2. 西北农林科技大学 农业农村部动物生物技术重点实验室, 杨凌 712100
2. Key Laboratory of Animal Biotechnology of the Ministry of Agriculture and Rural Affairs, Yangling 712100, China
内质网(endoplasmic reticulum, ER)是真核细胞蛋白质合成、折叠和运输的细胞器。当细胞受到各种不良刺激时,ER的蛋白质组装功能被扰乱,ER腔中出现大量未折叠或错误折叠的蛋白质,内质网应激(endoplasmic reticulum stress, ERS)激活,从而引发未折叠蛋白反应(unfolded protein response, UPR)[1]。UPR主要通过肌醇需求激酶1(inositol-requiring kinase 1, IRE1)、活化转录因子6(activating transcription factor, ATF6)和蛋白激酶样内质网激酶(protein kinase-like ER kinase, PERK)3条通路使内质网恢复稳态[2]。UPR是一种保守性的细胞自我保护措施,早期的UPR能及时有效的逆转内质网应激,增强细胞的存活能力,是细胞应对适度的内质网应激时启动的防御性反应[3]。当出现过度的ERS之后,则会引发内质网超负荷反应(endoplasmic reticulum-overload response,EOR),EOR主要通过激活NF-κB信号通路,诱导白细胞介素、肿瘤坏死因子、单核细胞趋化因子等炎性因子的表达,进而激活细胞凋亡、细胞炎症反应和细胞分化等相关信号途径[4]。有研究表明,在非酒精性脂肪肝形成过程中,UPR可导致炎性小体激活,当出现过度的ERS之后,甚至出现细胞凋亡的现象[5]。此外,在神经炎症和肠道炎症过程中也伴随着ERS的发生,越来越多的研究者将ERS作为炎症的一个新的研究靶点[6]。
反刍动物分娩后子宫出现细菌感染和组织损伤,是导致子宫内膜炎发生的主要原因,给养殖业带来巨大的经济损失;而子宫内膜上皮细胞是抵御细菌入侵的第一道防线,它们是对抗病原体的物理屏障,通过触发先天免疫反应在防御大多数炎症性疾病过程中扮演着关键角色[7]。反刍动物子宫被革兰阴性菌(如大肠杆菌)感染后,导致的反刍动物子宫内膜炎通常是由脂多糖(lipopolysaccharide, LPS)介导的[8]。大肠杆菌LPS可通过启动子宫内膜细胞的免疫反应引起子宫内膜炎症[9]。LPS可被细胞表面表达的Toll样受体4(toll-like receptor 4, TLR4)识别,触发细胞内的信号转导级联,导致核因子κB(nuclear factor kappa-B, NF-κB)、丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)和含NOD样受体热蛋白结构域相关蛋白3(NOD-like receptor thermal protein domain associated protein 3, NLRP3)炎性小体通路的激活,诱导IL-6和TNF-α等炎症介质的分泌[10-12]。本课题组的前期研究表明,ERS参与LPS刺激诱导的山羊子宫内膜基质细胞炎性反应并促进细胞凋亡[13]。有趣的是,在内皮细胞炎性反应中,抑制ERS和NF-κB信号通路也能够抑制内皮细胞炎性反应诱导的内皮细胞凋亡[14]。此外,在LPS刺激诱导的小鼠子宫内膜炎模型中,单磷酸腺苷活化蛋白激酶的激活能够抑制ERS相关的TXNIP/NLRP3炎症小体活化[15]。
大量的研究证实,ERS作为多种应激源的共同通路,通过UPR与细胞内炎性反应相偶联,参与机体多种炎症性疾病的病理过程,但目前关于ERS在反刍动物子宫内膜炎症反应调控中的作用机制仍未被完全阐明。因此,本研究以山羊子宫内膜上皮细胞(goat endometrial epithelial cells, gEECs)为试验材料,使用LPS处理构建体外细胞炎性反应模型,探究ERS在LPS诱导的gEECs炎性反应中的作用,为深入解析反刍动物子宫内膜炎的发病机理提供理论基础。
1 材料与方法 1.1 试剂衣霉素(tunicamycin,TM,ab120296)购自英国Abcam公司;脂多糖(lipopolysaccharide,LPS,L2630)和4-苯基丁酸(4-phenylbutyric acid, 4-PBA, 1821-12-1)购自美国Sigma公司;DMEM/F-12细胞培养液(SH30 023.01B)购自美国Hyclone公司;胎牛血清(Z7186FBS-500)购自美国ZETA LIFE公司;RNA Trizol试剂(9109)购自日本TaKaRa公司;ChamQ SYBR qPCR Master Mix(Q311-02)购自南京诺唯赞生物科技股份有限公司;反转录试剂盒(AG11711)购自湖南艾科瑞生物工程有限公司;全蛋白提取试剂盒(KGP2100)和BCA蛋白含量检测试剂盒(KGP902)购自江苏凯基生物技术股份有限公司;Anti-β-actin抗体(23660-1-AP)和Anti-NLRP3抗体(19771-1-AP)购自武汉三鹰生物技术有限公司;Anti-Phospho-NF-κB P65抗体(3033 T)和Anti-NF-κB P65抗体(8242)购自美国CST公司;Anti-TLR4抗体(sc-293072)购自美国Santa Cruz生物技术公司;HRP标记山羊抗兔IgG(PB001)和HRP标记山羊抗小鼠IgG(PB002)购自陕西中晖赫彩生物医药科技有限公司;蛋白Marker(M221-01)和ECL发光液(DE2002)购自北京康润诚业生物科技有限公司。
1.2 细胞培养永生化gEECs细胞系为本实验室前期构建并鉴定保存[16]。将本实验室保存的30代左右的gEECs置于培养条件为37 ℃,含5% CO2的细胞培养箱中,用含有10%胎牛血清的DMEM/F-12培养基进行培养,当细胞长至汇合度达80%~90%时进行传代。
1.3 体外细胞模型构建按照本实验室前期已经验证对细胞活性无影响的试验方法,构建不同的体外细胞模型[17-19]。即将gEECs按照2×105个·孔-1接种至6孔细胞培养板中进行培养,当细胞长至汇合度80%左右时,1)使用LPS(5 μg·mL-1)、TM(0.5 μmol·L-1)和4-PBA(1 mmol·L-1)分别单独处理6 h后收集细胞样品。2)使用TM(0.5 μmol·L-1)和4-PBA(1 mmol·L-1)分别预处理2 h,再用LPS(5 μg·mL-1)处理6 h,收集细胞样品,进行后续试验。
1.4 实时荧光定量PCR取“1.3”中收集的各组细胞样品,用Trizol进行细胞总RNA的提取,并利用反转录试剂盒合成cDNA。根据ChamQ SYBR qPCR Master Mix试剂盒说明书在无RNA酶且全程避光的条件下利用Bio-Rad CFX96 PCR仪进行RT-qPCR检测。试验所需引物见表 1。反应条件:95 ℃ 5 min;95 ℃ 10 s,60 ℃ 30 s,循环39次。采用2-ΔΔCt方法计算试验结果,以GAPDH为内参基因。
|
|
表 1 目的基因片段引物序列 Table 1 Primer sequences of target gene fragments |
取“1.3”中收集的各组细胞样品,使用细胞蛋白裂解Buffer按照细胞全蛋白提取试剂盒提取细胞全蛋白,利用BCA蛋白含量检测试剂盒测定蛋白浓度,然后加入5×Loading Buffer,充分混匀煮沸15 min。蛋白样品进行SDS-PAGE电泳,电泳结束后,将目的蛋白转移至PVDF膜,置于含10%脱脂奶粉的TBST中封闭2 h。经TBST洗涤后,分别置于Phospho-NF-κB P65(1∶1 000),NF-κB P65(1∶1 000),TLR4(1∶200),NLRP3(1∶1 000)和β-actin(1∶5 000)一抗中4 ℃孵育过夜;TBST洗涤后,置于HRP标记山羊抗兔IgG或HRP标记山羊抗小鼠IgG中室温孵育1 h;TBST洗涤后,置于ECL发光液中孵育30 s后,使用凝胶成像系统(GBox-Chem-XRQ)拍摄并保存。
1.6 数据统计与分析所有的试验均进行了至少3次独立的生物学重复,以“x±s”表示结果,利用GraphPad Prism 8软件进行试验数据的分析并绘制结果图,以单因素方差分析和显著性t检验对试验数据进行差异显著性分析,P<0.05为差异具有统计学意义。
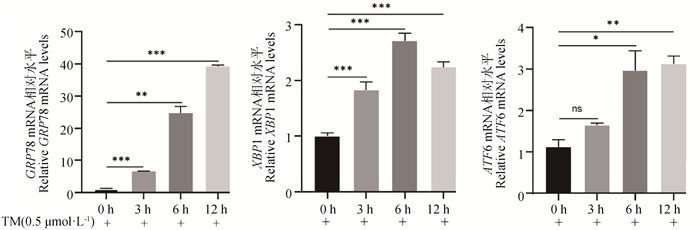
2 结果 2.1 TM处理条件筛选如图 1所示,与对照组相比,TM处理3 h后即可显著促进内质网应激通路关键基因GRP78和XBP1 mRNA的表达(P<0.001),但对ATF6的mRNA表达无显著影响(P>0.05)。随着TM处理时间的增加,当TM处理6 h后可以观察到GRP78、XBP1和ATF6的mRNA表达量均显著增加,且XBP1的mRNA表达量达到最高(P<0.001)。因此,后续试验将TM处理条件选择为终浓度0.5 μmol·L-1、处理6 h。
|
RT-qPCR检测GRP78、XBP1和AFT6 mRNA表达变化(ns. 无显著性差异;*. P<0.05;**. P<0.01;***. P<0.001。下同) Detection of GRP78, XBP1, and AFT6 mRNA expression by RT-qPCR (ns. No significance; *. P<0.05;**. P<0.01;***. P<0.001. The same as below) 图 1 TM处理条件筛选 Fig. 1 The appropriate conditions of TM treatment were screened |
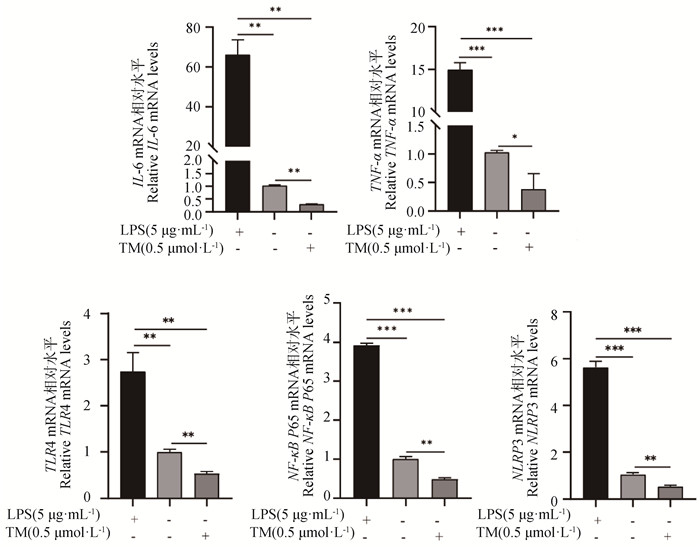
以本实验室前期构建的体外细胞炎性反应模型(5 μg·mL-1 LPS处理gEECs 6 h)为阳性对照,检测TM对gEECs炎症相关基因表达的影响。与对照组相比,TM处理组gEECs炎性细胞因子IL-6和TNF-α的mRNA表达量显著降低,同时显著低于LPS处理组炎性细胞因子的mRNA表达量(P<0.01)(图 2)。此外,如图 2所示,TM处理使经典炎症通路关键基因TLR4、NF-κB P65和NLRP3的mRNA表达量显著低于对照组及LPS处理组(P<0.01)。同时,Western blot结果进一步显示,TM处理显著抑制了NF-κB P65蛋白的磷酸化和NLRP3蛋白的表达(P<0.05)(图 3)。
|
RT-qPCR检测IL-6、TNF-α、TLR4、NF-κB P65和NLRP3的mRNA表达水平 Detection of IL-6, TNF-α, TLR4, NF-κB P65, and NLRP3 mRNA expression by RT-qPCR 图 2 TM处理对gEECs炎症相关基因表达的影响 Fig. 2 Effect of TM treatment on the expression of inflammation-related genes in gEECs |

|
图 3 Western blot检测TM处理后NF-κB P65蛋白磷酸化和NLRP3蛋白表达水平 Fig. 3 Detection of NF-κB P65 protein phosphorylation levels and NLRP3 protein expression levels by Western blot post TM treatment |
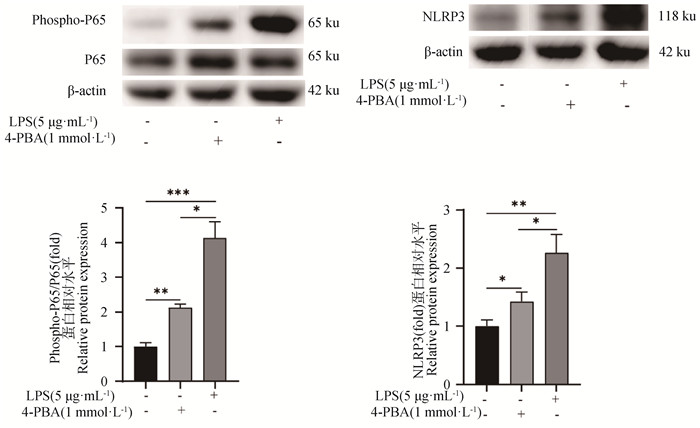
添加终浓度为1 mmol·L-1的4-PBA处理gEECs 6 h后,检测炎症相关基因的表达变化[16]。如图 4所示,与对照组相比,4-PBA处理组gEECs炎性细胞因子IL-6和TNF-α的mRNA表达量显著升高,但其表达量仍显著低于LPS处理组(P<0.01或P<0.001)。接着,对经典炎症通路关键基因进行检测,结果如图 4所示,4-PBA处理组TLR4和NLRP3的mRNA表达量显著高于对照组(P<0.01),而NF-κB P65的mRNA表达量与对照组相比无显著性差异(P>0.05);同时,对照组和4-PBA处理组炎症通路相关基因表达量均显著低于LPS处理组(P<0.01)。Western blot显示出相似的结果,如图 5所示,4-PBA处理显著促进了NF-κB P65蛋白的磷酸化和NLRP3蛋白的表达,但均显著低于LPS处理组(P<0.05)。

|
RT-qPCR检测IL-6、TNF-α、TLR4、NF-κB P65和NLRP3的mRNA表达水平 Detection of IL-6, TNF-α, TLR4, NF-κB P65, and NLRP3 mRNA expression by RT-qPCR 图 4 4-PBA处理对gEECs炎症相关基因表达的影响 Fig. 4 Effect of 4-PBA treatment on the expression of inflammation-related genes in gEECs |
|
图 5 Western blot检测4-PBA处理后NF-κB P65蛋白磷酸化和NLRP3蛋白表达水平 Fig. 5 Detection of NF-κB P65 protein phosphorylation levels and NLRP3 protein expression levels by Western blot post 4-PBA treatment |
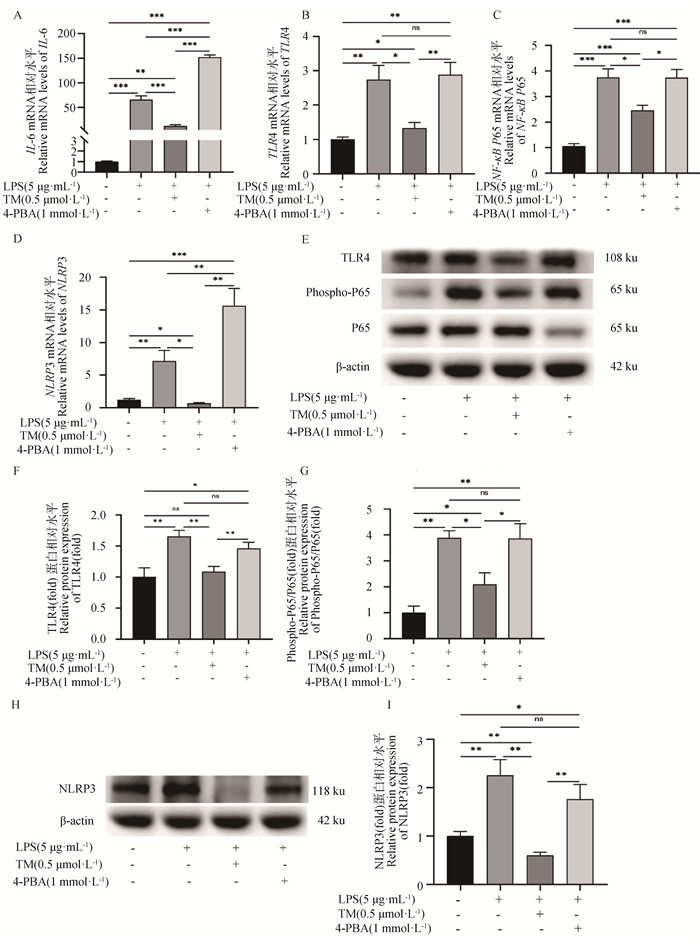
TM或4-PBA预处理2 h后,再使用LPS刺激,检测ERS对LPS诱导的gEECs炎性反应的影响。如图 6A所示,与LPS单独处理组相比,TM预处理显著抑制了LPS诱导的gEECs炎性细胞因子IL-6 mRNA的表达,而4-PBA预处理则显著促进了LPS诱导的IL-6 mRNA的表达,且3种不同处理组IL-6 mRNA表达量均显著高于对照组(P<0.05)。接着,检测了经典炎症通路关键基因的表达。如图 6B~D所示,TM预处理显著抑制了LPS诱导的gEECs炎性反应中TLR4、NF-κB P65和NLRP3 mRNA的表达(P<0.05);而4-PBA预处理则显著促进了TLR4、NF-κB P65和NLRP3 mRNA的表达(P<0.05)。Western blot结果进一步显示,TM预处理显著抑制了LPS诱导的NF-κB P65蛋白的磷酸化以及TLR4和NLRP3蛋白的表达(P<0.05);而4-PBA预处理则显著促进了NF-κB P65蛋白的磷酸化以及TLR4和NLRP3蛋白的表达(P<0.05)(图 6E~I)。
|
A~D. RT-qPCR检测IL-6、TLR4、NF-κB P65和NLRP3的mRNA表达水平;E~I. Western blot检测磷酸化NF-κB P65蛋白以及TLR4和NLRP3蛋白表达水平 A-D. Detection of IL-6, TLR4, NF-κB P65, and NLRP3 mRNA expression by RT-qPCR; E-I. Detection of NF-κB P65 protein phosphorylation levels and TLR4 and NLRP3 protein expression levels by Western blot 图 6 ERS对LPS诱导的gEECs炎性反应的影响 Fig. 6 Effect of ERS on LPS-induced inflammatory response in gEECs |
动物子宫是一个具有复杂免疫功能的器官,需要在半同种异体胎儿的发育过程中维持免疫耐受环境,同时保持其监测和响应感染因子的能力[20]。然而,分娩后子宫处于一种开放性状态,细菌感染和组织损伤等可引起子宫内膜炎的发生,给养殖业带来极大的经济损失[21]。目前大量研究证实子宫内膜上皮细胞是抵御病原体入侵的第一道防线,子宫内膜上皮细胞的Toll样受体(toll-like receptors, TLRs)能够检测到LPS等病原体相关分子模式(pathogen-associated molecular patterns, PAMP),激活细胞内NF-κB通路等炎症通路对其产生应答,对机体起保护作用[22]。此外,NOD样受体(NOD-like Receptors, NLRs)也存在于子宫内膜上皮细胞中,参与子宫抵抗病原微生物入侵的反应[23]。因此,更深入地了解子宫内膜上皮细胞在炎性反应中的功能,对反刍动物子宫内膜炎的防治来说至关重要。越来越多的研究发现ERS通过多种机制与炎症信号通路相偶联,包括NF-κB通路和JNK通路等,但ERS在反刍动物子宫内膜炎中的作用仍未被完全阐明[24]。本研究发现,TM诱导的ERS能够抑制gEECs中炎性细胞因子IL-6和TNF-α mRNA的表达;而用4-PBA处理后则促进了IL-6和TNF-α mRNA的表达。同时,在TM诱导的ERS中,NF-κB P65蛋白的磷酸化和NLRP3蛋白的表达同样受到抑制;而4-PBA则促进了gEECs中经典炎症通路关键基因的表达。这些结果表明,TM诱导的ERS能够抑制gEECs中炎症相关基因的表达,而使用4-PBA处理抑制生理条件下内质网应激的程度可能发挥促炎作用,提示ERS可能参与调节山羊子宫内膜炎的发生和发展。与本研究结果一致,ERS也可减轻游离脂肪酸诱导的3T3-L1脂肪细胞炎症,抑制NF-κB P65的磷酸化和炎性细胞因子的表达[25]。
ERS发生后激活UPR对外界的细胞不良应激进行应答,UPR信号通路能够通过对细胞转录以及翻译过程的改变来缓解ERS,以维持细胞内稳态,促进细胞存活[26]。最近的研究发现,UPR通过ERS诱导的信号通路与免疫反应之间的直接串扰发挥免疫作用[27]。此外,也有研究发现,TLR4-Myd88信号通路与PERK/eIF2α/CHOP通路的相互作用在坏死性小肠结肠炎的炎症发生中发挥着重要作用[28]。值得一提的是,作为适应性UPR的效应器,XBP1s可直接与细胞因子TNF-α和IL-6的启动子结合调节其表达[29]。在本研究中,作者首先确定了TM处理条件为终浓度0.5 μmol·L-1、处理6 h,此时,发现XBP1的表达量最高,同时内质网应激通路关键基因GRP78和ATF6 mRNA的表达量也显著升高但并非处于最大值,因此,推测TM可能诱导了适应性UPR的发生。有研究表明, 一定程度的内质网应激能激活适应性UPR的发生,从而激活机体保护机制发挥保护作用[30]。此外,Ishikawa等[31]在Sec63和XBP1s失活的基础上开发出了肾小管间质性肾病的遗传小鼠模型,该模型呈现间质炎症和进行性肾功能不全的症状,同时,XBP1s的异位表达可以减少由Sec63缺失引起的肾间质炎症,显示出ERS对机体局部炎症的抑制作用。本研究发现,TM预处理显著抑制了LPS诱导的gEECs中IL-6 mRNA的表达,同时显著抑制了LPS诱导的NF-κB P65蛋白的磷酸化和NLRP3蛋白的表达;而4-PBA预处理则促进了炎症相关基因的表达。提示TM诱导的ERS预适应激活了适应性UPR,从而减轻LPS诱导的gEECs炎性反应,其可能是通过抑制NF-κB通路和NLRP3炎性小体通路的激活来实现的。这与先前的研究一致,ERS激活UPR对其产生应答,能够抑制炎症和氧化应激,保护神经元细胞免受损伤[32]。此外,Ferrè等[33]也发现ERS能够促进肾炎和脓毒症等肾病的发生发展,导致肾损伤。总之,上述研究证据提示, ERS在炎症性疾病的发生发展中具有双重作用,它既可以抑制炎症,也可以促进炎症,造成这一现象的背后的原因仍待进一步研究。
4 结论TM诱导的ERS通过抑制NF-κB通路和NLRP3炎性小体通路的激活抑制LPS诱导的gEECs炎性反应;4-PBA处理抑制ERS能够促进LPS诱导的gEECs炎性反应。本试验结果为揭示ERS在山羊子宫内膜炎的发生发展中的作用提供了前期理论基础。
| [1] |
SCHWARZ D S, BLOWER M D. The endoplasmic reticulum: structure, function and response to cellular signaling[J]. Cell Mol Life Sci, 2016, 73(1): 79-94. DOI:10.1007/s00018-015-2052-6 |
| [2] |
HETZ C, ZHANG K Z, KAUFMAN R J. Mechanisms, regulation and functions of the unfolded protein response[J]. Nat Rev Mol Cell Biol, 2020, 21(8): 421-438. DOI:10.1038/s41580-020-0250-z |
| [3] |
KIM J H, PARK S J, KIM T S, et al. Testicular hyperthermia induces unfolded protein response signaling activation in spermatocyte[J]. Biochem Biophys Res Commun, 2013, 434(4): 861-866. DOI:10.1016/j.bbrc.2013.04.032 |
| [4] |
QU Z G, LU X C, QU Y, et al. Attenuation of the upregulation of NF-κB and AP-1 DNA-binding activities induced by tunicamycin or hypoxia/reoxygenation in neonatal rat cardiomyocytes by SERCA2a overexpression[J]. Int J Mol Med, 2021, 47(6): 113. DOI:10.3892/ijmm.2021.4946 |
| [5] |
LEBEAUPIN C, VALLÉE D, HAZARI Y, et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease[J]. J Hepatol, 2018, 69(4): 927-947. DOI:10.1016/j.jhep.2018.06.008 |
| [6] |
SPRENKLE N T, SIMS S G, SÁNCHEZ C L, et al. Endoplasmic reticulum stress and inflammation in the central nervous system[J]. Mol Neurodegener, 2017, 12(1): 42. DOI:10.1186/s13024-017-0183-y |
| [7] |
YIN P, ZHANG Z C, LI J D, et al. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NK-κB and MAPK pathway[J]. Res Vet Sci, 2019, 126: 164-169. DOI:10.1016/j.rvsc.2019.08.018 |
| [8] |
ZHANG S D, WANG D S, YAN Z T. Increasing of matrix metalloproteinase 3 in bovine endometritis[J]. Theriogenology, 2021, 175: 83-88. DOI:10.1016/j.theriogenology.2021.09.001 |
| [9] |
JIANG K F, YANG J, XUE G H, et al. Fisetin ameliorates the inflammation and oxidative stress in lipopolysaccharide-induced endometritis[J]. J Inflamm Res, 2021, 14: 2963-2978. DOI:10.2147/JIR.S314130 |
| [10] |
LIU C, TANG X, ZHANG W J, et al. 6-Bromoindirubin-3′-Oxime suppresses LPS-induced inflammation via inhibition of the TLR4/NF-κB and TLR4/MAPK signaling pathways[J]. Inflammation, 2019, 42(6): 2192-2204. DOI:10.1007/s10753-019-01083-1 |
| [11] |
LAI J L, LIU Y H, LIU C, et al. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways[J]. Inflammation, 2017, 40(1): 1-12. DOI:10.1007/s10753-016-0447-7 |
| [12] |
ZHAO W M, MA L, CAI C, et al. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-induced THP-1 macrophages[J]. Int J Biol Sci, 2019, 15(8): 1571-1581. DOI:10.7150/ijbs.34211 |
| [13] |
MOHAMED A A A, YANG D Q, LIU S Q, et al. Endoplasmic reticulum stress is involved in lipopolysaccharide-induced inflammatory response and apoptosis in goat endometrial stromal cells[J]. Mol Reprod Dev, 2019, 86(7): 908-921. DOI:10.1002/mrd.23152 |
| [14] |
JIANG M, WANG H M, LIU Z F, et al. Endoplasmic reticulum stress-dependent activation of iNOS/NO-NF-κB signaling and NLRP3 inflammasome contributes to endothelial inflammation and apoptosis associated with microgravity[J]. FASEB J, 2020, 34(8): 10835-10849. DOI:10.1096/fj.202000734R |
| [15] |
HU X Y, LI D P, WANG J X, et al. Melatonin inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in lipopolysaccharide-induced endometritis in mice[J]. Int Immunopharmacol, 2018, 64: 101-109. DOI:10.1016/j.intimp.2018.08.028 |
| [16] |
吴庆侠, 王爱华, 司永嘉, 等. 山羊子宫内膜上皮细胞转染pCI-neo-hTERT质粒后的永生化[J]. 中国兽医学报, 2010, 30(2): 228-232. WU Q X, WANG A H, SI Y J, et al. Immortalization of goat endometrial epithelial cells by telomerase reverse tran-scriptase transfection[J]. Chinese Journal of Veterinary Science, 2010, 30(2): 228-232. DOI:10.16303/j.cnki.1005-4545.2010.02.021 (in Chinese) |
| [17] |
杨迪琦. 内质网应激在激素调控山羊子宫内膜上皮细胞功能中的作用及其机制研究[D]. 杨凌: 西北农林科技大学, 2019. YANG D Q. The role of endoplasmic reticulum stress in goat endometrial epithelial cells function under hormone treatment[D]. Yangling: Northwest A&F University, 2019. (in Chinese) |
| [18] |
QI M Z, JIANG Q R, YANG S W, et al. The endoplasmic reticulum stress-mediated unfolded protein response protects against infection of goat endometrial epithelial cells by Trueperella pyogenes via autophagy[J]. Virulence, 2022, 13(1): 122-136. DOI:10.1080/21505594.2021.2021630 |
| [19] |
刘守勤. Ufmylation修饰系统在山羊子宫内膜炎症反应中的作用[D]. 杨凌: 西北农林科技大学, 2019. LIU S Q. The role of Ufmylation in the inflammatory response of goat endometrial epithelial cells[D]. Yangling: Northwest A&F University, 2019. (in Chinese) |
| [20] |
AGOSTINIS C, MANGOGNA A, BOSSI F, et al. Uterine immunity and microbiota: a shifting paradigm[J]. Front Immunol, 2019, 10: 2387. DOI:10.3389/fimmu.2019.02387 |
| [21] |
DAHIYA S, KUMARI S, RANI P, et al. Postpartum uterine infection & ovarian dysfunction[J]. Indian J Med Res, 2018, 148(S1): S64-S70. |
| [22] |
KING A E, CRITCHLEY H O D, KELLY R W. Innate immune defences in the human endometrium[J]. Reprod Biol Endocrinol, 2003, 1: 116. DOI:10.1186/1477-7827-1-116 |
| [23] |
MA X Y, LI Y Y, SHEN W X, et al. LPS mediates bovine endometrial epithelial cell pyroptosis directly through both NLRP3 classical and non-classical inflammasome pathways[J]. Front Immunol, 2021, 12: 676088. DOI:10.3389/fimmu.2021.676088 |
| [24] |
CHEN J, ZHANG M H, ZHU M M, et al. Paeoniflorin prevents endoplasmic reticulum stress-associated inflammation in lipopolysaccharide-stimulated human umbilical vein endothelial cells via the IRE1α/NF-κB signaling pathway[J]. Food Funct, 2018, 9(4): 2386-2397. DOI:10.1039/C7FO01406F |
| [25] |
WANG M, CHEN X, ZHENG Z D, et al. Beneficial effect of ER stress preconditioning in protection against FFA-induced adipocyte inflammation via XBP1 in 3T3-L1 adipocytes[J]. Mol Cell Biochem, 2020, 463(1-2): 45-55. DOI:10.1007/s11010-019-03627-3 |
| [26] |
XU W J, WANG C R, HUA J L. X-box binding protein 1 (XBP1) function in diseases[J]. Cell Biol Int, 2021, 45(4): 731-739. DOI:10.1002/cbin.11533 |
| [27] |
MOGILENKO D A, HAAS J T, LHOMME L, et al. Metabolic and innate immune cues merge into a specific inflammatory response via the UPR[J]. Cell, 2019, 177(5): 1201-1216. DOI:10.1016/j.cell.2019.03.018 |
| [28] |
AFRAZI A, BRANCA M F, SODHI C P, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis[J]. J Biol Chem, 2014, 289(14): 9584-9599. |
| [29] |
FANG P P, XIANG L X, HUANG S S, et al. IRE1α-XBP1 signaling pathway regulates IL-6 expression and promotes progression of hepatocellular carcinoma[J]. Oncol Lett, 2018, 16(4): 4729-4736. |
| [30] |
HETZ C, SAXENA S. ER stress and the unfolded protein response in neurodegeneration[J]. Nat Rev Neurol, 2017, 13(8): 477-491. |
| [31] |
ISHIKAWA Y, FEDELES S, MARLIER A, et al. Spliced XBP1 rescues renal interstitial inflammation due to loss of Sec63 in collecting ducts[J]. J Am Soc Nephrol, 2019, 30(3): 443-459. |
| [32] |
ZHANG G H, KAI J Y, CHEN M M, et al. Downregulation of XBP1 decreases serous ovarian cancer cell viability and enhances sensitivity to oxidative stress by increasing intracellular ROS levels[J]. Oncol Lett, 2019, 18(4): 4194-4202. |
| [33] |
FERRÈ S, DENG Y F, HUEN S C, et al. Renal tubular cell spliced X-box binding protein 1 (Xbp1s) has a unique role in sepsis-induced acute kidney injury and inflammation[J]. Kidney Int, 2019, 96(6): 1359-1373. |
(编辑 白永平)



