家鸡(Gallus gallus domesticus)包括肉鸡(产肉)和蛋鸡(产蛋),在我国具有巨大的农业生产价值。在生产中,鸡蛋是优选蛋白来源之一,含有人类全部的必需氨基酸且所含营养物质易吸收,是最主要的经济来源[1]。蛋鸡产蛋全程可分为3个阶段:产蛋前期、高峰期和后期。从产第一颗蛋到产蛋率达到80%之前的这段时间是产蛋前期,在此之后,蛋鸡产蛋率大幅度上升进入产蛋高峰期;高峰期维持一段时间之后,蛋鸡产蛋率会慢慢下降至80%,此时为产蛋后期。如今生产上一般通过调控外在因素来提高蛋鸡产蛋率,然而禽蛋生产仍未达到最优化。蛋鸡过快进入产蛋后期导致蛋鸡的产蛋率从产蛋高峰的90%急速下降到50%~70%。产蛋率下降的主要原因是等级前卵泡数量下降、闭锁和退化卵泡增加,进入等级的排卵前卵泡数量降低,从而导致排卵率降低[2]。在畜禽的研究中,表观遗传调控可能通过激活或者是沉默卵泡发育过程中的关键基因来调控这一过程,因此深入研究表观遗传调控机制可能对提高蛋鸡产蛋率有一定的指导意义。
1 蛋鸡卵泡的发育蛋鸡右侧卵巢在生长过程中发生退化,只有左侧卵巢正常发育并具有生殖能力。性成熟后左侧卵巢由大小不同的卵泡通过卵巢系膜韧带连接成一串葡萄状。卵泡根据发育程度不同分为原始卵泡、初级卵泡、次级卵泡和成熟卵泡。成熟卵泡主要由膜细胞层、颗粒细胞层和卵母细胞层组成,主要成分是卵黄蛋白原(vitellogenin, VTG)、极低密度脂蛋白y (very low-density lipoprotein yolk-targeted, VLDLy) 和活性蛋白(主要包括α、β、γ蛋白)[3]。在卵泡发育过程中,卵泡的不同细胞层也随之增殖分化。膜细胞层在次级卵泡中出现并分化成、内外两层,当卵泡发育至成熟卵泡后变厚,且内、外两层更为明显。在原始卵泡中,颗粒细胞层未分化,为扁平状,在次级卵泡中变为多层,当卵泡发育成成熟卵泡后又变为单层,且体积增大。卵母细胞层一直处于初级卵母细胞状态,直至卵泡发育至成熟卵泡后才发育成次级卵母细胞,之后卵泡破裂,卵母细胞逸出,遗留下来的组织被称为排卵后卵泡(postovulatory follicle, POF)[4]。在完成排卵之后,家禽POF不能像哺乳动物卵泡一样形成黄体,而是开始逐渐退化,并在几天后消失[5]。
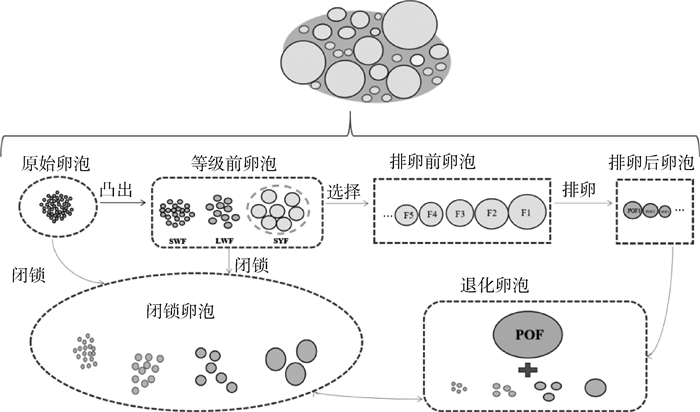
家禽卵泡发育是一个连续且分级的过程,原始卵泡发育后向卵巢皮质表面凸出,形成等级前卵泡,之后经卵泡选择进入等级卵泡期,然后依次进行排卵或卵泡闭锁终止,其发育过程如图 1所示。功能成熟的卵巢中含有数百个等级前卵泡[6],包括小白卵泡(small white follicles, SWFs)、大白卵泡(large white follicles, LWFs)、小黄卵泡(small yellow follicles, SYFs)和5~7个生长中的排卵前卵泡(其按体积顺序划分为FN、…、F4、F3、F2和F1),以及5~7个POF[7-8]。国内外大量研究发现,不同等级卵泡分类有着不同的方法,具体见表 1。在产蛋高峰期时,生殖活跃的母鸡的卵巢每天募集1个SYF发育至等级卵泡[9],等级卵泡继续快速生长发育,从F6卵泡发育到F1卵泡,直到排卵。然而母鸡一生中超过95%的卵泡没有发育至成熟排卵,它们在发育过程中发生退化、解体,最后被吸收[10]。鸡卵泡的生长发育在很大程度上是通过卵黄的积累完成的。卵黄蛋白通过血管化的卵泡细胞膜层进入卵泡后,穿过基膜,通过颗粒细胞(granulosa cells, GCs)之间的紧密连接进入卵母细胞[11],该过程是由极低密度脂蛋白(very low-density lipoprotein, VLDL)受体介导的胞吞作用完成的[12]。
|
图 1 家禽卵泡发育过程 Fig. 1 The process of follicular development in poultry |
|
|
表 1 卵泡的分类 Table 1 Classification of follicles |
当母鸡产蛋率到达产蛋后期时,母鸡卵巢中大多数卵泡(等级前卵泡)发生卵泡闭锁。细胞凋亡使禽类卵泡发生闭锁,且主要是颗粒层细胞的凋亡[20]。但最近的研究表明,自噬在卵泡发育中也起着重要的作用[21]。自噬是指在自噬基因的调控下自身细胞成分和受损细胞器受到溶酶体降解的过程,是生殖细胞的一种自我保护机制[22-23]。研究发现,卵泡刺激素(follicle-stimulating hormone, FSH)通过低氧诱导因子1α(hypoxic-inducible factor-1α, HIF-1α)信号诱导卵巢GCs自噬来促进卵泡闭锁[24],然而FSH也可以抑制卵巢GCs自噬来增强细胞活力[25]。因此,细胞凋亡和自噬对于蛋鸡卵泡发育至关重要,这将确保蛋鸡在产蛋期间有足够的卵泡可发育成成熟卵泡。
2 影响蛋鸡卵泡发育的主要因素蛋鸡卵泡发育过程受到许多内在和外在因素的调控,内在因素主要包括激素及细胞因子的调节,外在因素主要是营养水平的调节和氧化应激。
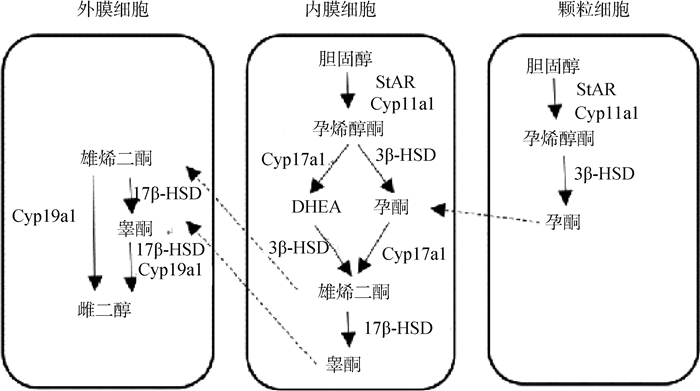
2.1 内在因素对卵泡发育的调节2.1.1 激素对卵泡发育的调节 鸡的卵泡发育受下丘脑-垂体-卵巢(hypothalamic-pituitary-ovarian, HPO)轴上的多种激素共同调控。下丘脑分泌的促性腺激素释放激素(gonadotrophin releasing hormone, GnRH)主要是通过控制促性腺激素的分泌而发挥对卵泡发育的调节作用[26]。垂体分泌的促性腺激素是黄体生成素(luteinizing hormone, LH)和FSH,主要影响卵巢类固醇激素的合成。在蛋鸡体内,适宜的FSH和LH浓度可以迅速地刺激卵泡生长、发育和排卵,从而增加产蛋量[27]。类固醇激素(如孕激素和雌激素)合成于卵巢和卵泡,是维持卵泡正常发育、增强动物繁殖性能所必需的,其合成途径如图 2所示。孕激素(progesterone, P)有促进排卵的作用。雌激素(estradiol, E2)是禽类雌性动物卵巢发育的关键调节物,具有调节性腺分化和发育、生殖行为及肝脏中卵黄前体物质合成的功能。蛋鸡卵泡发育还受其他内分泌激素的影响,比如胃促生长素(ghrelin)[28]、kisspeptin[29]、瘦素(leptin)[30]等。
2.1.2 细胞因子对卵泡发育的调节 转化生长因子β超家族(transforming growth factor-β, TGF-β)、表皮生长因子(epidermal growth factor, EGF)和其他细胞因子是影响蛋鸡卵泡发育的重要因素[31-32]。在鸡卵泡的发育过程中,Zhou等[33]研究发现TGF-β1可刺激GCs分泌胶原蛋白,促进膜细胞增殖,从而通过细胞间的通讯促进卵泡的发育。EGF可通过参与Smads信号通路、TACE/ADAM17信号通路、MAPK信号通路等多种与卵泡发育相关的信号通路来调控家禽卵泡的发育[34]。因此,这些细胞因子具有调控细胞增殖与分化、类固醇激素生成、卵泡选择、控制排卵率的作用[35]。
2.2 外在因素对卵泡发育的调节2.2.1 蛋鸡营养水平对卵泡发育的调节 日粮能量充足是蛋鸡生殖性能的保障。研究表明,在日粮中保持适当的能量水平可提供足够的能量和营养摄入,以满足其体重增加、骨骼和生殖系统发育的需求[36]。然而,营养过剩会导致鸡体重增加至过肥,引起多囊卵巢综合症[37]、脂肪肝[38]等代谢性疾病,从而导致鸡产蛋率急剧下降甚至死亡,最终造成巨大的经济损失。长期的高糖高脂饮食会造成机体高胰岛素血症和高脂血症,使卵巢卵泡发育受损[39],降低血清中类固醇激素(包括P、E2和睾酮)的水平[40]。相较于自由饮食,8~18周龄蛋鸡进行适当的能量限制(85.97%,2 450 kcal AMEn ·kg-1)可以提高蛋鸡整个产蛋期的蛋重和产蛋量[41]。由此可知,营养水平是蛋鸡产蛋性能的重要影响因素。
2.2.2 环境对卵泡发育的调节 卵巢氧化应激是影响蛋鸡卵泡发育的重要外界因素。Li等[42]研究发现,热应激通过激活FasL/Fas和肿瘤坏死因子(tumor necrosis factor-α, TNF-α)通路诱导细胞凋亡,导致等级卵泡数量减少,闭锁卵泡增加,蛋鸡产蛋率急剧下降,从而降低蛋鸡的产蛋性能。此外,遮光也可以造成蛋鸡卵巢氧化应激,其通过各种途径影响蛋鸡卵泡发育,从而降低其产蛋效率[43]。研究发现,抗氧化剂可以缓解蛋鸡卵巢的氧化应激,从而改善蛋鸡的卵泡发育,提高蛋鸡产蛋性能[44]。这可能是缓解环境对卵泡发育影响的有效措施之一。
3 卵泡发育的表观遗传调控机制动物卵泡的发育涉及基因有序的转录激活和抑制等一系列复杂的生命过程,这对雌性的繁殖性能至关重要。在DNA序列不改变的前提下,表观遗传修饰引起基因表达改变或细胞表型发生变化是卵泡发育中重要的调控机制之一[45]。表观遗传主要通过DNA甲基化、组蛋白修饰、非编码RNA调控、RNA修饰以及染色质重塑等5种方式在转录和转录后水平对卵泡发育相关基因的表达进行调控。
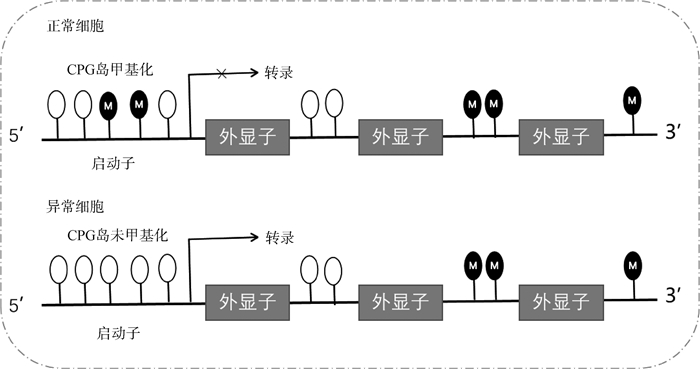
3.1 卵泡发育的DNA甲基化调控DNA甲基化是指在DNA甲基转移酶(DNA methyltransferases, DNMTs)的催化下,以S-腺苷甲硫氨酸(S-adenosine methionine, SAM)作为甲基供体,将胞嘧啶转化为5-甲基胞嘧啶(5-methylcytosine, 5mC)的过程[46]。这是一种在转录水平调控基因表达的表观遗传修饰方式,也是目前了解和研究最多的表观遗传调控机制之一。大量研究发现,DNA甲基化与卵泡发育之间存在密切联系。例如,大麻处理的小鼠卵巢颗粒细胞出现DNA甲基化水平增加,且其中三分之二的DNA甲基化差异位点影响基因的转录[47]。多囊卵巢综合征(polycystic ovarian syndrome, PCOS)患者的血清、卵巢、下丘脑、骨骼肌、脂肪组织均检测到基因的异常DNA甲基化,且这些基因所在的通路与PCOS的胰岛素抵抗、脂质代谢和卵泡发育密切相关[48]。另有研究表明,与类固醇合成相关基因[49-50]及与卵泡凋亡和细胞周期相关基因的异常DNA甲基化会导致卵泡发育异常[51]。DNA甲基化异常甚至可以导致细胞癌变,表现为总体上甲基化水平降低而局部甲基化水平升高[52]。DNA甲基化主要发生在启动子区域CpG岛。CpG差异甲基化区(differential methylated regions, DMRs)是重要的表观遗传修饰标记和参与基因转录的功能区[53]。正常情况下,GCs启动子区域的CpG岛发生甲基化,抑制基因的转录,细胞发育正常,而在卵巢癌中,CpG岛不发生甲基化,下游基因被激活,细胞发育异常(图 3)[54]。因此,蛋鸡也可以作为动物模型来研究人类卵巢癌[55]。
3.2 卵泡发育的组蛋白修饰调控组蛋白修饰也是调控卵泡发育的主要表观调控机制之一,主要通过组蛋白的N端发生乙酰化、甲基化、泛素化、磷酸化等修饰影响基因的转录[56]。其中甲基化和乙酰化修饰为调控蛋白提供附着位点影响染色质的结构和活性。组蛋白乙酰化是一种可逆的动态过程,乙酰化和去乙酰化分别由组蛋白乙酰转移酶和组蛋白去乙酰化酶调控[57]。研究发现,组蛋白乙酰化可促进转录,而去乙酰化可以促进基因沉默或抑制[58]。在鸡的卵泡发育过程中,Li等[59]利用不同等级卵泡的GCs通过ChIP-seq分析得到H3K27ac(活性增强子上的典型组蛋白标记物)图谱,之后通过ATAC-seq和scRNA-seq联合分析,发现基因表达和染色质结构变化是一致的。组蛋白乙酰化也会影响类固醇激素生成相关基因的表达,在小鼠卵巢GCs中,丁酸盐通过H3K9ac调控PPARγ和PGC1α信号通路上基因的表达促进类固醇激素生成[60]。
组蛋白甲基化是最稳定的组蛋白修饰,其中组蛋白H3和H4的赖氨酸(K)侧链上单、双、三甲基化在卵泡和卵母细胞的发育等生理过程中起着至关重要的作用[61-62]。研究表明,当特异性敲除卵母细胞中组蛋白去甲基化酶的编码基因Kdm2a后,其激素敏感性降低,且卵母细胞发育停滞、形态异常增多[63]。此外,衰老引起蛋鸡繁殖能力下降、卵泡发育受损的机制可能与组蛋白甲基化失调有关。在老年小鼠卵泡发育过程中,卵母细胞H3K36me3降低,线粒体凝集增加,细胞凋亡增加导致发情周期缩短,E2浓度降低,卵巢内卵泡数量减少,输卵管上皮组织结构紊乱[64]。
3.3 卵泡发育的非编码RNA调控非编码序列是一种基因转录后表达调控因子,在细胞增殖、分化、凋亡等生理过程中发挥着至关重要的作用[65]。大量关于非编码RNA调控蛋鸡卵泡发育的研究主要集中在miRNA(microRNA)、lnc-RNA(long non-coding RNA)、circRNA(circular RNA)。
miRNA是短链非编码RNA,在转录后调节基因表达。最近,许多研究揭示了卵巢卵泡发育的miRNA调控机制[66]。Song等[67]研究发现,雌性小鼠暴露于多种拟除虫菊酯类杀虫剂后,次级卵泡数量显著减少,闭锁卵泡数量增加,颗粒细胞凋亡增加,通过RNA-seq分析发现其卵巢内miR-152-3p、miR-450b-3p和miR-196a-5p水平显著上调。miRNA在蛋鸡卵巢发育过程中的调控作用也多有研究。相较于低产蛋鸡,高产蛋鸡卵巢的RNA-seq结果显示11个主要参与类固醇激素生物合成的miRNA表达增高,并且另外3个miRNA(gga-miR-34b、gga-miR-34c和gga-miR-216b)参与调控细胞增殖、周期、凋亡等过程[68]。另有研究表明,miR-1a和miR-21的表达量在鸡成熟期和未成熟期的卵巢及不同等级的卵泡中出现极显著变化[69]。gga-miR-449b-5p靶向GF2BP3基因调控鸡卵巢GCs类固醇激素的合成[70],miR-196b-5p表达量的降低会促进GCs的凋亡和抑制GCs的增殖[71],miR-143-3p靶向卵泡刺激素受体,对GCs分化和卵泡发育至关重要[72]。因此,miRNA通过调控卵巢发育和激素生成相关的靶基因发挥作用。
lncRNA是长度超过200个核苷酸,缺乏蛋白质编码功能的长链非编码RNA,在卵泡发育中发挥着不可或缺的作用[73]。据报道,高产蛋鸡(海兰褐)与低产蛋鸡(坝上长尾鸡)卵泡的RNA-seq结果发现了550种差异lncRNA,且这些lncRNA主要参与卵母细胞减数分裂、卵母细胞成熟和细胞周期等生物学过程[74]。卵泡发育抑制因子(inhibitory factor of follicular development, IFFD)是一个与卵泡发育相关的lncRNA,可以通过抑制GCs增殖和E2分泌促进GCs凋亡来抑制卵泡发育[75]。
circRNA是一种新型的非编码RNA,在蛋鸡的卵泡发育上也有相关研究。Wang等[72]对不同光照处理的蛋鸡SYFs构建GCs的circRNA图谱,发现这些circRNA主要富集在卵巢类固醇生成、MAPK和PI3K-Akt信号通路。
3.4 卵泡发育的RNA修饰RNA甲基化修饰包括N6-腺苷酸甲基化(N6-adenylate methylation, m6A)、N1-腺苷酸甲基化(N1-adenylate methylation, m1A)、胞嘧啶羟基化(5-methylcytosine, m5C)。m6A是一种普遍存在的RNA修饰,在细胞活力、增殖、周期中起着重要的调节作用[76]。在2019年,Fan等[77]首次运用高通量测序技术发现m6A在蛋鸡卵泡选择过程中的调控作用。MeRIP-seq的结果发现,m6A甲基化程度在蛋鸡的GCs和膜细胞中存在差异,并且Wnt通路上多个关键基因的mRNA甲基化程度与mRNA表达水平更高,表明m6A修饰可能通过调节Wnt通路发挥其重要作用。m6A修饰对其他动物卵泡发育的调控也有一定研究。m6A可以修饰牦牛卵巢中BNC1、HOMER1、BMP15、BMP6、GPX3和WNT11等与性激素分泌相关基因的mRNA甲基化程度,调节牛卵泡生长发育,影响牦牛的发情周期[78]。在猪的卵泡发育过程中,Cao等[79]对颗粒细胞构建m6A修饰图谱,表明m6A修饰可能调控GCs类固醇生成和卵子生成相关通路,以此来影响卵泡发育。
4 小结综上所述,在DNA序列不发生改变的情况下,表观遗传调控对蛋鸡卵泡发育起着重要的作用。在了解家禽卵泡发育如何受激素、细胞因子、环境及营养等影响的基础上,深入研究家禽卵泡发育的表观遗传调控机制,是后续提高蛋鸡产蛋性能和繁殖性能的重点。目前,表观遗传对蛋鸡卵泡发育的研究基本上都是在颗粒细胞层和颗粒细胞模型上进行的,且主要聚焦在非编码RNA的调控机制。因此,禽类卵泡发育的表观遗传调控机制可以在整体水平上多角度深入研究。可以借鉴小鼠上的研究思路,同时结合新的三代测序、代谢组、单细胞测序和空间转录组等新技术扩展表观遗传对卵泡发育调控机制的新思路。
| [1] |
KRALIK G, KRALIK Z. Poultry products enriched with nutricines have beneficial effects on human health[J]. Med Glas (Zenica), 2017, 14(1): 1-7. |
| [2] |
JOHNSON A L. Regulation of follicle differentiation by gonadotropins and growth factors[J]. Poult Sci, 1993, 72(5): 867-873. DOI:10.3382/ps.0720867 |
| [3] |
张俊楠, 孙志华, 徐桂云. 家禽卵泡发育与调控机制研究进展[J]. 中国家禽, 2021, 43(5): 1-7. ZHANG J N, SUN Z H, XU G Y. Research progress on development and regulatory mechanism of poultry follicles[J]. China Poultry, 2021, 43(5): 1-7. (in Chinese) |
| [4] |
李桂明. 热应激影响蛋鸡等级前卵泡发育的机理研究[D]. 泰安: 山东农业大学, 2020. LI G M. Studies on mechanisms of prehierarchical follicle development affected by heat stress in laying hens[D]. Taian: Shandong Agricultural University, 2020. (in Chinese) |
| [5] |
GURAYA S S, CHALANA R K. Morphology of the post-ovulatory follicle of house sparrow[J]. Acta Biol Acad Sci Hung, 1976, 27(4): 261-267. |
| [6] |
ONAGBESAN O, BRUGGEMAN V, DECUYPERE E. Intra-ovarian growth factors regulating ovarian function in avian species: a review[J]. Anim Reprod Sci, 2009, 111(2-4): 121-140. DOI:10.1016/j.anireprosci.2008.09.017 |
| [7] |
LIN X, LIU X T, GUO C Q, et al. Promotion of the prehierarchical follicle growth by postovulatory follicles involving PGE2-EP2 signaling in chickens[J]. J Cell Physiol, 2018, 233(11): 8984-8995. DOI:10.1002/jcp.26844 |
| [8] |
HRABIA A, SOCHA J K, SECHMAN A. Involvement of matrix metalloproteinases (MMP-2, -7, -9) and their tissue inhibitors (TIMP-2, -3) in the regression of chicken postovulatory follicles[J]. Gen Comp Endocrinol, 2018, 260: 32-40. DOI:10.1016/j.ygcen.2018.02.008 |
| [9] |
JOHNSON A L, WOODS D C. Dynamics of avian ovarian follicle development: cellular mechanisms of granulosa cell differentiation[J]. Gen Comp Endocrinol, 2009, 163(1-2): 12-17. DOI:10.1016/j.ygcen.2008.11.012 |
| [10] |
林欣. 蛋鸡退化卵泡衰退的机制及其功能的研究[D]. 杭州: 浙江大学, 2018. LIN X. Degradation and functions of the regressed ovarian follicles in the laying chicken[D]. Hangzhou: Zhejiang University, 2018. (in Chinese) |
| [11] |
MA Y F, YAO J W, ZHOU S, et al. Enhancing effect of FSH on follicular development through yolk formation and deposition in the low-yield laying chickens[J]. Theriogenology, 2020, 157: 418-430. DOI:10.1016/j.theriogenology.2020.07.012 |
| [12] |
LIN X, MA Y F, QIAN T F, et al. Basic fibroblast growth factor promotes prehierarchical follicle growth and yolk deposition in the chicken[J]. Theriogenology, 2019, 139: 90-97. DOI:10.1016/j.theriogenology.2019.07.025 |
| [13] |
GHANEM K, JOHNSON A L. Proteome profiling of chicken ovarian follicles immediately before and after cyclic recruitment[J]. Mol Reprod Dev, 2021, 88(8): 571-583. DOI:10.1002/mrd.23522 |
| [14] |
GHANEM K, JOHNSON A L. Relationship between cyclic follicle recruitment and ovulation in the hen ovary[J]. Poult Sci, 2019, 98(7): 3014-3021. DOI:10.3382/ps/pez100 |
| [15] |
DONG J, GUO C Q, ZHOU S, et al. Leukemia inhibitory factor prevents chicken follicular atresia through PI3K/AKT and Stat3 signaling pathways[J]. Mol Cell Endocrinol, 2022, 543: 111550. DOI:10.1016/j.mce.2021.111550 |
| [16] |
JIANG B Y, CAO B L, ZHOU Z C, et al. Characterization of chicken α2a-adrenoceptor: molecular cloning, functional analysis, and its involvement in ovarian follicular development[J]. Genes (Basel), 2022, 13(7): 1113. DOI:10.3390/genes13071113 |
| [17] |
STEPHENS C S, HILL-RICCIUTI A, FRANCOEUR L, et al. Feeding level is associated with altered liver transcriptome and follicle selection in hen[J]. Biol Reprod, 2022, 106(5): 943-952. DOI:10.1093/biolre/ioac013 |
| [18] |
SHEN M M, LI T T, ZHANG G X, et al. Dynamic expression and functional analysis of circRNA in granulosa cells during follicular development in chicken[J]. BMC Genomics, 2019, 20(1): 96. DOI:10.1186/s12864-019-5462-2 |
| [19] |
SHEN M, LI T, FENG Y, et al. Exploring the expression and preliminary function of chicken regulator of G protein signalling 3 (RGS3) gene in follicular development[J]. Br Poult Sci, 2022, 63(5): 613-620. DOI:10.1080/00071668.2022.2071597 |
| [20] |
ZHOU J W, PENG X W, MEI S Q. Autophagy in ovarian follicular development and atresia[J]. Int J Biol Sci, 2019, 15(4): 726-737. DOI:10.7150/ijbs.30369 |
| [21] |
BHARDWAJ J K, PALIWAL A, SARAF P, et al. Role of autophagy in follicular development and maintenance of primordial follicular pool in the ovary[J]. J Cell Physiol, 2022, 237(2): 1157-1170. DOI:10.1002/jcp.30613 |
| [22] |
LEOPARDO N P, VELAZQUEZ M E, CORTASA S, et al. A dual death/survival role of autophagy in the adult ovary of Lagostomus maximus (Mammalia-Rodentia)[J]. PLoS One, 2020, 15(5): e0232819. DOI:10.1371/journal.pone.0232819 |
| [23] |
GAWRILUK T R, HALE A N, FLAWS J A, et al. Autophagy is a cell survival program for female germ cells in the murine ovary[J]. Reproduction, 2011, 141(6): 759-765. DOI:10.1530/REP-10-0489 |
| [24] |
WU G, LI C Y, TAO J L, et al. FSH mediates estradiol synthesis in hypoxic granulosa cells by activating glycolytic metabolism through the HIF-1α-AMPK-GLUT1 signaling pathway[J]. J Biol Chem, 2022, 298(5): 101830. DOI:10.1016/j.jbc.2022.101830 |
| [25] |
MA L Z, ZHENG Y X, TANG X R, et al. miR-21-3p inhibits autophagy of bovine granulosa cells by targeting VEGFA via PI3K/AKT signaling[J]. Reproduction, 2019, 158(5): 441-452. DOI:10.1530/REP-19-0285 |
| [26] |
MAGGI R, CARIBONI A M, MARELLI M M, et al. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system[J]. Hum Reprod Update, 2016, 22(3): 358-381. DOI:10.1093/humupd/dmv059 |
| [27] |
PRASTIYA R A, MADYAWATI S P, SARI S Y, et al. Effect of follicle-stimulating hormone and luteinizing hormone levels on egg-laying frequency in hens[J]. Vet World, 2022, 15(12): 2890-2895. |
| [28] |
TENA-SEMPERE M. Ghrelin and reproduction: ghrelin as novel regulator of the gonadotropic axis[J]. Vitam Horm, 2007, 77: 285-300. |
| [29] |
NAVARRO V M. Metabolic regulation of kisspeptin-the link between energy balance and reproduction[J]. Nat Rev Endocrinol, 2020, 16(8): 407-420. DOI:10.1038/s41574-020-0363-7 |
| [30] |
CHRISTOFFERSEN B Ø, SANCHEZ-DELGADO G, JOHN L M, et al. Beyond appetite regulation: targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss[J]. Obesity (Silver Spring), 2022, 30(4): 841-857. DOI:10.1002/oby.23374 |
| [31] |
李敬. circEML1通过gga-miR-449a/IGF2BP3轴调控蛋鸡卵泡颗粒细胞类固醇激素合成和分泌的分子机制[D]. 郑州: 河南农业大学, 2022: 131. LI J. circEML1 modulates steroid synthesis and secretion of hen 's follicular granulosa cells via targeting the gga-miR-449a/IGF2BP3 axis[D]. Zhengzhou: Henan Agricultural University, 2022: 131. (in Chinese) |
| [32] |
李琴, 王启贵. TGF-β家族成员调控家禽卵泡发育的研究进展及展望[J]. 中国家禽, 2019, 41(19): 1-5. LI Q, WANG Q G. The role of TGF-β family members in avian follicle development[J]. China Poultry, 2019, 41(19): 1-5. DOI:10.16372/j.issn.1004-6364.2019.19.001 (in Chinese) |
| [33] |
ZHOU S, MA Y F, YAO J W, et al. TGF-β1-induced collagen promotes chicken ovarian follicle development via an intercellular cooperative pattern[J]. Cell Biol Int, 2021, 45(6): 1336-1348. DOI:10.1002/cbin.11580 |
| [34] |
陈林, 李刚, 徐光明, 等. 表皮生长因子影响家禽卵泡发育机制的研究进展[J]. 中国畜牧杂志, 2022, 58(12): 41-46. CHEN L, LI G, XU G M, et al. Research progress on the mechanism of epidermal growth factor (EGF) affecting follicle development in poultry[J]. Chinese Journal of Animal Science, 2022, 58(12): 41-46. (in Chinese) |
| [35] |
ONAGBESAN O, BRUGGEMAN V, DECUYPERE E. Intra-ovarian growth factors regulating ovarian function in avian species: a review[J]. Anim Reprod Sci, 2009, 111(2-4): 121-140. DOI:10.1016/j.anireprosci.2008.09.017 |
| [36] |
BERMUDEZ B, ISHⅡ T, WU Y H, et al. Energy balance and bone health: a nutrient availability perspective[J]. Curr Osteoporos Rep, 2023, 21(1): 77-84. DOI:10.1007/s11914-022-00765-4 |
| [37] |
WALZEM R L, CHEN S E. Obesity-induced dysfunctions in female reproduction: lessons from birds and mammals[J]. Adv Nutr, 2014, 5(2): 199-206. DOI:10.3945/an.113.004747 |
| [38] |
YAO Y, WANG H H, YANG Y, et al. Dehydroepiandrosterone activates the GPER-mediated AMPK signaling pathway to alleviate the oxidative stress and inflammatory response in laying hens fed with high-energy and low-protein diets[J]. Life Sci, 2022, 308: 120926. DOI:10.1016/j.lfs.2022.120926 |
| [39] |
ZHANG Q L, WANG Y, LIU J S, et al. Effects of hypercaloric diet-induced hyperinsulinemia and hyperlipidemia on the ovarian follicular development in mice[J]. J Reprod Dev, 2022, 68(3): 173-180. DOI:10.1262/jrd.2021-132 |
| [40] |
VARLAMOV O. Western-style diet, sex steroids and metabolism[J]. Biochim Biophys Acta (BBA): Mol Basis Dis, 2017, 1863(5): 1147-1155. DOI:10.1016/j.bbadis.2016.05.025 |
| [41] |
LU J, LI Y F, QU L, et al. Effects of energy-restricted feeding during rearing on sexual maturation and reproductive performance of Rugao layer breeders[J]. Poult Sci, 2021, 100(8): 101225. DOI:10.1016/j.psj.2021.101225 |
| [42] |
LI G M, LIU L P, YIN B, et al. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-α systems[J]. Poult Sci, 2020, 99(11): 6084-6093. DOI:10.1016/j.psj.2020.07.024 |
| [43] |
李先强, 王家乡, 吴艳, 等. 遮光诱导的氧化应激对蛋鸡卵泡发育的影响[J]. 中国畜牧杂志, 2022, 58(4): 188-194. LI X Q, WANG J X, WU Y, et al. Effects of oxidative stress induced by shading on follicle development in laying hens[J]. Chinese Journal of Animal Science, 2022, 58(4): 188-194. (in Chinese) |
| [44] |
CAO X H, GUO L Y, ZHOU C M, et al. Effects of N-acetyl-l-cysteine on chronic heat stress-induced oxidative stress and inflammation in the ovaries of growing pullets[J]. Poult Sci, 2023, 102(1): 102274. DOI:10.1016/j.psj.2022.102274 |
| [45] |
GONNELLA F, KONSTANTINIDOU F, DI BERARDINO C, et al. A systematic review of the effects of high-fat diet exposure on oocyte and follicular quality: a molecular point of view[J]. Int J Mol Sci, 2022, 23(16): 8890. DOI:10.3390/ijms23168890 |
| [46] |
CHEN Z Y, ZHANG Y. Role of mammalian DNA methyltransferases in development[J]. Annu Rev Biochem, 2020, 89: 135-158. DOI:10.1146/annurev-biochem-103019-102815 |
| [47] |
WEIZMAN N F, WYSE B A, MONTBRIAND J, et al. Cannabis significantly alters DNA methylation of the human ovarian follicle in a concentration-dependent manner[J]. Mol Hum Reprod, 2022, 28(7): gaac022. DOI:10.1093/molehr/gaac022 |
| [48] |
LIU Y N, QIN Y, WU B, et al. DNA methylation in polycystic ovary syndrome: emerging evidence and challenges[J]. Reprod Toxicol, 2022, 111: 11-19. DOI:10.1016/j.reprotox.2022.04.010 |
| [49] |
QU F, WANG F F, YIN R, et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells[J]. J Mol Med (Berl), 2012, 90(8): 911-923. DOI:10.1007/s00109-012-0881-4 |
| [50] |
SANG Q, LI X, WANG H J, et al. Quantitative methylation level of the EPHX1 promoter in peripheral blood DNA is associated with polycystic ovary syndrome[J]. PLoS One, 2014, 9(2): e88013. DOI:10.1371/journal.pone.0088013 |
| [51] |
LI S X, ZHU D Y, DUAN H M, et al. Differential DNA methylation patterns of polycystic ovarian syndrome in whole blood of Chinese women[J]. Oncotarget, 2017, 8(13): 20656-20666. DOI:10.18632/oncotarget.9327 |
| [52] |
KANWAL R, GUPTA K, GUPTA S. Cancer epigenetics: an introduction[M]//VERMA M. Cancer Epigenetics. New York: Humana Press, 2015: 3-25.
|
| [53] |
薛倩, 李国辉, 殷建玫, 等. 鸡繁殖性能近交衰退相关CpG岛差异甲基化基因的筛选[J]. 畜牧兽医学报, 2021, 52(4): 943-953. XUE Q, LI G H, YIN J M, et al. Screening of genes with differential methylated CpG island related to inbreeding depression of chicken reproduction[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(4): 943-953. (in Chinese) |
| [54] |
ZHAO J B, PAN H B, LIU Y, et al. Interacting networks of the hypothalamic-pituitary-ovarian axis regulate layer hens performance[J]. Genes (Basel), 2023, 14(1): 141. DOI:10.3390/genes14010141 |
| [55] |
BARUA A, BAHR J M. Ovarian cancer: applications of chickens to humans[J]. Annu Rev Anim Biosci, 2022, 10: 241-257. DOI:10.1146/annurev-animal-021419-084001 |
| [56] |
ROY S, SINHA N, HUANG B B, et al. Jumonji Domain-containing protein-3 (JMJD3/Kdm6b) is critical for normal ovarian function and female fertility[J]. Endocrinology, 2022, 163(5): bqac047. DOI:10.1210/endocr/bqac047 |
| [57] |
STRAHL B D, ALLIS C D. The language of covalent histone modifications[J]. Nature, 2000, 403(6765): 41-45. DOI:10.1038/47412 |
| [58] |
LAVOIE H A. Epigenetic control of ovarian function: the emerging role of histone modifications[J]. Mol Cell Endocrinol, 2005, 243(1-2): 12-18. DOI:10.1016/j.mce.2005.09.005 |
| [59] |
LI D Y, NING C Y, ZHANG J M, et al. Dynamic transcriptome and chromatin architecture in granulosa cells during chicken folliculogenesis[J]. Nat Commun, 2022, 13(1): 131. DOI:10.1038/s41467-021-27800-9 |
| [60] |
YE Q H, ZENG X F, WANG S, et al. Butyrate drives the acetylation of histone H3K9 to activate steroidogenesis through PPARγ and PGC1α pathways in ovarian granulosa cells[J]. FASEB J, 2021, 35(2): e21316. |
| [61] |
SHEN H J, XU W Q, LAN F. Histone lysine demethylases in mammalian embryonic development[J]. Exp Mol Med, 2017, 49(4): e325. DOI:10.1038/emm.2017.57 |
| [62] |
SHEN H J, XU W Q, LAN F. Histone lysine demethylases in mammalian embryonic development[J]. Exp Mol Med, 2017, 49(4): e325. DOI:10.1038/emm.2017.57 |
| [63] |
XIONG X R, ZHANG X J, YANG M Z, et al. Oocyte-specific knockout of histone lysine demethylase KDM2a compromises fertility by blocking the development of follicles and oocytes[J]. Int J Mol Sci, 2022, 23(19): 12008. DOI:10.3390/ijms231912008 |
| [64] |
LIU Y, KONG F, WANG W Y, et al. Low estrogen level in aged mice leads to abnormal oogenesis affecting the quality of surrounded nucleolus-type immature oocytes[J]. Reprod Fertil Dev, 2022, 34(15): 991-1001. DOI:10.1071/RD22120 |
| [65] |
HE C F, WANG K X, GAO Y Y, et al. Roles of noncoding RNA in reproduction[J]. Front Genet, 2021, 12: 777510. DOI:10.3389/fgene.2021.777510 |
| [66] |
ZHANG J B, XU Y X, LIU H L, et al. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis[J]. Reprod Biol Endocrinol, 2019, 17(1): 9. DOI:10.1186/s12958-018-0450-y |
| [67] |
SONG J Y, MA X C, LI F X, et al. Exposure to multiple pyrethroid insecticides affects ovarian follicular development via modifying microRNA expression[J]. Sci Total Environ, 2022, 828: 154384. DOI:10.1016/j.scitotenv.2022.154384 |
| [68] |
WU N, GAUR U, ZHU Q, et al. Expressed microRNA associated with high rate of egg production in chicken ovarian follicles[J]. Anim Genet, 2017, 48(2): 205-216. DOI:10.1111/age.12516 |
| [69] |
KANG L, CUI X X, ZHANG Y J, et al. Identification of miRNAs associated with sexual maturity in chicken ovary by Illumina small RNA deep sequencing[J]. BMC Genomics, 2013, 14: 352. DOI:10.1186/1471-2164-14-352 |
| [70] |
WU X, ZHANG N, LI J, et al. gga-miR-449b-5p regulates steroid hormone synthesis in laying hen ovarian granulosa cells by targeting the IGF2BP3 gene[J]. Animals (Basel), 2022, 12(19): 2710. |
| [71] |
WAN T, SUN H T, MAO Z L, et al. Vitamin D deficiency inhibits microRNA-196b-5p which regulates ovarian granulosa cell hormone synthesis, proliferation, and apoptosis by targeting RDX and LRRC17[J]. Ann Transl Med, 2021, 9(24): 1775. DOI:10.21037/atm-21-6081 |
| [72] |
WANG Y, GUO Z Y, ZI C, et al. CircRNA expression in chicken granulosa cells illuminated with red light[J]. Poult Sci, 2022, 101(4): 101734. DOI:10.1016/j.psj.2022.101734 |
| [73] |
陈亚楠, 李瑞梅, 陈晓丽, 等. 长链非编码RNA与卵泡发育相关性的研究进展[J]. 生殖医学杂志, 2020, 29(12): 1677-1681. CHEN Y N, LI R M, CHEN X L, et al. Research progress of correlation between LncRNAs and follicle development[J]. Journal of Reproductive Medicine, 2020, 29(12): 1677-1681. DOI:10.3969/j.issn.1004-3845.2020.12.025 (in Chinese) |
| [74] |
PENG Y D, CHANG L, WANG Y Q, et al. Genome-wide differential expression of long noncoding RNAs and mRNAs in ovarian follicles of two different chicken breeds[J]. Genomics, 2019, 111(6): 1395-1403. DOI:10.1016/j.ygeno.2018.09.012 |
| [75] |
ZHOU X F, HE Y T, PAN X C, et al. DNMT1-mediated lncRNA IFFD controls the follicular development via targeting GLI1 by sponging miR-370[J]. Cell Death Differ, 2023, 30(2): 576-588. |
| [76] |
MU H B, CAI S Y, WANG X F, et al. RNA binding protein IGF2BP1 meditates oxidative stress-induced granulosa cell dysfunction by regulating MDM2 mRNA stability in an m6A-dependent manner[J]. Redox Biol, 2022, 57: 102492. |
| [77] |
FAN Y, ZHANG C S, ZHU G Y. Profiling of RNA N6-methyladenosine methylation during follicle selection in chicken ovary[J]. Poult Sci, 2019, 98(11): 6117-6124. |
| [78] |
GUO S K, WANG X D, CAO M L, et al. The transcriptome-wide N6-methyladenosine (m6A) map profiling reveals the regulatory role of m6A in the yak ovary[J]. BMC Genomics, 2022, 23(1): 358. |
| [79] |
CAO Z B, ZHANG D D, WANG Y Q, et al. Identification and functional annotation of m6A methylation modification in granulosa cells during antral follicle development in pigs[J]. Anim Reprod Sci, 2020, 219: 106510. |
(编辑 郭云雁)