镉是一种广泛存在的环境污染物,不仅造成重大的经济损失也严重危害了人类健康[1],因此,镉污染的防治是一个不可忽视的环境问题。镉污染也确实引起了全世界的高度关注,但至今还没能彻底解决这个问题。早在1984年,联合国环境规划署提出了12种具有全球性意义的危险化学物质,镉列首位并被世界卫生组织确定为优先研究的食品污染物[2]。镉进入动物体内经血液循环转运到所有靶器官,蓄积后可造成肝、肾、骨骼等多器官的损伤,甚至引起死亡[3-6]。肝是镉重要的靶器官之一,被吸收的镉首先会与白蛋白结合,然后在肝门静脉循环的作用下被运送到肝窦毛细血管,并被肝细胞摄取,最终约有1/6的镉蓄积于肝中,导致肝细胞损伤或死亡[7]。就目前的研究来看,镉诱导肝细胞毒性的原因有很多,但是导致肝细胞损伤的确切机制仍是未知的。为了缓解镉的细胞毒性损伤,研究者们一直在寻找能拮抗镉毒性的有效药物。硒作为一种生命必需的微量元素,以硒代半胱氨酸的形式参与各种抗氧化酶的合成,发挥抗氧化和免疫调节等功能[8-9]。近些年的研究表明,硒具有拮抗镉毒性的作用[10]。但是传统无机硒的生理剂量与中毒剂量范围较窄,摄入过量的硒会引发毒性症状,而新型纳米硒因其毒性低且高效的特点,有望在临床中得到推广应用。因此,本文拟对镉致肝毒性的机制以及硒拮抗镉肝毒性的相关研究进行归纳综述,以期为镉污染的防治以及硒的临床应用提供理论依据。
1 镉诱导肝毒性损伤的作用机制镉诱导肝损伤的机制主要有:细胞自噬[11]、氧化应激[12]、线粒体损伤[13]、细胞凋亡[14]、炎性介质和黏附分子的产生[15]、必需金属元素的内稳态失衡[15]以及铁死亡等[16]。
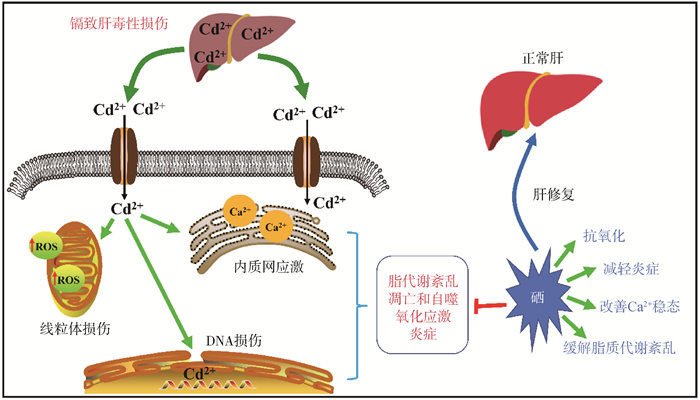
1.1 诱导细胞自噬自噬是由一系列刺激诱导的细胞成分降解,如错误折叠的蛋白质、受损的细胞器、脂滴等。自噬的分子机制与病理过程以及细胞损伤情况相关,据报道自噬还与镉诱导的细胞毒性有一定的关系[17-18]。p62蛋白在自噬被抑制时累积, 而激活时则会降解,因此p62被认为是自噬活性的标志[19]。
过度自噬将导致细胞损伤,而适当的自噬对机体是有益的。在高胆固醇饮食喂养的小鼠中,镉导致了自噬缺陷从而引起肝脂肪的堆积,因为自噬能够降低甘油三酯和脂滴的含量,抑制自噬将会减少甘油三酯的分解[20]。这种相互关系可能会使肝细胞陷入一个有害的循环,在这个循环中,自噬减少会促进脂质堆积,而脂质滞留又反过来抑制自噬。有研究显示,镉诱导的自噬抑制与溶酶体降解能力受损有关,原因是溶酶体膜通透性增加抑制了转录因子EB的核转位,以及由于Rap7蛋白表达减少阻止了自噬小体与溶酶体的融合[21]。例如在镉处理的HepG2细胞中,由于内质网钙离子动态平衡的改变抑制了溶酶体的降解途径,从而增加了甘油三酯的含量。据报道,AMPK/PPAR-γ/NF-κB通路在镉诱导的肝细胞自噬中具有重要作用[22],而线粒体自噬在肝损伤的早期也发挥了一定的作用[23]。
1.2 诱导氧化应激当细胞损伤较轻时,可通过线粒体自噬清除功能失调或多余的线粒体,从而微调线粒体数并保持能量代谢。当发生功能紊乱的线粒体数量较多时,会导致氧化与抗氧化系统失衡,从而发生氧化应激。氧化应激被认为是镉引起细胞损伤的重要机制之一[24]。正常状态下的动物机体具有一套包括谷胱甘肽过氧化物酶、过氧化氢酶、过氧化物酶和超氧化物歧化酶等的抗氧化防御系统,但是由于镉与巯基结合的亲和力强于其与氯化物、磷酸盐、羧基或氨基基团结合的亲和力,所以进入机体的镉通过与蛋白质上的巯基结合,使其失去活性后扰乱肝细胞内氧化还原状态,引起细胞活性氧(ROS)增加以及氧化应激[25-26],最终导致肝细胞损伤。
1.3 诱导线粒体损伤线粒体是细胞内最易损伤的细胞器也是产生ROS的主要场所,它可以显示细胞受损伤的程度。线粒体损伤时可导致细胞发生变性、ROS生成过多、细胞能量不足等[27]。线粒体是镉的主要靶标,镉暴露能够引起线粒体膜电位的改变和通透性增加,还能干扰呼吸链并增加自由基的产生,导致脂质过氧化以及细胞凋亡等,进而诱导肝的毒性损伤[28]。
1.4 诱导脂质稳态失衡脂质过氧化将导致脂质稳态的失衡,而近几年的研究表明,脂质稳态失衡也是镉致肝损伤的一个机制。肝病大多情况下开始于脂质过载,脂质过载有时是可逆的,此时对肝功能的影响比较小,但由于炎症的持续存在,肝病可能继续发展到很严重的状态[28]。有研究发现,氯化镉暴露造成的小鼠肝损伤中甘油三酯和胆固醇的含量明显增加,在大鼠体内试验中也有类似的发现,用大鼠暴露于氯化镉3个月后,观察到血清中甘油三酯、总胆固醇、高密度脂蛋白胆固醇、低密度脂蛋白胆固醇、谷胱甘肽和丙二醛明显增加,这与增加的ROS一起,可能导致癌症的发生[29]。事实上,胆固醇本身就可以为癌症的发展提供启动条件,而镉的存在会加大这种可能性。有研究表明,高胆固醇饲粮喂养的小鼠肝细胞中甘油三酯的含量增加,当这些肝细胞又暴露于镉时,甘油三酯的产生量得到更明显提高[30]。镉通过促进磷脂酰胆碱重塑和花生四烯酸的合成扰乱小鼠的脂质结构从而诱导肝功能发生障碍[31]。氯化镉还能刺激miR-34a/SIRT1/FXR/p53轴诱导大鼠的非酒精性脂肪肝[32]。另外,血清镉水平与脂肪变性以及纤维化之间也存在相关性。目前只在临床前的研究模型中发现镉会导致纤维化和肝癌,还需要进一步进行人类临床研究,找到将慢性接触镉与肝癌联系起来的确切证据,因为肝硬化和肝癌是肝进行性损害的最后阶段。
1.5 诱导细胞凋亡研究表明,无论是氧化应激还是脂质稳态失衡都可能继发细胞凋亡。细胞凋亡是一种由多个基因介导的程序性细胞死亡,包括外在途径和内在途径,细胞凋亡也是机体去除受损细胞以确保器官正常功能的一种重要措施[33]。镉暴露能够导致肝细胞内质网应激和胞内钙离子浓度升高,促进细胞凋亡诱导因子和核酸内切酶G的核移位[34-36]。此外,镉暴露也能显著增加细胞色素C的释放量以及ROS和Caspase-3的生成,降低线粒体膜电位,最终导致细胞凋亡[37]。另一方面,镉还能通过MAPK、NO、p53和Caspase-8等途径诱导肝细胞发生凋亡[38]。总之,镉暴露诱导肝细胞发生凋亡的途径有很多,而过度凋亡时也将导致肝的功能受损。
1.6 诱导炎性介质和黏附分子的产生镉暴露时肝细胞会释放出大量的炎性分子,而肝的巨噬细胞与中性粒细胞被认为是产生和释放促炎细胞因子的主要来源。促炎因子主要包括白介素1α(IL-1α)、白介素1β(IL-1β)、白介素18(IL-18)、肿瘤坏死因子α(TNF-α)和单核细胞趋化蛋白1(MCP-1)。其中IL-1β和TNF-α可以识别并激活肝细胞中的黏附分子,然后引发一系列细胞和体液反应,最终导致肝炎和继发性肝损伤。炎症小体NLRP3是一种蛋白质化合物,在炎症中发挥中介作用,镉暴露可激活大鼠肝细胞中NLRP3、NF-κB和MAPK信号通路,并增加促炎细胞因子的表达[39-40]。镉暴露还能诱导大鼠肝中一氧化氮(NO)的水平和过氧化物酶活性显著升高,而NO在炎症过程中发挥着重要作用。此外,镉暴露的大鼠肝组织中,抗炎细胞因子IL-10和miR-182-5p水平显著减少,而促炎细胞因子IL-6、TNF-α、IL-1β和TLR4显著增加[41],表明miR-182-5p/TLR4/炎症轴参与了镉的肝毒性。
1.7 诱导必需金属元素内稳态失衡铜、铁、锌等必需金属元素作为酶的辅助因子参与合成一些生物大分子,在生命活动中扮演着至关重要的角色[28]。镉暴露能够导致体内金属转运系统的紊乱以及一些必需金属元素的内稳态失衡,这也是镉毒性的机制之一[42]。现有研究表明,镉不会转变其氧化态,而是以二价阳离子的形式存在于环境中,并通过特定的转运体与必需金属元素竞争进入机体内,如二价金属转运体、ZIP和钙离子通道等[43]。镉的积累会改变二价金属的动态平衡,比如铁缺乏的原因可能是与镉竞争二价金属转运体有关。此外,ZIP8和ZIP14是促进细胞摄取铁、锰和锌的转运体,但它们也可以转运镉。特别是ZIP8转运蛋白表达的降低会减少镉的含量,但也会造成细胞内锌的缺乏,从而引起金属元素的内稳态失衡并影响组织细胞的生命过程[44]。铜转运蛋白是另一个与镉进入机体有关的转运蛋白,对人肝癌细胞系HepG2的研究表明,铜转运蛋白的过表达会导致镉摄取增加,而铜转运蛋白的沉默又会减少铜等金属元素进入细胞内[45]。另外,金属硫蛋白在必需金属元素特别是锌和铜的运输和调节中起着非常重要的作用。金属硫蛋白的巯基能够螯合镉并将之排出体外,从而降低镉离子对机体细胞的毒性,因此诱导金属硫蛋白的表达对镉的肝毒性具有保护作用[46]。总之,镉可以取代蛋白质中的一些必需金属元素,导致它们的功能障碍,从而干扰细胞膜金属转运、能量代谢等一系列细胞过程,这种方式被认为是镉肝毒性的主要分子机制之一。
1.8 诱导细胞铁死亡金属元素的内稳态失衡可能导致细胞的死亡,比如铁死亡。铁死亡是一种细胞程序性死亡方式,主要与铁代谢紊乱、氨基酸抗氧化系统失衡、脂质过氧化物集聚有关[47]。虽然肝损伤的类型与损伤的性质及严重程度有关,而且不同性质的肝疾病可由不同原因引起,但是肝的病变由肝炎、肝纤维化、肝硬化或原发性肝癌的演变进程是相似的。近几年的研究显示,铁死亡除了在肿瘤、帕金森病等疾病中发挥作用,在多种肝疾病中也发现了铁代谢紊乱和脂质过氧化物集聚的现象,并且调控铁死亡可以影响肝疾病进程[48-49]。陈敬宜等[50]的研究表明,镉暴露造成镉离子大量蓄积于肝中,引发铁死亡,进而引起肝组织炎性损伤。在He等[51]的研究中显示,镉能通过诱导氧化应激介导的自噬和内质网应激来激活铁死亡并引起肝损伤,这一过程中使用铁死亡抑制剂能够降低肝损伤的程度。也有研究表明,中药对肝细胞铁死亡具有一定的保护作用[52],那么硒作为镉的拮抗剂对镉诱导的肝细胞铁死亡是否具有一定的缓解作用呢?这是需要进一步研究并探讨的问题。此外,有文献报道镉暴露可通过激活PERK信号抑制胎盘来源的雌激素合成,从而损害胎儿的肝发育[53]。有意思的是,镉除了诱导肝损伤外还能抑制早期癌症的进展从而起到保护作用[54]。根据目前的报道,对镉致肝损伤机制的相关文献进行了汇总整理,具体如表 1所示。
|
|
表 1 镉致肝损伤的相关研究汇总 Table 1 Summary of related studies on cadmium induced liver damage |
硒是哺乳动物所必需的微量元素之一,具有广泛的生物活性,可通过形成硒蛋白调节细胞的氧化还原反应,维持机体内环境稳态[60-61]。硒的生理剂量与中毒剂量范围较窄,一旦摄入过量的硒则会引起乏力、食欲减退、精神不振以及头皮痒痛等症状[62]。常见的硒分为无机硒和有机硒,其中无机硒毒性较高而且很难控制用量,有机硒的吸收效率比无机硒高但是安全性也不强。在动物体内,硒以硒代半胱氨酸的形式结合到硒蛋白中,通过硒蛋白的表达来发挥生物学功能,在抗氧化、抗炎、免疫以及肝、肾等器官损伤的保护等方面具有重要作用[8]。此外,硒拮抗重金属毒性的功能已经被证实,并且能够从多种途径缓解镉对机体的毒性损伤,如通过AKT信号通路拮抗镉诱导的细胞凋亡,通过缓解氧化应激降低镉的肝毒性等[63]。但是传统的有机硒和无机硒本身也具有较大的毒性,因此制备安全有效的硒制剂很有必要,毒性低且高效的红色纳米硒有望解决这一问题。纳米硒是一种新型纳米级单质硒,稳定性好,与传统的硒化合物相比,安全性能高、具有更好的生物活性和抗氧化性[64]。纳米硒能够通过直接清除氧自由基、激活抗氧化酶活性、增强机体抗氧化防御体系中其它关键酶的活性等多种方式发挥其抗氧化作用,另外纳米硒在鸡上还能拮抗镉诱导的肝纤维化[55]。在镉导致的神经毒性和肝毒性方面,纳米硒具有一定的保护作用[65-66]。过量的纳米硒也能够造成机体的损伤,但是在畜禽的养殖过程中适当的补充硒可以降低重金属对机体造成毒性损伤的风险。
2.2 硒在镉致肝毒性损伤中的作用硒与肝的健康息息相关,肝病患者体内普遍缺硒,而补充硒能够提高肝细胞的抗氧化能力,抑制肝纤维化和损伤。虽然硒对重金属的拮抗作用在十几年前就有报道,但是其作用机制却还不是很明确。从现有报道的研究来看,在肝细胞中硒拮抗镉的机制可能有:减轻氧化应激[67]、缓解脂质代谢紊乱和线粒体损伤、抑制细胞凋亡和自噬[56]、减轻炎症反应以及改善细胞内游离Ca2+稳态等。此外,有研究显示硒对外因导致的细胞铁死亡具有保护作用[68-69],但是在镉诱导的肝细胞铁死亡中硒的作用机制还未明确。
线粒体质量控制是通过调节线粒体形态、迁移、分布和数量的平衡来实现线粒体稳态的关键机制,线粒体质量控制缺陷被认为是细胞衰老的主要因素。线粒体质量控制机制能够被镉破坏,硒通过减轻线粒体质量控制机制的紊乱来改善镉诱导的衰老[57]。Cong等[70]的报道表明,在鸡的肝组织中,硒通过细胞色素P450能够对镉诱导的线粒体损伤发挥保护作用[70]。另一方面,镉能够扰乱兔肝组织中微量元素的平衡,而补硒能够激活Nrf2信号通路调节线粒体相关蛋白的表达并平衡线粒体的分裂和融合,最终缓解镉诱导的肝细胞线粒体自噬以及内质网应激[58]。此外,硒通过干预脂质过氧化,改善细胞内游离Ca2+稳态和脂代谢紊乱,从而减轻镉诱导的肝细胞毒性损伤[71]。并且适当剂量的亚硒酸钠对氯化镉诱导的DNA损伤、细胞凋亡、细胞坏死具有一定的抑制作用[63]。El-Boshy等[72]用含镉水喂饲大鼠30 d后发现肝、肾受到了一定程度的损伤,而给予亚硒酸钠共处理则降低了镉引起的血清促炎因子水平,并且提高了肝中抗氧化酶活性从而缓解肝的毒性损伤。Cao等[59]发现,硒能够通过抑制鸭肝细胞中NLRP3炎症小体与HMGB1/NF-κB通路的相互作用,拮抗镉诱导的炎症和氧化应激。Wang等[73]在蛋鸡的研究中也发现硒通过抑制氧化应激和MAPK途径发挥镉肝毒性的拮抗作用。
由于镉致肝损伤的机制比较复杂,很难从单一的方面去阐述清楚其发病机制以及硒的保护作用,而采取联合治疗往往会取得更好的效果。对于联合用药方面,王跃[74]的研究显示,富硒酵母联合维生素E通过提高体内抗氧化酶的活性、抑制细胞凋亡以及减轻炎症反应,可以缓解镉导致的肝损伤。赵鸣飞[75]的研究表明,姜黄素-硒配合物对镉导致的大鼠肝氧化损伤和肝细胞凋亡等具有缓解作用,而L-肉碱与四氯化硒的联合使用也可以保护小鼠肝免受氯化镉诱导的组织结构损伤。根据目前报道,对硒拮抗镉肝毒性的文献进行了汇总整理,具体如表 2所示。硒在镉致肝毒性中的作用如图 1所示。
|
|
表 2 硒拮抗镉肝毒性的相关研究汇总 Table 2 Summary of related studies on selenium antagonizing cadmium hepatotoxicity |
|
图 1 硒在镉致肝毒性损伤中的作用示意图 Fig. 1 Schematic diagram of the role of selenium in cadmium induced hepatotoxicity injury |
综上所述,肝是镉主要的靶器官和蓄积部位,镉在肝中蓄积后能够造成毒性损伤,最终影响肝的功能。镉致肝损伤的机制比较多,各机制之间既相互独立又相互联系,而且可能还有潜在的机制未被发现。正是由于镉中毒的复杂性及其对人类健康造成的危害,镉污染的防控和治理是一直是政府努力解决的环境问题,而如何降低镉进入机体后造成的毒性损伤也是科学界研究的热点之一。硒作为哺乳动物必需的微量元素,具有广泛的生物活性,而且研究表明,硒对镉毒性具有一定的拮抗作用,但是由于传统硒的安全剂量范围比较窄,导致硒的应用受到了限制。近几年发现的红色纳米硒作为一种新形态的硒,因其具有低毒性和高生物活性的特点,有望成为新型的硒产品并在防治镉毒性中得到应用。然而,关于硒拮抗镉毒性的科学研究和应用仍然存在许多的困惑,例如目前报道的作用机制主要是硒的抗氧化性,但具有抗氧化性的产品有很多,而且镉对机体的毒性不止是氧化损伤一方面。因此,只有进一步研究硒、镉相互作用的机理以及硒发挥作用的关键靶点,才能促进相关硒产品更广泛的应用,这也是未来研究的方向。
| [1] |
LI W X, TAN M X, WANG H Q, et al. METTL3-mediated m6A mRNA modification was involved in cadmium-induced liver injury[J]. Environ Pollut, 2023, 331: 121887. DOI:10.1016/j.envpol.2023.121887 |
| [2] |
邬静, 周新华, 张明, 等. 镉诱导小鼠卵巢颗粒细胞凋亡时MDA含量及SOD活性变化[J]. 中国兽医杂志, 2008, 44(6): 24-25. WU J, ZHOU X H, ZHANG M, et al. Study of the change of madlondialdehyde contents and superoxide dismutase activities during Cadium induced apoptosis of mouse granulosa cells[J]. Chinese Journal of Veterinary Medicine, 2008, 44(6): 24-25. (in Chinese) |
| [3] |
ZHANG H, REYNOLDS M. Cadmium exposure in living organisms: a short review[J]. Sci Total Environ, 2019, 678: 761-767. DOI:10.1016/j.scitotenv.2019.04.395 |
| [4] |
JIA Y Z, YIN C Z, KE W B, et al. Alpha-ketoglutarate alleviates cadmium-induced inflammation by inhibiting the HIF1A-TNFAIP3 pathway in hepatocytes[J]. Sci Total Environ, 2023, 878: 163069. DOI:10.1016/j.scitotenv.2023.163069 |
| [5] |
CHOU X, LI X H, MIN Z, et al. Sirtuin-1 attenuates cadmium-induced renal cell senescence through p53 deacetylation[J]. Ecotoxicol Environ Saf, 2022, 245: 114098. DOI:10.1016/j.ecoenv.2022.114098 |
| [6] |
张雪晴, 仝锡帅, 王果帅, 等. 葛根素缓解镉对大鼠股骨胫骨中成骨细胞分化的抑制作用[J]. 畜牧兽医学报, 2022, 53(2): 628-636. ZHANG X Q, TONG X S, WANG G S, et al. Puerarin alleviates the inhibitory effect of cadmium on the femoral and tibial osteoblast differentiation of rat[J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(2): 628-636. (in Chinese) |
| [7] |
YUAN J Z, HUANG X Q, GU J H, et al. Honokiol reduces cadmium-induced oxidative injury and endosomal/lysosomal vacuolation via protecting mitochondrial function in quail (Coturnix japonica) liver tissues[J]. Sci Total Environ, 2023, 857: 159626. DOI:10.1016/j.scitotenv.2022.159626 |
| [8] |
PECORARO B M, LEAL D F, FRIAS-DE-DIEGO A, et al. The health benefits of selenium in food animals: a review[J]. J Anim Sci Biotechnol, 2022, 13(1): 58. DOI:10.1186/s40104-022-00706-2 |
| [9] |
ZHANG F, LI X L, WEI Y M. Selenium and selenoproteins in health[J]. Biomolecules, 2023, 13(5): 799. DOI:10.3390/biom13050799 |
| [10] |
DU H, ZHENG Y L, ZHANG W, et al. Nano-selenium alleviates cadmium-induced acute hepatic toxicity by decreasing oxidative stress and activating the Nrf2 pathway in male Kunming mice[J]. Front Vet Sci, 2022, 9: 942189. DOI:10.3389/fvets.2022.942189 |
| [11] |
NITURE S, LIN M H, QI Q, et al. Role of autophagy in cadmium-induced hepatotoxicity and liver diseases[J]. J Toxicol, 2021, 2021: 9564297. |
| [12] |
FANG J, YIN H, YANG Z Z, et al. Vitamin E protects against cadmium-induced sub-chronic liver injury associated with the inhibition of oxidative stress and activation of Nrf2 pathway[J]. Ecotoxicol Environ Saf, 2021, 208: 111610. DOI:10.1016/j.ecoenv.2020.111610 |
| [13] |
OKOYE C N, MACDONALD-JAY N, KAMUNDE C. Effects of bioenergetics, temperature and cadmium on liver mitochondria reactive oxygen species production and consumption[J]. Aquat Toxicol, 2019, 214: 105264. DOI:10.1016/j.aquatox.2019.105264 |
| [14] |
HU W, ZHU Q L, ZHENG J L, et al. Cadmium induced oxidative stress, endoplasmic reticulum (ER) stress and apoptosis with compensative responses towards the up-regulation of ribosome, protein processing in the ER, and protein export pathways in the liver of zebrafish[J]. Aquat Toxicol, 2022, 242: 106023. DOI:10.1016/j.aquatox.2021.106023 |
| [15] |
RANI A, KUMAR A, LAL A, et al. Cellular mechanisms of cadmium-induced toxicity: a review[J]. Int J Environ Health Res, 2014, 24(4): 378-399. DOI:10.1080/09603123.2013.835032 |
| [16] |
WANG Y, WU J, ZHANG M M, et al. Cadmium exposure during puberty damages testicular development and spermatogenesis via ferroptosis caused by intracellular iron overload and oxidative stress in mice[J]. Environ Pollut, 2023, 15(325): 121434. |
| [17] |
DUAN Y T, ZHAO Y M, WANG T, et al. Taurine alleviates cadmium-induced hepatotoxicity by regulating autophagy flux[J]. Int J Mol Sci, 2023, 24(2): 1205. DOI:10.3390/ijms24021205 |
| [18] |
闻双全, 王莉, 张文华, 等. Fas对镉暴露致大鼠大脑皮质自噬体形成的影响[J]. 畜牧兽医学报, 2022, 53(5): 1608-1614. WEN S Q, WANG L, ZHANG W H, et al. Effects of Fas on autophagosomes formation induced by cadmium exposure in rat cerebral cortex[J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(5): 1608-1614. (in Chinese) |
| [19] |
NODA N N. Cytoskeleton grows p62 condensates for autophagy[J]. Cell Res, 2022, 32(7): 607-608. DOI:10.1038/s41422-022-00671-5 |
| [20] |
QIAN H, CHAO X J, WILLIAMS J, et al. Autophagy in liver diseases: a review[J]. Mol Aspects Med, 2021, 82: 100973. DOI:10.1016/j.mam.2021.100973 |
| [21] |
ZHAO Y, LI Z F, ZHANG D, et al. Quercetin alleviates Cadmium-induced autophagy inhibition via TFEB-dependent lysosomal restoration in primary proximal tubular cells[J]. Ecotoxicol Environ Saf, 2021, 208: 111743. DOI:10.1016/j.ecoenv.2020.111743 |
| [22] |
WANG H, WANG A Q, WANG X Q, et al. AMPK/PPAR-γ/NF-κB axis participates in ROS-mediated apoptosis and autophagy caused by cadmium in pig liver[J]. Environ Pollut, 2022, 294: 118659. DOI:10.1016/j.envpol.2021.118659 |
| [23] |
SUN J, YU F, WANG T, et al. The role of DRP1- PINK1-Parkin-mediated mitophagy in early cadmium-induced liver damage[J]. Toxicology, 2022, 466: 153082. DOI:10.1016/j.tox.2021.153082 |
| [24] |
BRANCA J J V, FIORILLO C, CARRINO D, et al. Cadmium-induced oxidative stress: focus on the central nervous system[J]. Antioxidants (Basel), 2020, 9(6): 492. DOI:10.3390/antiox9060492 |
| [25] |
GAO M L, YANG Y J, LV M T, et al. Oxidative stress and DNA damage in zebrafish liver due to hydroxyapatite nanoparticles-loaded cadmium[J]. Chemosphere, 2018, 202: 498-505. DOI:10.1016/j.chemosphere.2018.03.146 |
| [26] |
SKIPPER A, SIMS J N, YEDJOU C G, et al. Cadmium chloride induces DNA damage and apoptosis of human liver carcinoma cells via oxidative stress[J]. Int J Environ Res Public Health, 2016, 13(1): 88. DOI:10.3390/ijerph13010088 |
| [27] |
ZANI A P, ZANI C P, DIN Z U, et al. Dibenzylideneacetone induces apoptosis in cervical cancer cells through Ros-Mediated mitochondrial damage[J]. Antioxidants (Basel), 2023, 12(2): 317. DOI:10.3390/antiox12020317 |
| [28] |
SOUZA-ARROYO V, FABIÁN J J, BUCIO-ORTIZ L, et al. The mechanism of the cadmium-induced toxicity and cellular response in the liver[J]. Toxicology, 2022, 480: 153339. DOI:10.1016/j.tox.2022.153339 |
| [29] |
SAMARGHANDIAN S, AZIMI-NEZHAD M, SHABESTARI M M, et al. Effect of chronic exposure to cadmium on serum lipid, lipoprotein and oxidative stress indices in male rats[J]. Interdiscip Toxicol, 2015, 8(3): 151-154. DOI:10.1515/intox-2015-0023 |
| [30] |
WANG Y W, JI X Q, DAI S Y, et al. Cadmium induced redistribution of cholesterol by upregulating ABCA1 and downregulating OSBP[J]. J Inorg Biochem, 2018, 189: 199-207. DOI:10.1016/j.jinorgbio.2018.09.016 |
| [31] |
GU J, KONG A Q, GUO C Z, et al. Cadmium perturbed lipid profile and induced liver dysfunction in mice through phosphatidylcholine remodeling and promoting arachidonic acid synthesis and metabolism[J]. Ecotoxicol Environ Saf, 2022, 247: 114254. DOI:10.1016/j.ecoenv.2022.114254 |
| [32] |
ALSHEHRI A S, EL-KOTT A F, EL-KENAWY A E, et al. Cadmium chloride induces non-alcoholic fatty liver disease in rats by stimulating miR-34a/SIRT1/FXR/p53 axis[J]. Sci Total Environ, 2021, 784: 147182. DOI:10.1016/j.scitotenv.2021.147182 |
| [33] |
OBENG E. Apoptosis (programmed cell death) and its signals-A review[J]. Braz J Biol, 2021, 81(4): 1133-1143. DOI:10.1590/1519-6984.228437 |
| [34] |
WANG J C, ZHU H L, LIU X Z, et al. Oxidative stress and Ca2+ signals involved on cadmium-induced apoptosis in rat hepatocyte[J]. Biol Trace Elem Res, 2014, 161(2): 180-189. DOI:10.1007/s12011-014-0105-6 |
| [35] |
LI K D, GUO C Z, RUAN J C, et al. Cadmium disrupted ER Ca2+ homeostasis by inhibiting SERCA2 expression and activity to induce apoptosis in renal proximal tubular cells[J]. Int J Mol Sci, 2023, 24(6): 5979. DOI:10.3390/ijms24065979 |
| [36] |
WANG J C, DING L L, WANG K, et al. Role of endoplasmic reticulum stress in cadmium-induced hepatocyte apoptosis and the protective effect of quercetin[J]. Ecotoxicol Environ Saf, 2022, 241: 113772. DOI:10.1016/j.ecoenv.2022.113772 |
| [37] |
AHAMED M, AKHTAR M J, ALHADLAQ H A. Influence of silica nanoparticles on cadmium-induced cytotoxicity, oxidative stress, and apoptosis in human liver HepG2 cells[J]. Environ Toxicol, 2020, 35(5): 599-608. DOI:10.1002/tox.22895 |
| [38] |
ZHANG R X, YI R, BI Y J, et al. The effect of selenium on the Cd-induced apoptosis via NO-mediated mitochondrial apoptosis pathway in chicken liver[J]. Biol Trace Elem Res, 2017, 178(2): 310-319. DOI:10.1007/s12011-016-0925-7 |
| [39] |
ARAB-NOZARI M, MOHAMMADI E, SHOKRZADEH M, et al. Co-exposure to non-toxic levels of cadmium and fluoride induces hepatotoxicity in rats via triggering mitochondrial oxidative damage, apoptosis, and NF-κB pathways[J]. Environ Sci Pollut Res Int, 2020, 27(19): 24048-24058. DOI:10.1007/s11356-020-08791-4 |
| [40] |
LIU C, ZHU Y H, LU Z X, et al. Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway[J]. Int J Environ Res Public Health, 2019, 17(1): 138. DOI:10.3390/ijerph17010138 |
| [41] |
HAO R L, GE J L, REN Y F, et al. Caffeic acid phenethyl ester mitigates cadmium-induced hepatotoxicity in mice: role of miR-182-5p/TLR4 axis[J]. Ecotoxicol Environ Saf, 2021, 207: 111578. DOI:10.1016/j.ecoenv.2020.111578 |
| [42] |
WANG C C, SI L F, GUO S N, et al. Negative effects of acute cadmium on stress defense, immunity, and metal homeostasis in liver of zebrafish: The protective role of environmental zinc dpre-exposure[J]. Chemosphere, 2019, 222: 91-97. DOI:10.1016/j.chemosphere.2019.01.111 |
| [43] |
GERASIMENKO T N, SENYAVINA N V, ANISIMOV N U, et al. A model of cadmium uptake and transport in caco-2 cells[J]. Bull Exp Biol Med, 2016, 161(1): 187-192. DOI:10.1007/s10517-016-3373-7 |
| [44] |
FUJIE T, ITO K, OZAKI Y, et al. Induction of ZIP8, a ZIP transporter, via NF-κB signaling by the activation of IκBα and JNK signaling in cultured vascular endothelial cells exposed to cadmium[J]. Toxicol Appl Pharmacol, 2022, 434: 115802. DOI:10.1016/j.taap.2021.115802 |
| [45] |
KWOK M L, LI Z P, LAW T Y S, et al. Promotion of cadmium uptake and cadmium-induced toxicity by the copper transporter CTR1 in HepG2 and ZFL cells[J]. Toxicol Rep, 2020, 7: 1564-1570. DOI:10.1016/j.toxrep.2020.11.005 |
| [46] |
HIRAO-SUZUKI M, TAKEDA S, SAKAI G, et al. Cadmium-stimulated invasion of rat liver cells during malignant transformation: Evidence of the involvement of oxidative stress/TET1-sensitive machinery[J]. Toxicology, 2021, 447: 152631. DOI:10.1016/j.tox.2020.152631 |
| [47] |
STOCKWELL B R. Ferroptosis turns 10:emerging mechanisms, physiological functions, and therapeutic applications[J]. Cell, 2022, 185(14): 2401-2421. DOI:10.1016/j.cell.2022.06.003 |
| [48] |
LIN F Y, CHEN W Y, ZHOU J H, et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury[J]. Cell Death Dis, 2022, 13(3): 271. DOI:10.1038/s41419-022-04708-w |
| [49] |
LI K, XU K, HE Y, et al. Functionalized Tumor-targeting nanosheets exhibiting Fe(Ⅱ) overloading and GSH consumption for Ferroptosis activation in liver tumor[J]. Small, 2021, 17(40): 2102046. DOI:10.1002/smll.202102046 |
| [50] |
陈敬宜, 于淼, 张金洋, 等. 铁死亡参与镉暴露鸡肝损伤的研究[J]. 畜牧兽医学报, 2023, 54(2): 787-802. CHEN J Y, YU M, ZHANG J Y, et al. Study on the involvement of Ferroptosis in liver injury of cadmium-exposed chickens[J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(2): 787-802. (in Chinese) |
| [51] |
HE Z Q, SHEN P, FENG L J, et al. Cadmium induces liver dysfunction and ferroptosis through the endoplasmic stress-ferritinophagy axis[J]. Ecotoxicol Environ Saf, 2022, 245: 114123. DOI:10.1016/j.ecoenv.2022.114123 |
| [52] |
WU Q B, CHEN Z H, DING Y, et al. Protective effect of traditional Chinese medicine on non-alcoholic fatty liver disease and liver cancer by targeting ferroptosis[J]. Front Nutr, 2022, 9: 1033129. DOI:10.3389/fnut.2022.1033129 |
| [53] |
FU Y T, ZHANG J, LIU W B, et al. Gestational cadmium exposure disrupts fetal liver development via repressing estrogen biosynthesis in placental trophoblasts[J]. Food Chem Toxicol, 2023, 176: 113807. DOI:10.1016/j.fct.2023.113807 |
| [54] |
ZHANG H L, YAN J, XIE Y, et al. Dual role of cadmium in rat liver: inducing liver injury and inhibiting the progression of early liver cancer[J]. Toxicol Lett, 2022, 355: 62-81. DOI:10.1016/j.toxlet.2021.11.004 |
| [55] |
SUN X H, LV M W, ZHAO Y X, et al. Nano-selenium antagonized cadmium-induced liver fibrosis in chicken[J]. J Agric Food Chem, 2023, 71(1): 846-856. DOI:10.1021/acs.jafc.2c06562 |
| [56] |
ZHANG C, LIN J, GE J, et al. Selenium triggers Nrf2-mediated protection against cadmium-induced chicken hepatocyte autophagy and apoptosis[J]. Toxicol in Vitro, 2017, 44: 349-356. DOI:10.1016/j.tiv.2017.07.027 |
| [57] |
XIONG Z W, YANG F, XU T F, et al. Selenium alleviates cadmium-induced aging via mitochondrial quality control in the livers of sheep[J]. J Inorg Biochem, 2022, 232: 111818. DOI:10.1016/j.jinorgbio.2022.111818 |
| [58] |
ZHANG L W, YANG F, LI Y, et al. The protection of selenium against cadmium-induced mitophagy via modulating nuclear xenobiotic receptors response and oxidative stress in the liver of rabbits[J]. Environ Pollut, 2021, 285: 117301. DOI:10.1016/j.envpol.2021.117301 |
| [59] |
CAO Z Y, YANG F, LIN Y Q, et al. Selenium antagonizes cadmium-induced inflammation and oxidative stress via suppressing the interplay between NLRP3 inflammasome and HMGB1/NF-κB pathway in duck hepatocytes[J]. Int J Mol Sci, 2022, 23(11): 6252. DOI:10.3390/ijms23116252 |
| [60] |
NADERI M, PUAR P, ZONOUZI-MARAND M, et al. A comprehensive review on the neuropathophysiology of selenium[J]. Sci Total Environ, 2021, 767: 144329. DOI:10.1016/j.scitotenv.2020.144329 |
| [61] |
KAYROUZ C M, HUANG J, HAUSER N, et al. Biosynthesis of selenium-containing small molecules in diverse microorganisms[J]. Nature, 2022, 610(7930): 199-204. DOI:10.1038/s41586-022-05174-2 |
| [62] |
ZWOLAK I, ZAPOROWSKA H. Selenium interactions and toxicity: a review[J]. Cell Biol Toxicol, 2012, 28(1): 31-46. DOI:10.1007/s10565-011-9203-9 |
| [63] |
ZHANG J Q, ZHENG S F, WANG S C, et al. Cadmium-induced oxidative stress promotes apoptosis and necrosis through the regulation of the miR-216a-PI3K/AKT axis in common carp lymphocytes and antagonized by selenium[J]. Chemosphere, 2020, 258: 127341. DOI:10.1016/j.chemosphere.2020.127341 |
| [64] |
ALI H F H, EL-SAYED N M, KHODEER D M, et al. Nano selenium ameliorates oxidative stress and inflammatory response associated with cypermethrin-induced neurotoxicity in rats[J]. Ecotoxicol Environ Saf, 2020, 195: 110479. DOI:10.1016/j.ecoenv.2020.110479 |
| [65] |
AL-KAHTANI M, MORSY K. Ameliorative effect of selenium nanoparticles against aluminum chloride-induced hepatorenal toxicity in rats[J]. Environ Sci Pollut Res Int, 2019, 26(31): 32189-32197. DOI:10.1007/s11356-019-06417-y |
| [66] |
VICAS S I, LASLO V, TIMAR A V, et al. Nano selenium-enriched probiotics as functional food products against cadmium liver toxicity[J]. Materials (Basel), 2021, 14(9): 2257. DOI:10.3390/ma14092257 |
| [67] |
JIHEN E H, IMED M, FATIMA H, et al. Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver of the rat: effects on the oxidative stress[J]. Ecotoxicol Environ Saf, 2009, 72(5): 1559-1564. DOI:10.1016/j.ecoenv.2008.12.006 |
| [68] |
ALIM I, CAULFIELD J T, CHEN Y X, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke[J]. Cell, 2019, 177(5): 1262-1279.e25. DOI:10.1016/j.cell.2019.03.032 |
| [69] |
INGOLD I, BERNDT C, SCHMITT S, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis[J]. Cell, 2018, 172(3): 409-422.e21. DOI:10.1016/j.cell.2017.11.048 |
| [70] |
CONG Y M, CHI Q R, TENG X H, et al. The protection of selenium against cadmium-induced mitochondrial damage via the cytochrome P450 in the livers of chicken[J]. Biol Trace Elem Res, 2019, 190(2): 484-492. DOI:10.1007/s12011-018-1557-x |
| [71] |
YIIN S J, CHERN C L, SHEU J Y, et al. Cadmium-induced liver, heart, and spleen lipid peroxidation in rats and protection by selenium[J]. Biol Trace Elem Res, 2000, 78(1/3): 219-230. |
| [72] |
EL-BOSHY M E, RISHA E F, ABDELHAMID F M, et al. Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats[J]. J Trace Elem Med Biol, 2015, 29: 104-110. DOI:10.1016/j.jtemb.2014.05.009 |
| [73] |
WANG Y, CHEN H W, CHANG W H, et al. Protective effects of selenium yeast against cadmium-induced necroptosis via inhibition of oxidative stress and MAPK pathway in chicken liver[J]. Ecotoxicol Environ Saf, 2020, 206: 111329. DOI:10.1016/j.ecoenv.2020.111329 |
| [74] |
王跃. 富硒酵母联合维生素E减轻镉诱导小鼠肝损伤的研究[D]. 宜春: 宜春学院, 2022: 1-26. WANG Y. Selenium-enriched yeast combined with vitamin E attenuates cadmium-induced liver injury in mice[D]. Yichun: Yichun University, 2022: 1-26. (in Chinese) |
| [75] |
赵鸣飞. 姜黄素—硒配合物的合成及对镉染毒大鼠肝损伤的干预作用[D]. 唐山: 华北理工大学, 2015: 1-40. ZHAO M F. Synthesis of curcumin-selenium coordination compound and intervention on liver injury exposed to Cadmiun in rats[D]. Tangshan: North China University of Science and Technology, 2015: 1-40. (in Chinese) |
| [76] |
LI J L, JIANG C Y, LI S, et al. Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium[J]. Ecotoxicol Environ Saf, 2013, 96: 103-109. DOI:10.1016/j.ecoenv.2013.07.007 |
| [77] |
SLENCU B G, CIOBANU C, CUCIUREANU R, et al. Protective effects of selenium on hepatotoxicity caused by subacute experimental combined exposure to cadmium and lead in rats[J]. Farmacia, 2018, 66(5): 866-876. DOI:10.31925/farmacia.2018.5.18 |
| [78] |
ZHANG C, GE J, LV M W, et al. Selenium prevent cadmium-induced hepatotoxicity through modulation of endoplasmic reticulum-resident selenoproteins and attenuation of endoplasmic reticulum stress[J]. Environ Pollut, 2020, 260: 113873. DOI:10.1016/j.envpol.2019.113873 |
| [79] |
ZHANG R X, LIU Y H, XING L, et al. The protective role of selenium against cadmium-induced hepatotoxicity in laying hens: Expression of Hsps and inflammation-related genes and modulation of elements homeostasis[J]. Ecotoxicol Environ Saf, 2018, 159: 205-212. DOI:10.1016/j.ecoenv.2018.05.016 |
| [80] |
ABU-EL-ZAHAB H S H, HAMZA R Z, MONTASER M M, et al. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice[J]. Ecotoxicol Environ Saf, 2019, 173: 419-428. DOI:10.1016/j.ecoenv.2019.02.041 |
| [81] |
WANG Y, LIU J F, CHEN R, et al. The antagonistic effects of selenium yeast (SeY) on cadmium-induced inflammatory factors and the heat shock protein expression levels in chicken livers[J]. Biol Trace Elem Res, 2020, 198(1): 260-268. DOI:10.1007/s12011-020-02039-5 |
| [82] |
ZOIDIS E, PAPADOMICHELAKIS G, PAPPAS A C, et al. Effects of selenium and cadmium on breast muscle fatty-acid composition and gene expression of liver antioxidant proteins in broilers[J]. Antioxidants (Basel), 2019, 8(5): 147. DOI:10.3390/antiox8050147 |
(编辑 范子娟)



