2. 青海大学畜牧兽医科学院, 西宁 810016;
3. 内蒙古农业大学动物科学学院, 呼和浩特 010018
2. Academy of Animal Science and Veterinary Medicine, Qinghai University, Xining 810016, China;
3. College of Animal Science, Inner Mongolia Agricultural University, Huhhot 010018, China
动物皮毛颜色是畜牧生产中的重要性状,更是育种工作中品种鉴定、品系划定的关键依据[1-2],因此探究动物毛色形成机制意义重大。本质上,动物肤色和毛色与皮肤中黑色素细胞(melanin cell,MC)数量和黑色素种类(真黑色素和褐黑色素)密切相关[3]。黑色素细胞起源于神经嵴细胞[4],由背外侧路径迁移至外胚层定植,并增殖分化为成熟黑色素细胞。黑色素细胞中黑素小体合成的色素颗粒由胞吐等方式运输至周围细胞[5],发挥吸收散射紫外线和清除自由基等作用[6-7]。黑色素生成涉及复杂的调控过程,受多个基因、转录因子、非编码RNA等的影响[8-9]。大量研究表明,酪蛋白酶(tyrosinase,TYR)、酪蛋白酶相关蛋白酶1(tyrosinase related protein 1,TYRP1)、酪蛋白酶相关蛋白酶2(tyrosinase related protein 2,TYRP2)、小眼畸形相关转录因子(microphthalmia-associated transcription factor,MITF)等基因在黑色素生成的过程中发挥了重要作用[10]。其中TYR编码的蛋白是黑色素合成过程的限速酶,可将机体内酪氨酸氧化形成L-二羟基苯丙氨酸(L-dihydroxyphenylalanine,L-DOPA),TYRP1、TYRP2则与其协同作用,共同参与黑色素的生成[11-12]。MITF是调控哺乳动物黑色素沉积的重要转录因子,与黑色素细胞的发育、存活、迁移、增殖及分化有关,是多条黑色素合成相关信号通路的共同作用位点[13-14]。MITF基因参与黑色素合成主要的信号通路有Wnt/β-catenin、EDN-1/EDNRB、SCF/c-kit、NO等[15]。
近年来,越来越多的miRNA被证实参与调控哺乳动物毛色的形成。Tian等[16]通过测序筛选鉴定出了48个在棕色和白色羊驼皮肤组织中差异表达的miRNAs,Wu等[17]对被毛颜色为白色和黑色的山羊毛囊组织进行miRNA测序,筛选鉴定出6个差异表达miRNAs,其中5个在黑毛毛囊中表达上调,暗示这些差异miRNA可能参与了毛色或肤色的调控。进一步研究发现,miR-125b-5p能够通过靶向MITF下调黑色素的生成[18]。此外,Wu等[19]在小鼠皮肤中注射pre-miR-434-5p后发现皮肤中TYR表达量显著下调,黑色素合成过程被抑制,导致小鼠毛发颜色变白。Dong等[20]研究也证实,miR-137过表达会导致小鼠毛色由黑变黄。
本试验通过对肤色有差异的酉州乌羊(Youzhou dark goat,YZDG)和川东白山羊(Chuandong white goat,CDWG),毛色有差异的大足黑山羊(Dazu black goat,DBG)和成年内蒙古绒山羊(Inner Mongolia cashmere goat,IMCG)分别进行小RNA测序,取两组测序差异miRNA交集,筛选黑色素生产的核心miRNA。并以B16-F10细胞为模型,以共同差异miR-129-5p为对象,通过转染其模拟物(mimics)、抑制剂(inhibitor)及相应对照组(mimics NC、inhibitor NC)进一步探究miRNA在细胞黑色素生成中的作用。
1 材料与方法 1.1 主要试剂苏木素-伊红染色试剂盒(Sigma,中国),FBS(Thermo,美国),RPMI 1640(Thermo,美国),Lipofectamine 2000(Thermo,美国),Trizol(TaKaRa,日本),PrimeScriptTM RT Master Mix(TaKaRa,日本),MiR-XTM miRNA FirstStrand Synthesis(TaKaRa,日本),TB Green Premix Ex TaqII(TaKaRa,日本),RIPA蛋白裂解液(康为世纪,中国),MITF、TYR、TYRP1一抗(Bioss ANTIBODIES,中国),辣根过氧化物酶标记山羊抗兔IgG(H+L)(碧云天,中国),黑色素标准品(Aladdin,中国)。
1.2 样本采集选择相同饲养环境下健康、纯种酉州乌羊(n=3)和川东白山羊(n=3)妊娠母羊,在其妊娠期第100天进行剖腹产手术,采集胎儿肩胛骨后缘3~5 cm处皮肤组织。选择相同饲养环境下2~3周岁的大足黑山羊(n=3)和内蒙古绒山羊(n=3),手术采集山羊左侧肩胛骨后缘5~10 cm处皮肤样本。将采集的每一份皮肤组织剪成两份,一份经液氮保存后用于小RNA测序,一份保存在含4%多聚甲醛离心管中用于组织切片试验。
细胞试验部分所用的B16-F10皮肤黑色素瘤细胞系来自重庆市畜牧科学院。
1.3 皮肤切片制作4%多聚甲醛固定的皮肤样品经梯度浓度酒精(75%、85%、90%、95%和100%)脱水、二甲苯透明后进行石蜡包埋。利用切片机进行平行于皮肤表面的横切和垂直于皮肤表面的纵切,切片厚度为7 μm。切片用常规苏木精-伊红(hematoxylin-eosin,HE)染色,中性树胶封片,于倒置显微镜下观察组织学形态并捕获组织学图像。
1.4 小RNA测序及分析取液氮冻存的皮肤样本,研磨后使用Trizol提取总RNA。利用1%琼脂糖凝胶电泳检测RNA完整度及污染,Nanodrop检测RNA浓度及纯度。检测合格的RNA样品经打断、末端修复、加A尾、接头和PCR富集等过程构建文库后,于Illumina NovaSeq 6000测序平台进行测序。对原始测序数据进行质量控制后,将对比上参考基因组的reads序列和数据库miRBase(v22)中成熟miRNA序列进行比对,并利用miRDeep2软件进行新miRNA的预测。使用FPKM[21]法对表达量进行归一化,以获得miRNA相对表达量。基于筛选条件P-value < 0.05、FC≥1.5或FC≤0.66获得差异miRNAs。利用Venn分析,获得两个测序数据集的差异miRNA交集,以筛选关键miRNAs。
1.5 B16-F10细胞复苏与转染冻存的B16-F10细胞37 ℃水浴复苏,1 500 r·min-1离心5 min,弃去冻存液。使用预热37 ℃的PBS清洗3次,10% FBS的RPMI 1640培养基重悬,细胞接种到培养基及六孔板中,在37 ℃、5% CO2培养箱中培养。每48 h换液。六孔板中培养细胞融合度达到70%左右时进行转染处理,转染的模拟物及抑制剂终浓度为33 nmol·L-1,初次更换培养基时间记为0 h,分别继续培养18、27和36 h后,用qPCR检测转染效率,以寻找最高转染效率的时间。
1.6 总RNA提取、第一链合成及引物设计使用Trizol提取细胞总RNA,使用核酸测定仪检测RNA浓度和OD260 nm/OD280 nm值,对RNA完整性进行琼脂糖凝胶电泳检测,用无酶水调整RNA浓度至500 ng·μL-1,使用相应反转录试剂盒进行mRNA及miRNA反转录。利用NCBI和Primer Premier 5.0设计β-actin、TYR、MITF、TYRP1和miR-129-5p引物序列,交由华大公司合成,U6及miRNAs下游通用引物均来自于miRNA反转试剂盒。引物序列见表 1。
|
|
表 1 引物序列 Table 1 The sequence of primers |
以相应cDNA为模板进行PCR反应,反应总体系为10 μL:包含TB Green Premix Ex TaqII 5 μL,cDNA 1 μL,上、下游引物各0.4 μL和ddH2O 3.2 μL。在Biorad CFX96实时荧光定量系统中执行热循环反应:95 ℃预变性30 s;95 ℃ 5 s,59 ℃退火30 s,共循环40次。基于定量系统自动生成的阈值线,获得Ct值。基于参考基因,应用2-ΔΔCt法计算mRNA及miRNA的相对表达量。依据qPCR产物的熔解曲线判断循环反应的特异性。
1.8 Western blotting检测黑色素相关蛋白转染处理的B16-F10细胞,使用蛋白酶混合物及RIPA裂解液提取总蛋白。蛋白样品通过10% SDS-PAGE分离并转移到PVDF膜上。使用5%脱脂奶粉封闭1 h,TBST摇晃清洗3次(5 min·次-1),一抗(MITF、TYR、TYRP1)按比例稀释后常温孵育1 h,4 ℃孵育过夜。再次使用TBST清洗PVDF膜3次后,二抗(辣根过氧化物酶标记山羊抗兔IgG(H+L))孵育2 h,孵育结束后TBST清洗二抗,并进行显影。
1.9 黑色素含量测定黑色素标准曲线制作:称量黑色素标准品,用1 mol·L-1的NaOH液将其配制成2 mg·mL-1的黑色素标准液;标准液经梯度稀释后,获得不同浓度(20、40、60、80、100 μg·mL-1)黑色素溶液;在酶标仪上测定各吸光值,以制作成标准曲线。在待测的黑色素细胞沉淀中加入适量1 mol·L-1 NaOH于37 ℃金属浴中孵育1 h充分裂解,然后测定其在A500处的吸光值,用黑色素标准曲线计算黑色素含量,每个样品设置3个技术重复。
1.10 统计学分析本研究结果用SPSS 25.0软件进行单因素方差分析(One-way ANOVA),用LSD法进行多重比较。试验结果以“平均值±标准误”表示。
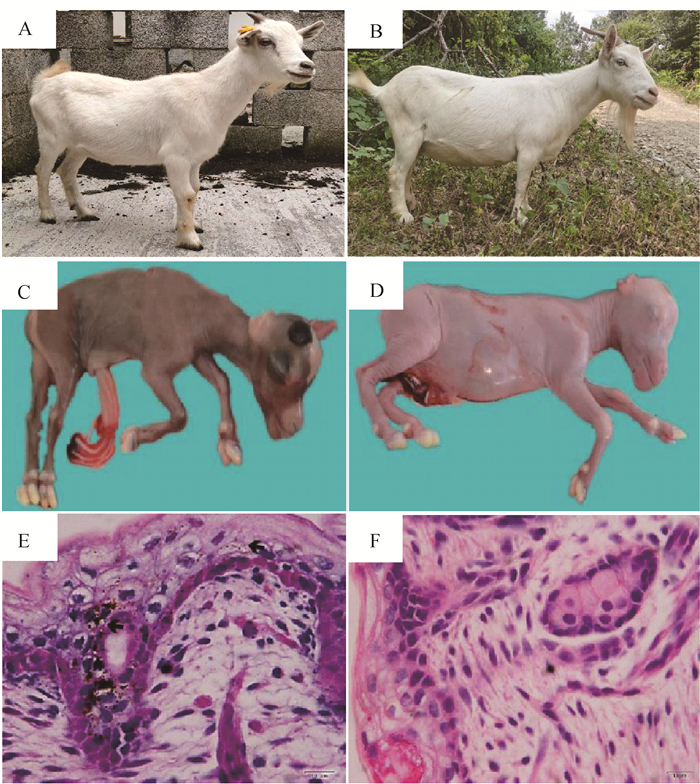
2 结果 2.1 黑色素含量在山羊皮肤组织中差异表达观察发现,两个山羊品种YZDG和CDWG的被毛以白色为主(图 1A-B),但YZDG胎羊全身皮肤为乌色(图 1C)、CDWG肤色为白色(图 1D)。皮肤切片染色结果显示,YZDG胎羊皮肤中可明显观察到黑色素颗粒沉积,而CDWG皮肤中无明显黑色素颗粒存在(图 1E-F)。
|
A、B.YZDG及CDWG品种照;C、D.100日龄YZDG及CDWG胎羊;E、F.100日龄YZDG及CDWG胎羊皮肤HE染色切片(400×) A, B. The representative breed photographs of YZDG and CDWG; C, D. 100-day-old YZDG and CDWG fetal; E, F. HE-stained sections of skin from 100-day-old YZDG and CDWG fetal(400×) 图 1 酉州乌羊和川东白山羊的皮毛表型观察 Fig. 1 Dermatologic and skin histological observation of YZDG and CDWG |
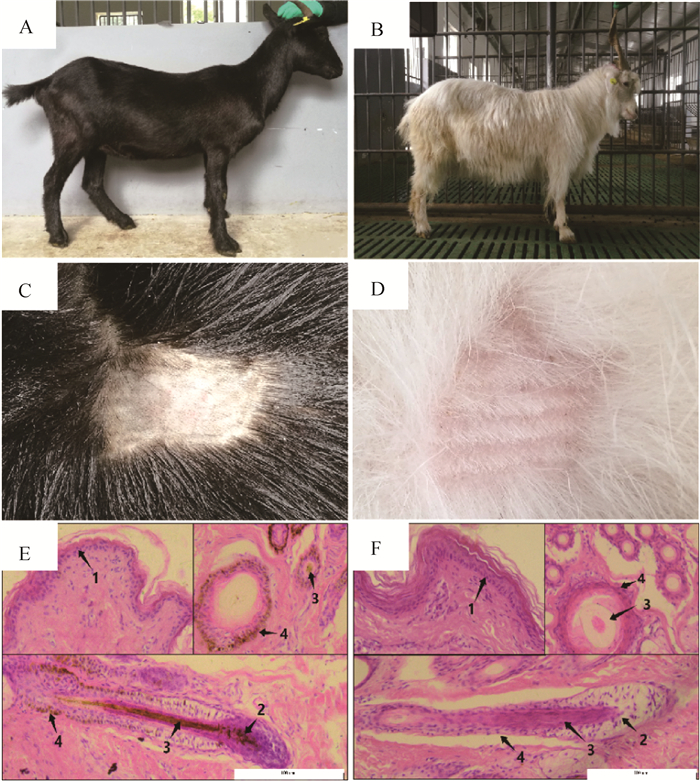
在另外一组对比试验中,DBG黑色被毛、白色皮肤(图 2A、C),而IMCG白色被毛、白色皮肤(图 2B、D)。皮肤HE染色结果也显示,DBG及IMCG表皮中皆无明显黑色素颗粒沉积;但在DBG毛囊组织的毛球、毛干及外根鞘等部位沉积量较大,与其黑色被毛的表型一致,IMCG毛囊中几乎没有黑色素沉积(图 2E-F)。
|
A、B.DBG和IMCG品种照;C、D.DBG和IMCG被毛图像;E、F.DBG及IMCG皮肤HE染色切片(1.表皮;2.毛球;3.毛干;4.外根鞘)(200×) A, C. The representative breed photographs of DBG and IMCG; B, D. The skin surface of DBG and IMCG; E, F. HE stained sections of DBG and IMCG skin(1.Epidermis; 2.Bulb; 3.Hair shaft; 4.Out root sheath)(200×) 图 2 大足黑山羊和内蒙古绒山羊的皮毛表型观察 Fig. 2 Dermatologic and skin histological observation of IMCG and DBG |
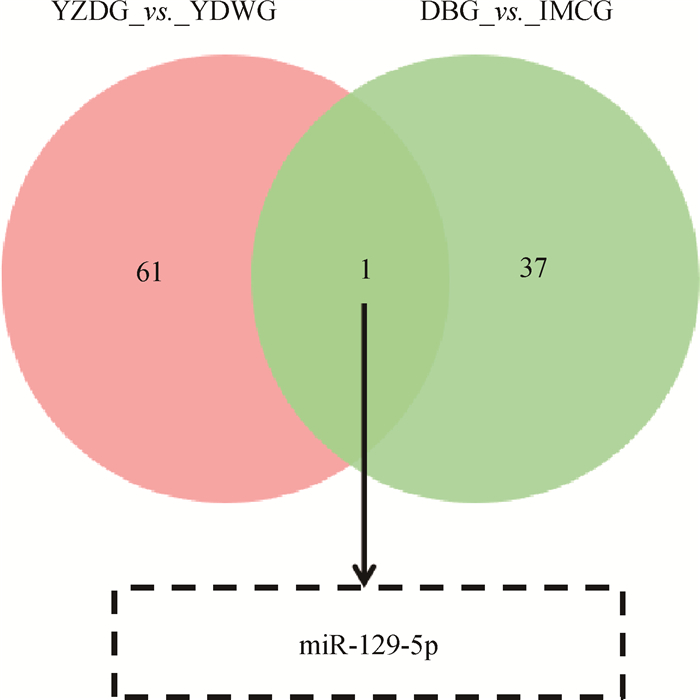
本研究通过对具有肤色差异的YZDG、CDWG,和具有毛色差异的DBG、IMCG分别进行小RNA测序。结果显示,在乌皮和白皮胎羊皮肤样本中,鉴定到62个差异表达miRNAs,其中31个在乌皮山羊皮肤中表达上调,31个在白皮山羊中表达下调,表 2为部分差异miRNAs。在黑色被毛及白色被毛山羊皮肤测序中,筛选到38个差异miRNAs,其中10个在黑色被毛山羊中上调,28个在白色被毛山羊皮肤中下调,表 3为部分差异表达miRNAs。对两组测序数据进行Venn分析,发现miR-129-5p在乌色皮肤及黑色被毛山羊皮肤组织中均高表达(图 3)。故进一步进行细胞试验,以验证miR-129-5p对黑色素生成的作用。
|
|
表 2 酉州乌羊、川东白山羊胎儿皮肤中差异表达miRNAs Table 2 The differentially expressed miRNAs in fetal skin samples of YZDG and CDWG |
|
|
表 3 大足黑山羊、内蒙古绒山羊皮肤中差异表达miRNAs Table 3 The differentially expressed miRNAs in skin samples of DBG and IMCG |
|
图 3 两组小RNA测序的共同差异miRNAs Fig. 3 The overlaping differential miRNAs between two small RNA sequencing data |
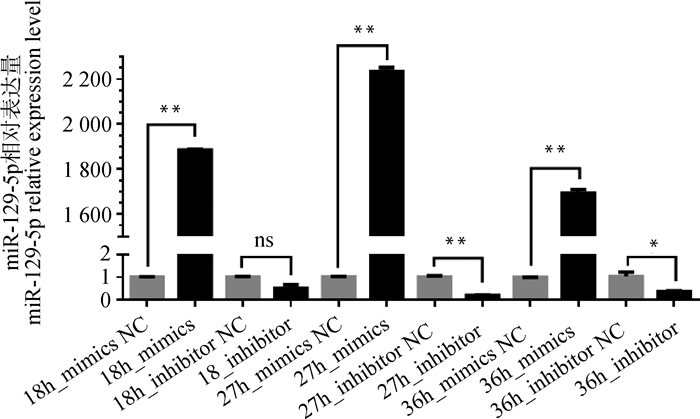
为了确定miR-129-5p对黑色素生成的影响,进行过表达和减低表达miR-129-5p,检测黑色素含量、基因、蛋白表达量的变化情况。转染结果显示,除转染18 h的inhibitor组无显著变化外,其余各转染组都出现相应过表达和减低表达情况(图 4),而在27 h miR-129-5p过表达和减低表达效果最为显著(P < 0.01),故后续试验均以此时间进行。
|
图 4 最佳转染时间筛选 Fig. 4 The time screening of optimal transfection |
为检测转染处理后黑色素生成量变化,使用碱溶法提取细胞黑色素。离心后miR-129-5p mimics组细胞沉淀呈灰黑色,颜色较其它3组深,而其它3组细胞沉淀呈灰色(图 5A),黑色素含量检测结果也显示miR-129-5p mimics组的黑色素含量比NC组高18.9%(P < 0.05);而inhibitor组黑色素含量与其NC组无显著差异(P>0.05)(图 5B)。
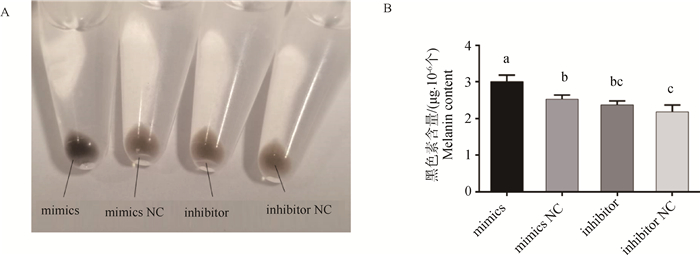
|
A.不同转染组的细胞沉淀;B.不同转染组B16-F10细胞的黑色素相对含量 A. Cell sedimentation in different transfection groups; B. Relative melanin content in B16-F10 cells in different transfection groups 图 5 不同转染组黑色素细胞的黑色素含量 Fig. 5 Melanin content in melanocytes in different transfection groups |
为揭示黑色素含量变化的原因,对转染处理后细胞黑色素生成相关基因表达情况进行检测。结果如图 6所示,与空白对照组相比,miR-129-5p mimics、inhibitor组MITF mRNA相对表达量无显著差异(P>0.05)(图 6A),而mimics组的TYR及TYRP1 mRNA表达量相对空白对照组分别上调57.3%和16.5%(P < 0.05)(图 6B、C),inhibitor组TYR mRNA表达量显著下调38.9%(P < 0.05),而TYRP1 mRNA表达量无显著变化(P>0.05)(图 6B、C)。
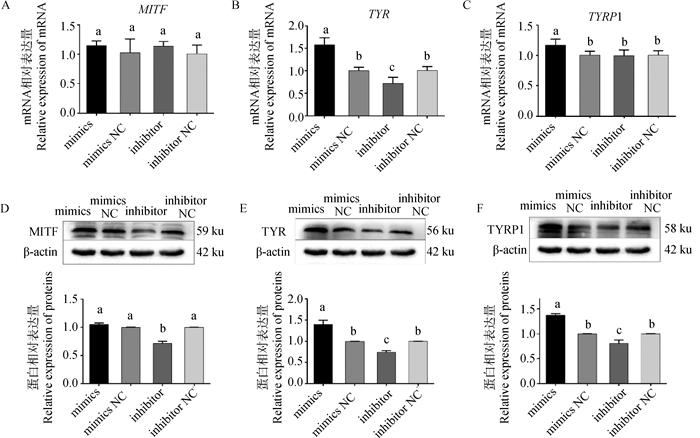
|
A~C.不同转染组MITF、TYR、TYRP1基因相对表达量;D~F.不同转染组MITF、TYR、TYRP1蛋白印迹及蛋白相对表达量 A-C. Relative expression of MITF, TYR, and TYRP1 genes in different transfection groups; D-F. Protein blotting and relative protein expression of MITF, TYR, and TYRP1 in different transfection groups 图 6 转染处理后黑色素相关基因mRNA及蛋白表达 Fig. 6 Melanin-related genes mRNA and proteins expression after transfection treatment |
蛋白定量结果显示,MITF蛋白在inhibitor组中表达量显著下调28.4%(P < 0.05),而mimics组MITF蛋白的表达量没有显著差异(图 6D);mimics组TYR、TYRP1蛋白相较NC组分别显著上调49.2%及40.2%(P < 0.05),inhibitor组TYR、TYRP1蛋白水平则分别下调21.1%及25.3%,差异显著(P < 0.05),两个基因受调控情况一致(图 6E、F)。
3 讨论皮肤中黑色素的沉积量是不同肤色和毛色形成的关键。大量研究指出,黑色表型的绵羊[22]、猪[23]、獭兔以及牦牛[24]等动物皮肤中黑色素沉积量高于白色表型个体。本研究同样发现,黑色素颗粒在乌皮山羊的表皮及黑毛山羊的毛球、毛干、外根鞘等部位大量沉积,但在白皮、白毛山羊的表皮及毛囊组织中沉积量很少,表明黑色素含量与肤色及毛色的深浅呈正相关。而黑色素的沉积不仅涉及调控黑色素细胞的成熟、增殖、迁移和黑色素产生的多个基因及信号通路,还受到miRNAs相互作用形成的复杂调控网络影响[25]。本研究通过分别对肤色及毛色有差异的4个品种山羊进行小RNA测序,发现miR-129-5p是两组测序共同差异miRNA,且在黑色表型个体中高表达。已有研究显示,miR-129-5p广泛参与癌细胞抑制、炎症反应、血管再生和神经发育等生物学过程,已知的靶基因包括高迁移率族蛋白B1(High mobility group protein B1,HMGB1)[26]、同源框C13(Homeobox C13,HOXC13)[27]、转录因子4(Transcription factor 4,TCF4)[28]等,然而在调控黑色素合成方面暂无具体报道。故本研究通过细胞试验进一步验证miR-129-5p对黑色素生成的影响。结果显示,过表达miR-129-5p会显著增加细胞黑色素沉积量,会显著上调TYR、TYRP1等黑色素合成相关基因的mRNA和蛋白表达,对黑色素生成有促进作用,而抑制miR-129-5p则显著下调TYR mRNA及蛋白表达,而TYRP1 mRNA表达水平无显著差异,仅蛋白表达显著下调。前人研究证明,TYR基因家族的3个成员TYR、TYRP1、TYRP2都对黑色素的合成有影响[29-30],并且在动物表皮和毛囊中大量表达[31]。Zhao等[32]研究也发现,在小鼠黑色素细胞中过表达及抑制miR-27a-3p,对靶基因WNT3A mRNA水平无显著影响,但对WNT3A蛋白表达有显著调节作用,且影响细胞黑色素生成。张利环等[33]研究同样证明,miR-96-5p仅调节MITF蛋白表达,但对细胞黑色素生成有显著影响,说明miR-129-5p确实可通过调节TYR与TYRP1基因或蛋白的表达影响黑色素的沉积。但miR-129-5p是以怎样的形式促进黑色素的沉积,仍需进一步探索。
经典的分子生物学理论认为,miRNA主要是通过与胞质中靶基因mRNA的3′UTR的5~8个碱基进行配对,进而抑制靶基因表达,与靶基因表达呈负相关关系。miR-101a-3p和miR-144a-3p等可通过靶向负调控MITF基因下调羊驼皮肤黑色素沉积[34],但本试验中miR-129-5p表达与TYR、TYRP1等黑色素相关基因表达及黑色素沉积量呈正相关。因此推测,miR-129-5p有两种调节黑色素沉积的可能机制,其一是miR-129-5p能通过靶向抑制黑色素生成通路的上游的某个因子,进而促进TYR及TYRP1的表达。Wang等[35]的研究即证明,miR-21a-5p可通过抑制SRY-box转录因子5(SRY-box transcription factor 5,SOX5)基因的表达,使MITF、TYR的mRNA和蛋白水平显著上调,黑色素生成增加。其二是随着研究的深入,科研工作者发现miRNAs不一定是抑制基因表达。如细胞在G0期时,miRNA活性将从翻译抑制转为翻译激活[36],另一报道显示,miR-10a还可以与编码核糖体蛋白(ribosomal protein,RP)的mRNA 5′UTR区结合,增强其表达[37]。除此之外,还有研究表明成熟的miRNA存在于细胞核中[38],且核内miRNAs还被报道能与靶基因启动子、增强子等元件结合以促进基因转录。如miR-205被证实在前列腺癌细胞中能靶向肿瘤抑制因子白介素24(interleukin-24,IL-24)和IL-32启动子中的特定位点激活其表达[39]。miR-24-1也被发现与多个相邻编码基因的增强子结合转录出增强子RNAs(enhancer RNAs,eRNAs),进而增强基因转录[40]。miRNAs的转录激活作用已被大量报道,科学家推测这或许是一种待发掘的疾病治疗新方法。本研究中,miR-129-5p在细胞中过表达后能显著的提高黑色素相关基因TYR、TYRP1等mRNA和蛋白水平的表达,能显著提高细胞黑色素含量,是黑色素合成通路的重要调控因子,但其在调控过程中是否发挥黑色素合成上游基因沉默或下游基因翻译增强、转录激活等作用还需更进一步的研究。
4 结论本研究结果表明,miR-129-5p在乌皮山羊及黑色被毛山羊中高表达,可通过调控TYR、TYRP1等关键基因的表达进而影响黑色素的生成,是山羊肤色和毛色形成过程中的重要调节因子。
| [1] |
常洪. 山羊的毛色遗传[J]. 西安联合大学学报, 1999, 2(2): 1-4. CHANG H. Genetics of hair color in goats[J]. Journal of Xi'an United University, 1999, 2(2): 1-4. (in Chinese) |
| [2] |
杜小龙, 王麒, 张乐超, 等. 山羊DCT基因启动子区甲基化水平、SNP与毛色特征关系研究[J]. 畜牧兽医学报, 2019, 50(2): 271-279. DU X L, WANG Q, ZHANG L C, et al. Study on the relationship between methylation level, SNP in promoter region of DCT gene and hair color in goat[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(2): 271-279. (in Chinese) |
| [3] |
LIANG D, ZHAO P J, SI J F, et al. Genomic analysis revealed a convergent evolution of LINE-1 in coat color: a case study in water buffaloes (Bubalus bubalis)[J]. Mol Biol Evol, 2021, 38(3): 1122-1136. DOI:10.1093/molbev/msaa279 |
| [4] |
LI M N, KNAPP S K, IDEN S. Mechanisms of melanocyte polarity and differentiation: What can we learn from other neuroectoderm-derived lineages?[J]. Curr Opin Cell Biol, 2020, 67: 99-108. DOI:10.1016/j.ceb.2020.09.001 |
| [5] |
KIM J Y, KIM J, AHN Y, et al. Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes[J]. Pigment Cell Melanoma Res, 2020, 33(3): 403-415. DOI:10.1111/pcmr.12838 |
| [6] |
CAO W, ZHOU X H, MCCALLUM N C, et al. Unraveling the Structure and Function of Melanin through Synthesis[J]. J Am Chem Soc, 2021, 143(7): 2622-2637. DOI:10.1021/jacs.0c12322 |
| [7] |
BRENNER M, HEARING V J. The protective role of melanin against UV damage in human skin[J]. Photochem Photobiol, 2008, 84(3): 539-549. DOI:10.1111/j.1751-1097.2007.00226.x |
| [8] |
SCHALLREUTER K U, KOTHARI S, CHAVAN B, et al. Regulation of melanogenesis-controversies and new concepts[J]. Exp Dermatol, 2008, 17(5): 395-404. DOI:10.1111/j.1600-0625.2007.00675.x |
| [9] |
章誉兴, 吴宏, 于黎. 哺乳动物毛色调控机制及其适应性进化研究进展[J]. 遗传, 2021, 43(2): 118-133. ZHANG Y X, WU H, YU L. Progress on coat color regulation mechanism and its association with the adaptive evolution in mammals[J]. Hereditas (Beijing), 2021, 43(2): 118-133. (in Chinese) |
| [10] |
JIA X B, DING P, CHEN S Y, et al. Analysis of MC1R, MITF, TYR, TYRP1, and MLPH genes polymorphism in four rabbit breeds with different coat colors[J]. Animals (Basel), 2021, 11(1): 81. |
| [11] |
LAI X L, WICHERS H J, SOLER-LOPEZ M, et al. Structure and function of human tyrosinase and tyrosinase-related proteins[J]. Chem Eur J, 2018, 24(1): 47-55. DOI:10.1002/chem.201704410 |
| [12] |
ZHOU S H, ZENG H L, HUANG J H, et al. Epigenetic regulation of melanogenesis[J]. Ageing Res Rev, 2021, 69: 101349. DOI:10.1016/j.arr.2021.101349 |
| [13] |
BAUER G L, PRAETORIUS C, BERGSTEINSDÓTTIR K, et al. The role of MITF phosphorylation sites during coat color and eye development in mice analyzed by bacterial artificial chromosome transgene rescue[J]. Genetics, 2009, 183(2): 581-594. DOI:10.1534/genetics.109.103945 |
| [14] |
CHEN T Z, ZHAO B L, LIU Y, et al. MITF-M regulates melanogenesis in mouse melanocytes[J]. J Dermatol Sci, 2018, 90(3): 253-262. DOI:10.1016/j.jdermsci.2018.02.008 |
| [15] |
D'MELLO S A N, FINLAY G J, BAGULEY B C, et al. Signaling pathways in melanogenesis[J]. Int J Mol Sci, 2016, 17(7): 1144. DOI:10.3390/ijms17071144 |
| [16] |
TIAN X, JIANG J B, FAN R W, et al. Identification and characterization of microRNAs in white and brown alpaca skin[J]. BMC Genomics, 2012, 13: 555. DOI:10.1186/1471-2164-13-555 |
| [17] |
WU Z Y, FU Y H, CAO J H, et al. Identification of differentially expressed miRNAs between white and black hair follicles by RNA-sequencing in the goat (Capra hircus)[J]. Int J Mol Sci, 2014, 15(6): 9531-9545. DOI:10.3390/ijms15069531 |
| [18] |
WANG X C, WU Y F, DU P, et al. Study on the mechanism of miR-125b-5p affecting melanocyte biological behavior and melanogenesis in vitiligo through regulation of MITF[J]. Dis Markers, 2022, 2022: 6832680. |
| [19] |
WU D T, CHEN J S, CHANG D C, et al. Mir-434-5p mediates skin whitening and lightening[J]. Clin Cosmet Investig Dermatol, 2008, 1: 19-35. |
| [20] |
DONG C S, WANG H D, XUE L L, et al. Coat color determination by miR-137 mediated down-regulation of microphthalmia-associated transcription factor in a mouse model[J]. RNA, 2012, 18(9): 1679-1686. DOI:10.1261/rna.033977.112 |
| [21] |
DJEBALI S, DAVIS C A, MERKEL A, et al. Landscape of transcription in human cells[J]. Nature, 2012, 489(7414): 101-108. DOI:10.1038/nature11233 |
| [22] |
SHI X L, WU J P, LANG X, et al. Comparative transcriptome and histological analyses provide insights into the skin pigmentation in Minxian black fur sheep (Ovis aries)[J]. PeerJ, 2021, 9: e11122. DOI:10.7717/peerj.11122 |
| [23] |
YUAN W, QIN H, BI H, et al. Ssc-mir-221-3p regulates melanin production in Xiang pigs melanocytes by targeting the TYRP1 gene[J]. BMC Genomics, 2023, 24(1): 369. DOI:10.1186/s12864-023-09451-w |
| [24] |
唐朋, 凌笑笑, 高泽成, 等. 不同毛色牦牛皮肤组织学观察及MC1R基因功能验证[J]. 畜牧兽医学报, 2018, 49(7): 1377-1386. TANG P, LIN X X, GAO Z C, et al. Skin histological observation of yak with different coat colors and MC1R functional verification[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(7): 1377-1386. (in Chinese) |
| [25] |
HUSHCHA Y, BLO I, OTON-GONZALEZ L, et al. microRNAs in the regulation of melanogenesis[J]. Int J Mol Sci, 2021, 22(11): 6104. DOI:10.3390/ijms22116104 |
| [26] |
TANG P, ZHOU J J, LIU H G, et al. Depletion of lncRNA MEG3 ameliorates imatinib-induced injury of cardiomyocytes via regulating miR-129-5p/HMGB1 axis[J]. Anal Cell Pathol (Amst), 2023, 2023: 1108280. |
| [27] |
YAO F, ZHAO B H, HU S S, et al. miR-129-5p participates in hair follicle growth by targeting HOXC13 in rabbit[J]. Genes (Basel), 2022, 13(4): 679. DOI:10.3390/genes13040679 |
| [28] |
YIN C, TIAN Y, YU Y, et al. miR-129-5p inhibits bone formation through TCF4[J]. Front Cell Dev Biol, 2020, 8: 600641. DOI:10.3389/fcell.2020.600641 |
| [29] |
MO X, KAZMI H R, PRESTON-ALP S, et al. Interferon-gamma induces melanogenesis via post-translational regulation of tyrosinase[J]. Pigment Cell Melanoma Res, 2022, 35(3): 342-355. DOI:10.1111/pcmr.13036 |
| [30] |
HELSING P, NYMOEN D A, ROOTWELT H, et al. MC1R, ASIP, TYR, and TYRP1 gene variants in a population-based series of multiple primary melanomas[J]. Genes Chromosomes Cancer, 2012, 51(7): 654-661. DOI:10.1002/gcc.21952 |
| [31] |
赵若阳, 李超, 图格琴, 等. 越背花毛蒙古马皮肤组织中色素生成相关基因TYR、TYRP1及DCT的表达分析[J]. 农业生物技术学报, 2019, 27(6): 1042-1050. ZHAO R Y, LI C, TU G Q, et al. Expression analysis of melanogenesis related genes TYR, TYRP1 and DCT in Tobiano Mongolian Horse (Equus caballus) skin tissue[J]. Journal of Agricultural Biotechnology, 2019, 27(6): 1042-1050. (in Chinese) |
| [32] |
ZHAO Y Y, WANG P C, MENG J Z, et al. MicroRNA-27a-3p Inhibits Melanogenesis in Mouse Skin Melanocytes by Targeting Wnt3a[J]. Int J Mol Sci, 2015, 16(5): 10921-10933. |
| [33] |
张利环, 马悦悦, 刘文艳, 等. microRNA-96-5p靶向调控羊驼黑色素细胞中MITF基因的表达[J]. 畜牧兽医学报, 2020, 51(6): 1229-1237. ZHANG L H, MA Y Y, LIU W Y, et al. microRNA-96-5p targets MITF gene in Alpaca melanocytes[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(6): 1229-1237. (in Chinese) |
| [34] |
ZHU Z W, MA Y Y, LI Y, et al. Comparison of miRNA-101a-3p and miRNA-144a-3p regulation with the key genes of alpaca melanocyte pigmentation[J]. BMC Mol Biol, 2019, 20(1): 19. DOI:10.1186/s12867-019-0137-8 |
| [35] |
WANG P C, ZHAO Y Y, FAN R W, et al. MicroRNA-21a-5p functions on the regulation of melanogenesis by targeting Sox5 in mouse skin melanocytes[J]. Int J Mol Sci, 2016, 17(7): 959. DOI:10.3390/ijms17070959 |
| [36] |
VASUDEVAN S, TONG Y C, STEITZ J A. Cell-cycle control of microRNA-mediated translation regulation[J]. Cell Cycle, 2008, 7(11): 1545-1549. DOI:10.4161/cc.7.11.6018 |
| [37] |
ØROM U A, NIELSEN F C, LUND A H. MicroRNA-10a binds the 5'UTR of ribosomal protein mRNAs and enhances their translation[J]. Mol Cell, 2008, 30(4): 460-471. DOI:10.1016/j.molcel.2008.05.001 |
| [38] |
GAGNON K T, LI L D, CHU Y J, et al. RNAi factors are present and active in human cell nuclei[J]. Cell Rep, 2014, 6(1): 211-221. DOI:10.1016/j.celrep.2013.12.013 |
| [39] |
MAJID S, DAR A A, SAINI S, et al. MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer[J]. Cancer, 2010, 116(24): 5637-5649. DOI:10.1002/cncr.25488 |
| [40] |
XIAO M, LI J, LI W, et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger[J]. RNA Biol, 2017, 14(10): 1326-1334. DOI:10.1080/15476286.2015.1112487 |
(编辑 郭云雁)



