℃恒温培养箱。处理组培养基分别添加10、20、40 μmol·L-1 SITO(将1 g SITO粉末溶于0.5 mL DMSO+29.64 mL培养基中,制得80 mmol·L-1母液,再分别稀释至10、20、40 μmol·L-1),含0.16% DMSO的培养基为对照组。培养46 h后,将COCs置于0.1%透明质酸酶中,反复吹打脱去颗粒细胞,以排出第一极体作为成熟的标志,统计成熟率(成熟率=排出第一极体卵母细胞数/培养卵母细胞总数×100)。
1.2.2 孤雌激活和体外胚胎培养 在5 mmol·L-1 ion中孵育4 min激活成熟卵母细胞,再在2 mmol·L-1 6-DMAP中培养3~5 h,然后在PZM-3中进行培养,培养条件为5% CO2+5% O2、38.5 ℃恒温培养箱。将孤雌激活当天记为第0天,于第2天统计卵裂率,第7天统计囊胚率(卵裂率=卵裂胚胎数/排出第一极体卵母细胞数×100,囊胚率=囊胚数/卵裂胚胎数×100)。
1.2.3 活性氧含量检测 根据DCFH-DA ROS检测试剂盒说明书,将成熟卵母细胞用0.1% PBS-PVA洗涤3次,置于10 mmol·L-1 DCFH-DA染色液中,37 ℃避光染色20 min。染色完成后再洗涤3次,封片,倒置荧光显微镜下拍照观察。
1.2.4 细胞凋亡检测及线粒体膜电位ΔΨ m检测 根据Annexin V-FITC细胞凋亡检测试剂盒说明书,将成熟卵母细胞用0.1% PBS-PVA洗涤3次,然后加入188 μL Annexin V-FITC结合液、5 μL Annexin V-FITC、2 μL Mito tracker Red CMXROs染色液、5 μL Hoechst 33342染色液,轻轻混合,室温避光孵育30 min,随后置于冰浴,染色完成再洗涤3次后封片。根据线粒体膜电位检测试剂盒(JC-1)说明书,将成熟卵母细胞用0.1% PBS-PVA洗涤3次后,在10 mmol·L-1 JC-1中于38.5 ℃、5% CO2下孵育30 min,染色完成再洗涤3次后封片,在共聚焦显微镜下观察。
1.2.5 实时荧光定量PCR 将成熟卵母细胞用0.1% PBS-PVA洗涤3次,转移到Cell to Signal Lysis Buffer中,待其完全裂解,根据反转录试剂盒说明书将其反转为cDNA。以cDNA为模板,使用SYBR Green PCR试剂盒进行qRT-PCR检测。以GAPDH为内参基因,特异性qRT-PCR引物序列见表 1。反应程序:95 ℃ 30 s,75 ℃ 10 s,60 ℃ 30 s,循环40次。反应体系:cDNA 1 μL,2×ChamQ SYBR qPCR Master Mix 5 μL,引物0.4 μL,RNase Free ddH2O 3.6 μL,总计10 μL。
|
|
表 1 引物序列 Table 1 Primers sequence |
1.2.6 囊胚免疫荧光染色 收集发育至第7天的囊胚,用0.1% PBS-PVA洗涤3次,使用500 μL DAPI染色5 min,再洗涤3次后封片,在荧光显微镜下观察。
1.2.7 数据分析 利用Image J软件分析图像荧光强度,使用GraphPad-prism 8绘制图表并进行数据处理,当P<0.05时认为差异显著。每组试验均重复3次。
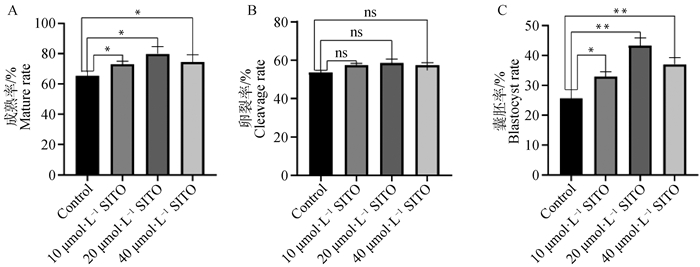
2 结果 2.1 SITO对猪卵母细胞成熟及早期胚胎发育的影响与对照组相比,处理组卵母细胞成熟率显著增加(P<0.05, 图 1A),10与40 μmol·L-1组之间差异不显著(P>0.05)(对照组:(65.28±3.13)%,n=183;10 μmol·L-1 SITO:(73.1±1.93)%,n=174;20 μmol·L-1 SITO:(78.89±4.61)%,n=169;40 μmol·L-1 SITO:(74.55±4.66)%,n=150)。各处理组与对照组卵裂率差异不显著(P>0.05)(图 1B),但囊胚率均显著增加(P<0.05, 图 1C);(对照组:(25.60±2.99)%,n=25;10 μmol·L-1 SITO:(32.95±1.55)%,n=33;20 μmol·L-1 SITO:(43.39±2.49)%,n=43;40 μmol·L-1 SITO:(37.08±2.17)%,n=32)。结果表明,在IVM培养基中添加SITO可以促进猪卵母细胞体外成熟和囊胚发育能力,20 μmol·L-1的作用最为显著。
|
A.对照组与处理组卵母细胞成熟率;B.对照组与处理组卵裂率;C.对照组与处理组囊胚率 A. Oocyte mature rate in control group and treatment group; B. Cleavage rate of control group and treatment group; C. Blastocyst rate of control group and treatment group 图 1 SITO促进卵母细胞成熟和囊胚形成 Fig. 1 SITO promotes oocyte maturation and blastocyst formation |
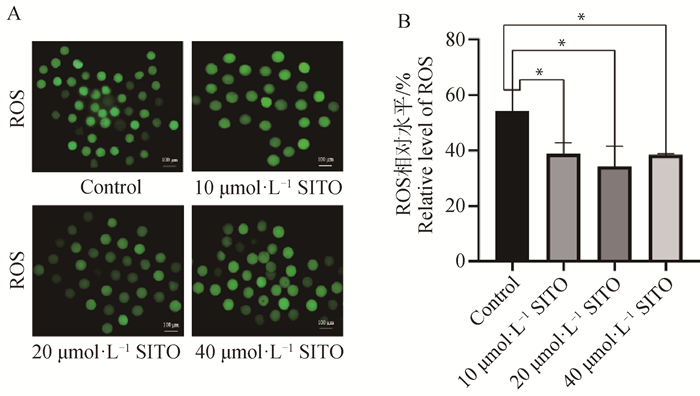
如图 2所示,各处理组ROS荧光强度均显著低于对照组(P<0.05),说明SITO对于减少卵母细胞ROS水平具有显著作用。其中20 μmol·L-1 SITO处理的荧光强度降低最为明显,因此选择添加20 μmol·L-1 SITO用于后续试验。
|
A.对照组与处理组的ROS荧光图像(Bar=100 μm);B.对照组与处理组卵母细胞ROS荧光值分析 A. ROS fluorescence images of control group and treatment group(Bar=100 μm); B. Analysis of ROS fluorescence values of oocytes in control group and treatment group 图 2 SITO降低猪卵母细胞中的ROS水平 Fig. 2 SITO decreases ROS levels in pig oocytes |
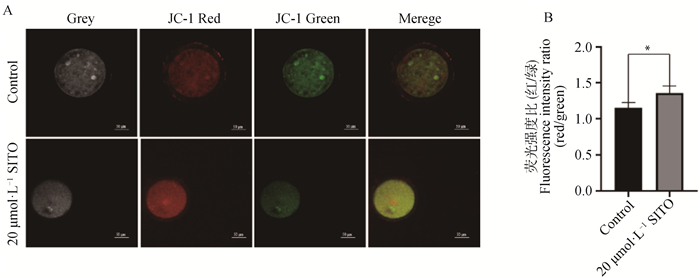
如图 3所示,碘化丙啶(PI)为红色荧光,Annexin V-FITC为绿色荧光,20 μmol·L-1 SITO处理的卵母细胞红色荧光强度显著高于对照组(P<0.05),绿色荧光强度显著低于对照组(P<0.05),说明SITO处理使猪卵母细胞凋亡显著减少。如图 4所示,在线粒体膜电位较高时产生红色荧光,在线粒体膜电位较低时产生绿色荧光,红/绿荧光比则说明了ΔΨ m的水平,20 μmol·L-1 SITO处理卵母细胞的红/绿荧光强度比高于对照组(P<0.05),表明20 μmol·L-1 SITO具有增强线粒体膜电位,改善线粒体功能的作用。

|
A. 对照组和20 μmol·L-1处理组激光共聚焦图像(Bar=50 μm);B. 对照组和20 μmol·L-1处理组红色荧光强度数据分析;C. 对照组和20 μmol·L-1处理组绿色荧光强度数据分析 A. Laser confocal images of control group and 20 μmol·L-1 treatment group(Bar=50 μm); B. Analysis of red fluorescence intensity data in control group and 20 μmol·L-1 treatment group; C. Analysis of green fluorescence intensity data in control group and 20 μmol·L-1 treatment group 图 3 SITO减少卵母细胞凋亡 Fig. 3 SITO decreased apoptosis of porcine oocytes |
|
A.对照组和20 μmol·L-1处理组卵母细胞的JC-1荧光图像(Bar=50 μm);B.JC-1荧光强度定量分析 A. JC-1 fluorescence images of control group and 20 μmol·L-1 treatment group(Bar=50 μm); B. Quantitative analysis of fluorescence intensity of JC-1 图 4 SITO增加猪卵母细胞ΔΨ m Fig. 4 SITO increased ΔΨ m in porcine oocytes |
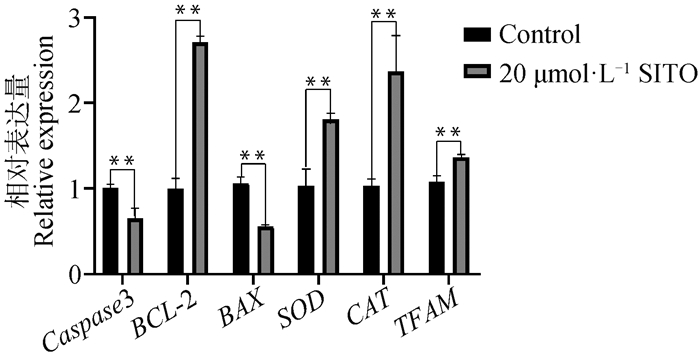
如图 5所示,20 μmol·L-1 SITO处理后BCL-2、SOD、CAT、TFAM基因的表达上调,Caspase 3和BAX的表达下调。表明20 μmol·L-1 SITO处理具有抗凋亡、抗氧化以及促进线粒体DNA复制和转录的作用。
|
图 5 SITO处理对卵母细胞相关基因表达的影响 Fig. 5 Effect of SITO treatment on oocyte related genes expression |
成熟培养液中添加20 μmol·L-1 SITO使囊胚率明显提高(图 1C),经过囊胚免疫荧光染色,如图 6A、B所示,20 μmol·L-1 SITO组的囊胚细胞数显著高于对照组(P<0.05),说明20 μmol·L-1 SITO可以提高囊胚细胞总数。

|
A.对照组和20 μmol·L-1处理组囊胚及免疫荧光染色图(Bar=50 μm;B.对照组和20 μmol·L-1处理组囊胚细胞数 A.Blastocyst and immunofluorescence staining of control group and 20 μmol·L-1 treatment group(Bar=50 μm); B. Total blastocysts in control group and 20 μmol·L-1 treatment group 图 6 SITO增加猪囊胚细胞数 Fig. 6 SITO increases the total number of pig blastocysts |
卵母细胞在正常生理条件下通过线粒体氧化磷酸化产生ROS,但它的过度积累会导致氧化和抗氧化功能的紊乱。ROS会影响细胞活力、破坏细胞结构,甚至导致细胞死亡,最终对胚胎发育产生负面影响,在培养基中添加抗氧化剂或ROS清除剂有助于减少氧化应激,利于胚胎存活和囊胚的形成[22-23],氧化还原相关基因的表达也与体外成熟卵母细胞中的ROS水平有关[24]。过氧化氢酶(CAT)和超氧化物歧化酶(SOD)在抵抗氧化应激中起着重要的作用,具有抵抗自由基的攻击、保护细胞免受损害的作用[25],在本研究中,SITO可以显著降低猪卵母细胞的ROS水平,同时CAT和SOD基因的表达被SITO激活,这与之前报道的SIRT1通过调控氧化还原状态参与卵母细胞成熟的结果一致[26]。还有研究表明,SITO可以通过减少DNA损伤来延长细胞寿命[27],通过增强AMPK磷酸化来激活脂肪体,并具有较强的自由基清除活性[28],自由基是机体生命活动中各种氧化反应的代谢产物,这进一步说明了SITO具有抗氧化的能力。
众所周知,氧化应激超载可以调节与卵母细胞凋亡相关的BAX/BCL-2比值[29-30]。在线粒体介导的凋亡途径中,细胞内异常信号导致BAX活化,抑制BCL-2的表达,诱导线粒体释放细胞色素c,形成凋亡小体或启动caspase级联激活,从而导致细胞凋亡的发生[31]。在SITO方面,有报道称其可诱导细胞外信号调节激酶(ERK)和p38丝裂原活化蛋白激酶(MARK)的磷酸化,下调PI3K/Akt,从而促进细胞凋亡和细胞死亡[32],为了验证这一点,本研究检测了Caspase 3、BAX和BCL-2的转录水平,结果显示SITO降低了猪卵母细胞Caspase 3基因和促凋亡基因BAX的表达,促进了抗凋亡基因BCL-2的表达。结果表明,SITO抑制了卵母细胞的凋亡,这与Moon等[32]的结果不符,推测这是由于SITO的抗氧化能力使卵母细胞的氧化应激显著减少,从而抑制了细胞凋亡的发生。这也反映出抗氧化作用对于抑制细胞凋亡能够产生明显影响。
线粒体是细胞质中必不可少的细胞器,被认为是评价卵母细胞质量的重要标志[33-34],线粒体膜电位ΔΨ m是ATP产生的驱动力。在卵母细胞发育成熟的过程中,对能量的需求是递增的,直到排卵时达到高峰,此时线粒体发生氧化磷酸化产生ATP,同时也产生一定量的ROS[35-37]。本研究结果表明,添加SITO后ΔΨ m有所增加,这表明SITO具有提高线粒体膜电位,促进ATP产生的作用,这有利于卵母细胞成熟。线粒体的生物合成和功能主要受线粒体DNA复制和转录的直接调节因子TFAM的调控[38-39],在本研究中,SITO处理使TFAM表达上调,因此,认为SITO可能通过激活线粒体功能减少细胞凋亡。
另外,囊胚细胞总数体现了早期胚胎发育的质量[40],本研究结果显示,SITO处理的囊胚细胞总数显著高于对照组,这表明成熟液中添加SITO能显著提高体外成熟卵母细胞的早期胚胎发育潜力。
4 结论综上所述,本研究通过在IVM培养基中添加SITO,结合免疫荧光染色,说明SITO对猪卵母细胞体外发育起重要作用,SITO增强了猪卵母细胞抗氧化和抗凋亡能力,增强了线粒体膜电位和ATP的含量。因此,在猪卵母细胞IVM中添加β-谷甾醇对支持和改善卵母细胞质量和早期胚胎发育具有重要作用。
| [1] |
JAROUDI K A, HOLLANDERS J M G, ELNOUR A M, et al. Embryo development and pregnancies from in-vitro matured and fertilized human oocytes[J]. Hum Reprod, 1999, 14(7): 1749-1751. DOI:10.1093/humrep/14.7.1749 |
| [2] |
TELFER E E, ANDERSEN C Y. In vitro growth and maturation of primordial follicles and immature oocytes[J]. Fertil Steril, 2021, 115(5): 1116-1125. DOI:10.1016/j.fertnstert.2021.03.004 |
| [3] |
ITO K, TAKAE S, NAKAMURA K, et al. The study of the efficiency of in vitro maturation of ovarian tissue oocytes in pediatric patients[J]. J Assist Reprod Genet, 2023, 40(12): 2787-2797. DOI:10.1007/s10815-023-02958-x |
| [4] |
WANG L, TANG J H, WANG L, et al. Oxidative stress in oocyte aging and female reproduction[J]. J Cell Physiol, 2021, 236(12): 7966-7983. DOI:10.1002/jcp.30468 |
| [5] |
LI Y, ZHANG Z Z, HE C J, et al. Melatonin protects porcine oocyte in vitro maturation from heat stress[J]. J Pineal Res, 2015, 59(3): 365-375. DOI:10.1111/jpi.12268 |
| [6] |
ZHANG M Q, MIAO Y L, CHEN Q, et al. BaP exposure causes oocyte meiotic arrest and fertilization failure to weaken female fertility[J]. FASEB J, 2018, 32(1): 342-352. DOI:10.1096/fj.201700514r |
| [7] |
徐沛欣, 陈红, 王惠增, 等. 活性氧在胚胎和生殖细胞中的研究进展及其检测方法[J]. 生命的化学, 2023, 43(10): 1599-1608. XU P X, CHEN H, WANG H Z, et al. Research progress and detection methods of reactive oxygen species in embryo and germ cells[J]. Chemistry of Life, 2023, 43(10): 1599-1608. (in Chinese) |
| [8] |
IWATA H. Resveratrol enhanced mitochondrial recovery from cryopreservation-induced damages in oocytes and embryos[J]. Reprod Med Biol, 2021, 20(4): 419-426. DOI:10.1002/rmb2.12401 |
| [9] |
GHORBANMEHR N, SALEHNIA M, AMOOSHAHI M. The effects of sodium selenite on mitochondrial dna copy number and reactive oxygen species levels of in vitro matured mouse oocytes[J]. Cell J, 2018, 20(3): 396-402. |
| [10] |
黄竹涛, 袁玉国, 朱家桥. 纳米顺铂对小鼠卵母细胞体外成熟的影响[J]. 动物医学进展, 2023, 44(11): 47-52. HUANG Z T, YUAN Y G, ZHU J Q. Effect of cisplatin nanoparticles on in vitro maturation of oocytes in mice[J]. Progress in Veterinary Medicine, 2023, 44(11): 47-52. (in Chinese) |
| [11] |
MENDES A F, PUELKER R Z, DE SOUZA L F A, et al. In vitro maturation in synthetic oviductal fluid increases gene expression associated with quality and lipid metabolism in bovine oocytes[J]. Zygote, 2023, 31(6): 582-587. DOI:10.1017/S0967199423000473 |
| [12] |
李一, 马述海, 彭华, 等. 外源性抗氧化剂对卵母细胞体外成熟的影响[J]. 中国畜禽种业, 2023, 19(7): 114-120. LI Y, MA S H, PENG H, et al. Effects of exogenous antioxidants on in vitro maturation of oocytes[J]. The Chinese Livestock and Poultry Breeding, 2023, 19(7): 114-120. (in Chinese) |
| [13] |
IMAI S, MURATA T, FUJIOKA S, et al. Isolation of β-sitosterol-β-D-glucoside from the leaves of Lycium chinense mill[J]. Yakugaku Zasshi, 1963, 83(11): 1092. DOI:10.1248/yakushi1947.83.11_1092 |
| [14] |
ZHOU X, XU G, WANG Q. Chemical constituents in the roots of Lycium chinense Mill[J]. Zhongguo Zhong Yao Za Zhi, 1996, 21(11): 675-676. |
| [15] |
KHAN Z, NATH N, RAUF A, et al. Multifunctional roles and pharmacological potential of β-sitosterol: emerging evidence toward clinical applications[J]. Chem Biol Interact, 2022, 365: 110117. DOI:10.1016/j.cbi.2022.110117 |
| [16] |
HAH Y S, LEE W K, LEE S, et al. β-sitosterol attenuates dexamethasone-induced muscle atrophy via regulating FoxO1-dependent signaling in C2C12 cell and mice model[J]. Nutrients, 2022, 14(14): 2894. DOI:10.3390/nu14142894 |
| [17] |
FAN Y T, SHEN J L, LIU X L, et al. β-sitosterol suppresses lipopolysaccharide-induced inflammation and lipogenesis disorder in bovine mammary epithelial cells[J]. Int J Mol Sci, 2023, 24(19): 14644. DOI:10.3390/ijms241914644 |
| [18] |
GUMEDE N M, LEMBEDE B W, BROOKSBANK R L, et al. β-sitosterol shows potential to protect against the development of high-fructose diet-induced metabolic dysfunction in female rats[J]. J Med Food, 2020, 23(4): 367-374. DOI:10.1089/jmf.2019.0120 |
| [19] |
CHEN C, SHEN J L, LIANG C S, et al. First discovery of beta-sitosterol as a novel antiviral agent against white spot syndrome virus[J]. Int J Mol Sci, 2022, 23(18): 10448. DOI:10.3390/ijms231810448 |
| [20] |
WANG H Y, WANG Z, ZHANG Z H, et al. β-sitosterol as a promising anticancer agent for chemoprevention and chemotherapy: mechanisms of action and future prospects[J]. Adv Nutr, 2023, 14(5): 1085-1110. DOI:10.1016/j.advnut.2023.05.013 |
| [21] |
VIVANCOS M, MORENO J J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages[J]. Free Radic Biol Med, 2005, 39(1): 91-97. DOI:10.1016/j.freeradbiomed.2005.02.025 |
| [22] |
SHARMA M, PUNETHA M, SAINI S, et al. Mito-Q supplementation of in vitro maturation or in vitro culture medium improves maturation of buffalo oocytes and developmental competence of cloned embryos by reducing ROS production[J]. Anim Reprod Sci, 2024, 260: 107382. DOI:10.1016/j.anireprosci.2023.107382 |
| [23] |
TAYLOR C T. Antioxidants and reactive oxygen species in human fertility[J]. Environ Toxicol Pharmacol, 2001, 10(4): 189-198. DOI:10.1016/S1382-6689(01)00099-0 |
| [24] |
PANG Y W, ZHAO S J, SUN Y Q, et al. Protective effects of melatonin on the in vitro developmental competence of bovine oocytes[J]. Anim Sci J, 2018, 89(4): 648-660. DOI:10.1111/asj.12970 |
| [25] |
GRZEGORZEWSKA A K, WOLAK D, HRABIA A. Effect of tamoxifen treatment on catalase (CAT) and superoxide dismutase (SOD) expression and localization in the hen oviduct[J]. Theriogenology, 2024, 214: 73-80. DOI:10.1016/j.theriogenology.2023.10.008 |
| [26] |
XING X P, ZHANG J J, WU T, et al. SIRT1 reduces epigenetic and non-epigenetic changes to maintain the quality of postovulatory aged oocytes in mice[J]. Exp Cell Res, 2021, 399(2): 112421. DOI:10.1016/j.yexcr.2020.112421 |
| [27] |
SHARMILA R, SINDHU G. Evaluate the antigenotoxicity and anticancer role of β-sitosterol by determining oxidative DNA damage and the expression of phosphorylated mitogen-activated protein kinases', C-fos, C-jun, and endothelial growth factor receptor[J]. Pharmacogn Mag, 2017, 13(49): 95-101. DOI:10.4103/0973-1296.203975 |
| [28] |
LIN W S, CHEN J Y, WANG J C, et al. The anti-aging effects of Ludwigia octovalvis on Drosophila melanogaster and SAMP8 mice[J]. Age (Dordr), 2014, 36(2): 689-703. DOI:10.1007/s11357-013-9606-z |
| [29] |
WANG L Y, WANG D H, ZOU X Y, et al. Mitochondrial functions on oocytes and preimplantation embryos[J]. J Zhejiang Univ Sci B, 2009, 10(7): 483-492. DOI:10.1631/jzus.B0820379 |
| [30] |
黄循铷, 蒋赵艳, 陈亚乐, 等. 不同剂量乙醇灌胃对大鼠心功能和Bcl-2/Bax表达的影响[J]. 广西医科大学学报, 2023, 40(11): 1802-1806. HUANG X R, JIANG Z Y, CHEN Y L, et al. Effects of different doses of ethanol administration on cardiac function and Bcl-2/Bax in rats[J]. Journal of Guangxi Medical University, 2023, 40(11): 1802-1806. (in Chinese) |
| [31] |
ELMORE S. Apoptosis: a review of programmed cell death[J]. Toxicol Pathol, 2007, 35(4): 495-516. DOI:10.1080/01926230701320337 |
| [32] |
MOON D O, LEE K J, CHOI Y H, et al. β-Sitosterol-induced-apoptosis is mediated by the activation of ERK and the downregulation of Akt in MCA-102 murine fibrosarcoma cells[J]. Int Immunopharmacol, 2007, 7(8): 1044-1053. DOI:10.1016/j.intimp.2007.03.010 |
| [33] |
李钰浚, 何翃闳, 杨丽雪, 等. 线粒体自噬调控哺乳动物胚胎发育的研究进展[J]. 畜牧兽医学报, 2024, 55(3): 905-912. LI Y J, HE H H, YANG L X, et al. Advances in regulation of mammalian embryonic development by mitochondrial autophagy[J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(3): 905-912. (in Chinese) |
| [34] |
金君学, 綦新月, 刘子瑜, 等. Mito-Tempo修复过氧化氢诱导的猪卵母细胞线粒体损伤机制研究[J]. 东北农业大学学报, 2023, 54(6): 28-34. JIN J X, QI X Y, LIU Z Y, et al. H2O2-induced mitochondrial damage rescued by Mito-Tempo in porcine oocytes[J]. Journal of Northeast Agricultural University, 2023, 54(6): 28-34. (in Chinese) |
| [35] |
MAY-PANLOUP P, BOUCRET L, CHAO DE LA BARCA J M, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles[J]. Hum Reprod Update, 2016, 22(6): 725-743. DOI:10.1093/humupd/dmw028 |
| [36] |
孙立晨, 许晓玲, 白佳桦, 等. 哺乳动物体外成熟卵母细胞的线粒体调控机制研究进展[J]. 畜牧与兽医, 2023, 55(10): 116-120. SUN L C, XU X L, BAI J H, et al. Progress in research on mitochondrial regulation mechanism of mammalian in vitro maturation oocyte[J]. Animal Husbandry & Veterinary Medicine, 2023, 55(10): 116-120. (in Chinese) |
| [37] |
ANGELOVA P R, ABRAMOV A Y. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration[J]. FEBS Lett, 2018, 592(5): 692-702. DOI:10.1002/1873-3468.12964 |
| [38] |
ROTH Z. Symposium review: reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function[J]. J Dairy Sci, 2018, 101(4): 3642-3654. DOI:10.3168/jds.2017-13389 |
| [39] |
ZHAO M, WANG Y Z, LI L, et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance[J]. Theranostics, 2021, 11(4): 1845-1863. DOI:10.7150/thno.50905 |
| [40] |
ROSS P J, GOISSIS M D, MARTINS J P N, et al. Blastocyst cell number and allocation affect the developmental potential and transcriptome of bovine somatic cell nuclear transfer embryos[J]. Stem Cells Dev, 2023, 32(17-18): 515-523. DOI:10.1089/scd.2022.0292 |
(编辑 郭云雁)



