卵巢卵泡是决定雌性哺乳动物生殖寿命、不可再生的基本功能单位[1]。在卵泡中,卵丘细胞(cumulus cells,CCs)是包裹卵母细胞的重要体细胞,属于颗粒细胞(granulosa cells,GCs),对卵巢发育、卵母细胞成熟和胚胎发育有重要调控作用[2]。CCs与卵母细胞形成卵丘-卵母细胞复合体(cumulus-oocyte complex,COCs),通过缝隙连接进行物质交换来调控卵母细胞的成熟,为早期胚胎的发育提供物质基础[3]。CCs的增殖与卵泡发育密切相关,凋亡可引起卵泡闭锁,其增殖和凋亡之间的相对平衡对卵巢发育和卵母细胞成熟有至关重要的影响[4]。
在细胞生长和分化过程中,存在广泛的表观遗传修饰。组蛋白甲基化作为一种重要的表观调控机制,在基因的表达及沉默、细胞分化、异染色质形成、基因印迹等方面发挥着重要作用[5]。组蛋白甲基化也参与卵泡发育和卵母细胞成熟的调控[6-7],可以改变卵泡的发育命运。缺失的、小的、同源异形1(absent,small,or homeotic 1-like,ASH1L)是组蛋白赖氨酸甲基转移酶,由位于染色体带1q22的ash1 l基因编码,首次发现于果蝇[8],因其基因突变导致的同源异形的表型与三胸腔结构蛋白(trithorax,Trx)的突变体相似而被归类于TrxG蛋白家族[9]。牛ASH1L蛋白含有2 965个氨基酸,包含SET(su(var)3-9,enhancer-of-zeste and trithorax)、AWS(associate with SET)、Post-SET、Bromo和BAH(bromo adjacent homology)等已知结构域,其中SET域具有催化功能,负责将一个甲基从S-腺苷甲硫氨酸(S-adenosyl methionine,SAM)辅助因子转移到赖氨酸底物[10]。在哺乳动物中,ASH1L可特异性催化H3K36甲基化,是染色质的激活标记[9, 11]。在果蝇的活性基因中,ASH1能通过干扰核小体上多梳抑制复合体2(polycomb repressive complex2,PRC2)的活性来抑制H3K27甲基化,从而促进基因表达[12]。Ash1 l突变导致部分仔鼠出生后死亡,存活的突变小鼠则存在小鼠附睾(雄性)和子宫(雌性)发育缺陷导致的生长不足和不育[13]。在牛CCs中,下调ASH1 L表达可降低H3K36 me1/2/3水平,抑制细胞增殖,诱导细胞凋亡[14]。在小鼠中,Ash1 l通过调控Hox(Homebox gene)和Smad3(drosophila mothers against decapentaplegic protein 3)基因的表达调节骨髓间充质干细胞的成骨分化,促进细胞的生长发育[15]。通过调节小鼠减数分裂前期I中p63和p-CHK2的表达,Ash1 l可保护卵母细胞基因组的完整性,清除具有严重DNA损伤的卵母细胞;过表达Ash1 l可通过调节DNA损伤而诱导细胞凋亡,进而导致胎儿中卵母细胞的急剧丢失和原始卵泡池的容量减少[16]。
综上所述,ASH1L甲基转移酶的相关研究主要集中于果蝇、小鼠及人,而其在调控家畜细胞分化和胚胎发育方面的作用还未被揭示。本研究以牛CCs为试验对象,研究ASH1L过表达对牛CCs中H3K36甲基化状态、细胞增殖和细胞凋亡的影响,为深入研究组蛋白甲基化酶ASH1L对牛CCs生长的调控作用提供依据,为卵泡发育、卵母细胞成熟和胚胎发育提供重要的理论基础。
1 材料与方法 1.1 试验材料如无特殊说明,本研究所用试剂均购自西格玛奥德里奇(上海)贸易有限公司;DMEM/F12培养基、TCM199、0.25%胰酶、血清和DPBS购自赛默飞世尔科技(中国)有限公司;ASH1L兔多克隆抗体购自Bethyl Laboratories公司;辣根过氧化物酶(HRP)标记的山羊抗兔IgG购自北京中杉金桥公司;高灵敏度化学发光液测试试剂盒购自北京聚合美生物科技有限公司;H3K36 me1/2/3兔多克隆抗体购自艾博抗(上海)贸易有限公司;AlexaFluor 488标记的山羊抗兔IgG购自Cell Signaling Technology(CST)公司。
1.2 ASH1L过表达载体的构建参考Xia等[17]的报道,ASH1L SET结构域具有甲基转移酶功能;本试验按照NCBI提供的牛ASH1L基因的编码序列(登录号:NC_037 330.1),扩增包括SET结构域的第2 141位氨基酸到终止密码子的ASH1L片段,长度为2 478 bp。根据目的片段设计携带kozak(GCCACC)、起始密码子(ATG)及Nhe Ⅰ(CTAGCTAGC)和Not Ⅰ(ATAGTTTAGCGGCCGC)酶切位点的引物,由北京六合华大基因科技有限公司合成,引物序列见表 1。以牛CCs的cDNA为模板,采用PCR扩增目的片段,PCR反应条件为:98 ℃预变性5 min;98 ℃变性10 s,60 ℃退火1 min,68 ℃延伸1.5 min,35个循环;68 ℃延伸5 min,4 ℃保持。扩增产物经酶切后,鉴定获得序列长度符合预期的片段。T4连接酶将该片段连接到经Nhe Ⅰ /Not Ⅰ双酶切的pcDNA3.1载体上,转化细菌,并挑菌测序。测序正确的质粒命名为pcDNA3.1+ASH1L,并用去内毒素质粒DNA提取试剂盒(OMEGA,美国)提取、纯化质粒,-20 ℃保存,用于后续试验。
|
|
表 1 引物序列表 Table 1 Sequences of primers |
参照Wang等[18]的文献,从当地屠宰场收集牛卵巢,用含100 IU ·mL-1青霉素和100 mg ·mL-1硫酸链霉素(1% PS)的30 ℃生理盐水洗涤,2~3 h内运回实验室。从2~6 mm卵泡中抽出COCs,用含10%血清(FBS)、0.01 IU ·mL-1卵泡刺激激素(FSH)、1 IU ·mL-1黄体生成素(LH)、1 μg ·mL-1雌二醇(E2)、10 μg ·mL-1肝素(HP)、40 ng ·mL-1胰岛素生长因子(IGF)和50 ng ·mL-1上表皮生长因子(EGF)的TCM199配制成的成熟液于38.5 ℃、5% CO2、饱和湿度条件下培养22 h。用0.1%透明质酸酶消化成熟后的COCs,2 min后加入预热、含10% FBS的TCM199终止消化。将CCs悬浮液移入离心管,1 000 r ·min-1离心5 min,弃上清。用DPBS离心洗涤两次后,DMEM/F12培养基(含10% FBS和1% PS)重悬细胞沉淀,混匀移入六孔板,于38.5 ℃、5% CO2、饱和湿度条件下培养。
1.4 细胞转染六孔板中接种的细胞生长至70%~80%汇合度时进行细胞转染。先用Opti-MEMTM培养基稀释LipofectamineTM 3 000试剂,充分混匀后室温静置5 min;同时用Opti-MEMTM培养基稀释质粒DNA,制备DNA预混液,添加P3000TM增强剂,充分混匀;然后按1 ∶1的比例混和已稀释的LipofectamineTM3000和质粒DNA,形成DNA-脂质复合物,室温孵育10~15 min。随后将DNA-脂质复合物加入细胞培养孔中,于38.5 ℃、5% CO2、饱和湿度条件下培养,6 h后更换为DMEM/F12培养基(含10% FBS和1% PS),24 h收集细胞用于后续试验。转染pcDNA3.1+ASH1L载体的细胞为过表达组(ASH1L-OE),未转染载体的细胞为对照组(Control)。
1.5 荧光定量PCR(quantitative RT-PCR,RT-qPCR)载体质粒转染后24 h,分别收集对照组和过表达组细胞,参照动物细胞RNA提取试剂盒说明书(QIAGEN,德国)提取RNA,并反转录成cDNA,利用CFX96TM实时荧光定量PCR仪(BIO-RAD,美国)进行基因表达水平的定量检测。选择的基因包括牛ASH1 L、BCL-2、BCL-2相关X蛋白(BCL-2 associated X,BAX)、半胱氨酸天冬氨酸蛋白酶3(cysteinyl aspartate specific proteinase,CASPASE-3)、增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)、细胞分裂周期蛋白42(cell division cycle 42,CDC42)、周期素D2(cyclin D2,CCND2)和GAPDH。以上基因的引物参考Tian等[19]的研究,并由北京六合华大基因科技有限公司合成,引物序列见表 1。反应体系为20 μL:上、下游引物各0.4 μL,cDNA模板2 μL,TB green Premix EX Taq(Tli RNaseH Plus)10 μL,ddH2O 6.8 μL,ROX Reference Dye Ⅱ 0.4 μL。反应程序为:95 ℃预变性30 s;95 ℃变性5 s,60 ℃退火30 s,共40个循环。每个样品重复3次,以GAPDH为内参基因,采用2-ΔΔCt法计算基因的相对表达量,ΔΔCt=[(Ctgene-CtGAPDH)]过表达组-[(Ctgene-CtGAPDH)]对照组。
1.6 免疫荧光染色将细胞预先接种到细胞共聚焦培养皿中,细胞汇合度达到70%~80%时转染过表达载体质粒,24 h后将对照组和过表达组细胞进行免疫荧光染色。用DPBS清洗细胞3次,4%多聚甲醛室温固定1 h,并于0.2% Triton X-100中通透40 min。用含1% BSA的DPBS(DPBS+1% BSA)清洗3次,每次5 min,细胞于DPBS+1% BSA中37 ℃封闭1 h。用含0.02% Triton X-100和1%BSA的DPBS清洗3次后,细胞于一抗(兔来源H3K36 me1/2/3抗体的稀释比例为1 ∶200,兔来源ASH1L抗体的稀释比例为1 ∶50)中4 ℃孵育过夜;清洗3次,于二抗(Alexa Fluor 488标记的山羊抗兔IgG稀释比例为1 ∶1 000)中37 ℃孵育1 h。清洗3次后,细胞于1.5 μg ·mL-1 DAPI液中室温、避光孵育5 min;清洗后,于激光共聚焦显微镜(Leica,德国)下观察,并收集图像。采用Image J软件对免疫荧光图片的信号进行分析。每个试验至少进行3次重复,数据值的平均值即为该组的荧光强度值。以对照组荧光强度值为参考,采用归一法进行相对荧光强度分析。
1.7 蛋白免疫印迹(western blotting,WB)载体质粒转染后24 h,分别收集对照组和过表达组细胞,加入200 μL含1%抑制剂PMSF的细胞裂解液,冰上静置20~30 min,振荡使细胞充分裂解,细胞悬液于4 ℃、12 000 r ·min-1离心10 min,利用BCA蛋白浓度测定试剂盒(金浦来,中国)测定蛋白浓度。取上清与缓冲液(1 ∶5)混合,震荡混匀,100 ℃变性10 min。变性蛋白经4%~15%梯度SDA-PAGE凝胶电泳分离,之后300 V、200 mA将蛋白转移至硝酸纤维素膜上。用5%的脱脂奶粉室温封闭1 h,滤膜于兔源ASH1L一抗(稀释比例1 ∶1 000)中4 ℃孵育过夜;TBST清洗3次后,滤膜于HRP标记的二抗(稀释比例1 ∶5 000)中37 ℃孵育1 h,TBST清洗3次后,用ECL发光液试剂盒显影,于化学发光仪(天能,中国)中曝光,拍照保存。
1.8 细胞增殖检测根据CCK-8试剂盒(碧云天,中国)说明书,贴壁CCs用0.25%胰酶消化、DPBS洗涤后,按照2×103个·mL-1的密度,把细胞接种到96孔板中。培养24 h后,将pcDNA3.1+ASH1L质粒转染CCs,并分别于转染0、24、36、48、72 h,每孔加入10 μL WST-8试剂,避光孵育2 h,用酶标记仪(Tecan,瑞士)检测450 nm处的吸光值。对照组细胞也进行同样处理,记录各时间段的吸光值。
1.9 细胞凋亡检测采用Annexin V-FITC/碘化丙啶(Propidium iodide,PI)荧光双染细胞凋亡检测试剂盒(碧云天,中国)进行凋亡分析。载体质粒转染后24 h,将对照组和过表达组CCs用0.25%胰酶消化,终止消化后收集细胞悬液,1 000 r ·min-1离心5 min。PBS洗涤细胞,离心获得的细胞用Annexin V-FITC/PI室温染色10 min。采用FACSVerse流式细胞仪(Becton Dickinson,美国)进行活细胞率、坏死细胞率、早期凋亡率和晚期凋亡率的计算分析,凋亡率为早期凋亡率与晚期凋亡率之和。
1.10 统计分析每个试验至少重复3次,试验结果以“平均数±标准差(SD)”表示。所有数据均采用SPSS 22.0软件进行显著性分析,用单因素方差分析(One-way ANOVA)判断不同处理间的差异显著性,P < 0.05为差异显著,P < 0.01为差异极显著。
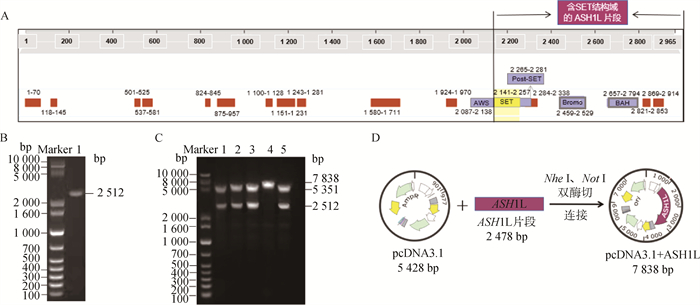
2 结果 2.1 ASH1L过表达载体的构建和鉴定牛ASH1L cDNA全长接近9 kb,构建携带该基因全长序列的过表达载体较为困难。通过对ASH1L各结构域的位置和功能分析(图 1A),本研究拟从其SET结构域的起始氨基酸位点(第2 141)开始,终止密码子氨基酸为终点,扩增ASH1L基因片段,替代ASH1L全长序列。以牛CCs cDNA为模板,PCR扩增获得包含牛ASH1L基因片段(长2 487 bp)、kozak序列、双酶切位点及其保护碱基的序列(长2 512 bp,图 1B),并将其连接于pcDNA3.1载体。成功连接的载体经Nhe Ⅰ/Not Ⅰ双酶切,获得长度分别为5 351和2 512 bp的两条片段(图 1C),与预期结果一致;对酶切鉴定正确的载体进行测序,序列正确的载体即为成功构建的ASH1L过表达载体,命名为pcDNA3.1+ASH1L,全长7 838 bp(图 1D),可用于后续试验。
|
A.牛ASH1L蛋白的氨基酸序列及包含的结构域;B.以牛CCs cDNA为模板,PCR扩增目的片段,长2 512 bp;C.过表达载体pcDNA3.1+ASH1L双酶切鉴定,泳道1、2、3、5为载体酶切后获得的两条片段,泳道4为未能被酶切的载体;D.过表达载体pcDNA3.1+ASH1L构建策略 A. Amino acid sequences and domains contained in the bovine ASH1L protein; B. PCR amplification of the target fragment with bovine CCs cDNA as a template, the length is 2 512 bp; C. Identification of the pcDNA3.1+ASH1L vector with double enzyme digestion, lane 1, 2, 3 and 5 indicate two fragments obtained after enzyme digestion, lane 4 is vector without enzyme digestion; D. Construction scheme of pcDNA3.1+ASH1L vector 图 1 牛ASH1L过表达载体的构建和鉴定 Fig. 1 Construction and identification of overexpression vector of bovine ASH1L |
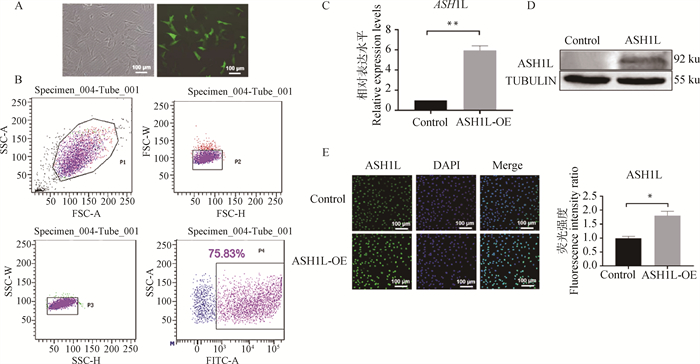
为评估转染效率,将上述pcDNA3.1+ASH1L质粒和pmaxGFP质粒共转染牛CCs。24 h后,镜下可见绿色荧光(图 2A),通过流式分选出阳性细胞的比例约为75.83%(图 2B),可见转染效率较高。pcDNA3.1+ASH1L转染CCs,24 h收集过表达组细胞(ASH1L-OE);同时收集常规培养的CCs作为对照组(Control),采用RT-qPCR、WB和免疫荧光染色检测细胞中ASH1L mRNA及其蛋白的表达。结果显示,过表达组细胞中ASH1L mRNA水平极显著高于对照组(P < 0.01,图 2C)。与内源性ASH1L蛋白的分子量不同,外源ASH1L蛋白的分子量约为92 ku,过表达组细胞中可检测到92 ku的目的蛋白条带,而对照组则没有检测到(图 2D),说明过表达组细胞中有外源ASH1L蛋白的表达。此外,过表达组细胞中ASH1L蛋白的免疫荧光信号强度显著升高(P < 0.05,图 2E)。可见,本试验构建的pcDNA3.1+ASH1L载体能在CCs中表达,可用于后续试验。
|
A.质粒pcDNA3.1+ASH1L和pmaxGFP共转染牛CCs 24 h后可观察到绿色荧光;B.转染24 h后流式分选出阳性细胞;C.过表达载体转染后,ASH1L mRNA表达水平;D.过表达载体转染后,ASH1L蛋白表达量;E.过表达载体转染后,ASH1L甲基转移酶的荧光染色及相对荧光强度。*表示差异显著(P < 0.05),**表示差异极显著(P < 0.01), 下同 A. Green fluorescence of the bovine CCs co-transfected with pmaxGFP and pcDNA3.1+ASH1L plasmids for 24 h after transfection; B. Positive cells screened by flow sorting for 24 h after transfection; C. Expression level of bovine ASH1L mRNA in CCs transfected with overexpression vector; D. Protein expression level of bovine ASH1L in CCs transfected with overexpression vector; E. Immunofluorescence staining of ASH1L methyltransferase and its relative fluorescence intensity in CCs transfected with overexpression vector. * indicates significant difference (P < 0.05), ** indicates highly significant difference (P < 0.01), the same as below 图 2 牛CCs中ASH1L过表达效果的验证 Fig. 2 Validation of ASH1L overexpression in bovine CCs |
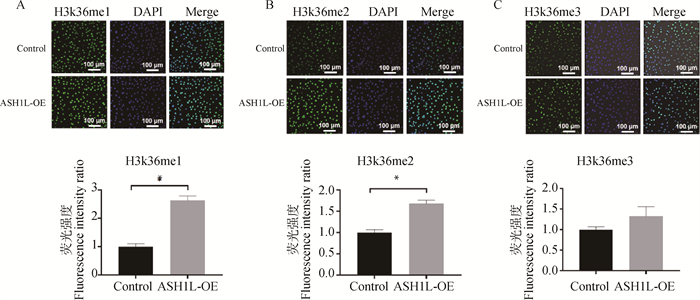
ASH1L过表达载体转染牛CCs 24 h后,细胞中H3K36 me1/2甲基化水平显著升高(P < 0.05,图 3A,3B),H3K36 me3水平高于对照组,但差异不显著(P>0.05,图 3C)。
|
A, B, C.过表达ASH1L后,牛CCs中H3K36me1/2/3甲基化的荧光染色及其相对荧光强度 A, B, C. Immunofluorescence staining of H3K36me1/2/3 and its relative fluorescence intensity in bovine CCs with ASH1L overexpression 图 3 过表达ASH1L对牛CCs中H3K36me1/2/3甲基化状态的影响 Fig. 3 Effects of ASH1L overexpression on the methylation levels of H3K361/2/3 in bovine CCs |
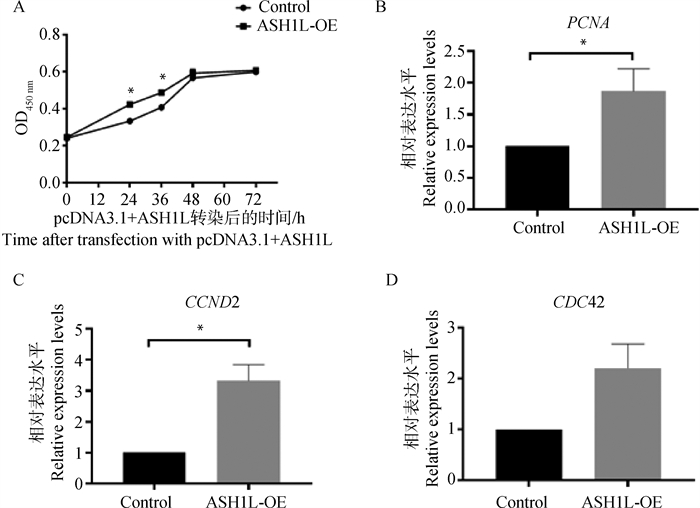
ASH1L过表达载体转染CCs 24和36 h后,细胞增殖率显著高于对照组细胞(P < 0.05);但在转染48 h时,两组间的细胞增殖率差异不显著(P>0.05,图 4A)。ASH1L表达上调后,细胞增殖相关基因PCNA、CCND2 mRNA表达水平均显著高于对照组细胞(P < 0.05,图 4B、4C);而CDC42表达水平在两组间差异不显著(P>0.05,图 4D)。
|
A.过表达ASH1L对牛卵丘细胞增殖活性的影响;B,C,D.过表达ASH1L后,细胞增殖相关基因PCNA、CCND2、CDC42的相对表达量 A. Proliferation activity of bovine CCs with ASH1L overexpression; B, C, D. Relative expression levels of cell proliferation-related genes PCNA, CCND2 and CDC42 in bovine CCs with ASH1L overexpression 图 4 过表达ASH1L对牛CCs增殖的影响 Fig. 4 Effects of ASH1L overexpression on bovine CCs proliferation |
ASH1L过表达组的活细胞率((91.85±1.25)%)显著高于对照组((87.39±1.71)%),而细胞凋亡率((8.08±1.21)%)则显著低于对照组((12.51±1.72)%,P < 0.05)(图 5A)。相应地,过表达组细胞中促凋亡基因BAX和CASPASE-3 mRNA表达水平分别极显著(P < 0.01)和显著地(P < 0.05)低于对照组,而抗凋亡基因BCL-2 mRNA表达水平则极显著地高于对照组(P < 0.01,图 5B)。
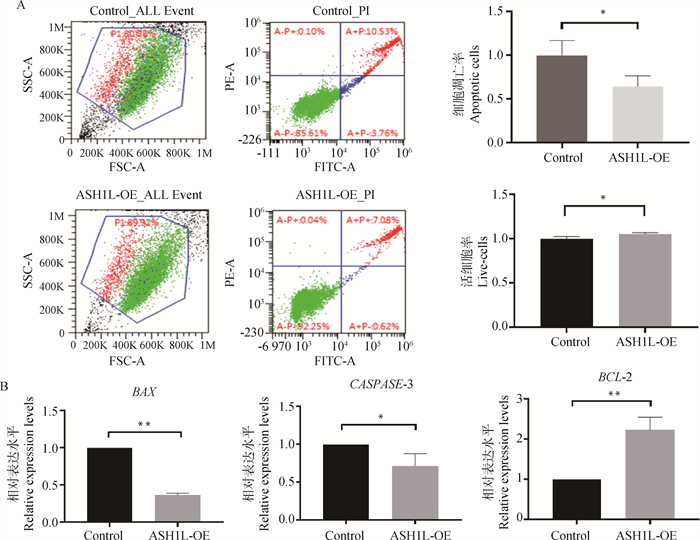
|
A.过表达ASH1L对牛CCs活细胞率和凋亡细胞率的影响:A-P+(左上象限)代表坏死细胞; A+P+(右上象限)代表晚期凋亡细胞; A-P-(左下象限)代表活细胞; A+P-(右下象限)代表早期凋亡细胞; 凋亡率为早期凋亡率与晚期凋亡细胞率之和。B.过表达ASH1L后,CCs中凋亡相关基因BAX、CASPASE-3、BCL-2的相对表达量 A. Rate of live cells and apoptosis of bovine CCs with ASH1L overexpression: A-P+ (upper left quadrant) represents necrotic cell; A+P+ (upper right quadrant) represents late apoptotic cell; A-P-(lower left quadrant) represents live cell; A+P-(lower right quadrant) represents early apoptotic cell, and the apoptotic rate is the sum of early and late apoptotic cell rates. B. Relative expression of apoptosis-related genes BAX, CASPASE-3 and BCL-2 in CCs with ASH1L overexpression 图 5 过表达ASH1L对CCs凋亡的影响 Fig. 5 Effects of ASH1L overexpression on CCs apoptosis |
一般组蛋白甲基转移酶均包含SET结构域,可催化组蛋白氨基酸的甲基化修饰。ASH1L首次发现于果蝇,具有保守的SET结构域,属于三胸腔结构蛋白(TrxG)家族,能催化H3K4和H3K36甲基化激活基因表达[20],从而调节细胞生长、胚胎发育及自身免疫应答等过程[21]。本试验通过构建过表达载体上调牛CCs中ASH1L表达,并对细胞中组蛋白甲基化修饰、细胞凋亡与增殖,及其相关基因表达的变化进行深入研究。
据报道,小鼠ASH1L蛋白SET结构域中第2 212位氨基酸的突变能破坏其甲基转移酶活性,导致H3K4甲基化水平降低,成骨和软骨形成受阻,所以ASH1L SET结构域催化H3K4甲基化活性对于成骨和软骨细胞分化是不可或缺的[22]。另外,含有Ash1L SET结构域片段的载体能达到过表达的效果,并诱导H3K4甲基转移酶活性,增强去泛素化酶的表达,抑制白细胞介素-6和肿瘤坏死因子的产生[17];然而,ASH1L SET结构域的抑制剂AS-99在白血病细胞中表现出了显著的抗白血病活性,可显著阻断人白血病细胞增殖,诱导细胞凋亡和分化[23]。通过扩增ASH1L C端的部分片段(1 892~2 969aa)成功构建了ASH1L过表达质粒来替代人的ASH1L的全长载体[24]。以上研究表明,ASH1L蛋白甲基化修饰的活性是由其中的SET结构域来维持的。所以,为了克服由于牛ASH1L cDNA较长而难以构建全长载体的问题,本研究扩增了含有SET结构域的牛ASH1L基因片段,构建了ASH1L过表达载体。该载体导入牛CCs后,细胞中H3K36甲基化水平和细胞增殖率升高,细胞凋亡率降低,与已有的研究结果一致。可见,牛ASH1L基因中SET结构域的片段可代替全长序列用于基因过表达研究。
ASH1L在哺乳动物神经系统的正常发育[25]、肌肉萎缩症发病机制[21]和免疫细胞发育[26]等方面均发挥重要作用。组蛋白赖氨酸的甲基化修饰与基因的转录调控密切相关。在果蝇和哺乳动物中,ASH1L可甲基化转录激活标记H3K36,调控发育基因的表达[21]。在人的分化神经祖细胞(neuroepithelial cells,NPC)中,ASH1L能催化H3K36me2甲基化,其缺失降低了参与大脑发育的基因表达,这表明受损的神经发育相关基因表达可能是连接ASH1L的关键分子机制[27]。在人的前列腺癌细胞中过表达ASH1L显著增加了H3K36me2的总体水平,但对H3K4me2/3水平没有显著影响[24]。ASH1L已被确定为驱动混合谱系白血病(Mixed linkage leukemia,MLL1)的致癌复合物的关键成分。在这种恶性肿瘤中,ASH1L被H3K36me2标记,这些标记被读取蛋白(如晶状体上皮衍生生长因子)“读取”,导致关键白血病驱动因素(如HOX基因)的激活[28]。作为转录延伸因子,果蝇TrxG家族的KISMET与基因激活有关,其通过促进ash1l催化H3K36me2/3甲基化,从而激活转录发生[29]。在利什曼原虫中,多诺瓦尼乳杆菌能可通过Ash1L介导的H3K4甲基转移酶调控宿主的巨噬细胞极化;在感染后的巨噬细胞中敲低Ash1L基因表达显著抑制了H3K4me3活性和细胞增殖,显著减缓了炎症发生[30]。在MLL-AF9诱导的白血病中,ASHIL可通过直接结合基因启动子,并修饰局部组蛋白H3K36me2水平来激活MLL-AF9靶基因的表达[31]。此外,ASH1L基因的低表达导致了牛CCs中H3K36me1/2/3甲基化水平均显著降低以及PRC2蛋白相关亚基EZH2和SUZ12基因表达量的升高[32]。本研究过表达ASH1L基因后,牛CCs中H3K3单甲基化、二甲基化水平均显著升高,三甲基化水平也升高但不显著,说明ASH1L可调控H3K36甲基化修饰,与之前的研究结果一致。
ASH1L广泛表达于大脑、心脏和肾脏等多种组织器官[33]。在小鼠骨质疏松的骨骼中,Ash1 l表达水平显著降低;野生型(wild type,WT)小鼠中敲降Ash1 l基因可导致小鼠关节炎,并抑制小鼠成骨和软骨形成[22]。ASH1L表达下调导致MLL白血病细胞凋亡和生长停滞,并在体内终止了MLL白血病的发展[34]。在人中,ASH1L通常位于细胞核和细胞间紧密连接处,通过甲基化组蛋白H3K4和H3K36促进基因表达,并拮抗由Polycomb蛋白介导的基因沉默。敲低ASH1L诱导了前列腺癌细胞的周期停滞和凋亡[24]。同样地,牛CCs中ASH1L基因的表达下调后,促凋亡相关基因CASPASE-3和BAX表达量、细胞凋亡率均显著升高,说明ASH1L表达的缺失可促进细胞凋亡[14]。相反地,在小鼠的生殖系统中,Ash1l过表达导致DNA双链断裂修复的缺失,卵母细胞减数分裂前期中p63和磷酸化检查点激酶2(p-CHK2)异常上调,诱导细胞凋亡导致卵母细胞大量丢失;而凋亡抑制剂则可以挽救过表达Ash1l引起的卵母细胞丢失[16]。本试验过表达ASH1L显著降低了牛CCs中CASPASE-3及BAX mRNA表达量、细胞凋亡率,显著升高了BCL-2 mRNA表达量和活细胞率,所以上调ASH1L表达促进细胞生长,抑制细胞凋亡,但具体的作用机制还有待于进一步研究。
细胞增殖参与生物体的生长、发育、繁殖等许多重要的生命活动[35]。在卵泡发育过程中,PCNA表达水平的升高是颗粒细胞增殖的最早标记物[36]。细胞周期蛋白D2(cyclin D2,CCND2)的缺乏会阻碍卵巢中的颗粒增殖和卵泡生长,导致雌性小鼠不育[37]。上调细胞分裂周期蛋白42(cell division cycle protein 42,CDC42)可抑制鸡GCs细胞凋亡,促进细胞增殖[38]。ASH1L过表达增强了乳腺癌和肝癌细胞的增殖,并导致侵袭性疾病的突发[34, 39]。ASH1L基因表达与人和小鼠成肌细胞的融合呈正相关,其缺失会导致肌肉细胞中选择性成肌细胞融合缺陷,不利于骨骼肌组织的形成、生长和修复[21]。在受伤小鼠中,ASH1L的突变使其表达异常升高后,会导致表皮细胞分化紊乱,角质细胞过度增殖,伤口愈合缺陷及皮肤增生[40]。本研究结果表明,上调牛CCs中ASH1L表达,则细胞增殖率、增殖相关基因PCNA、CCND2 mRNA表达量均显著升高,与已有的研究结果一致。
4 结论本研究成功构建了牛ASH1L过表达载体,并建立过表达细胞模型。在牛CCs中,过表达ASH1L显著提高了H3K36me1/2甲基化水平,促进细胞增殖,抑制细胞凋亡,为揭示ASH1L甲基转移酶在家畜CCs生长和卵泡发育中的功能和调控机制提供了理论基础。
| [1] |
WANG C, ZHOU B, XIA G L. Mechanisms controlling germline cyst breakdown and primordial follicle formation[J]. Cell Mol Life Sci, 2017, 74(14): 2547-2566. DOI:10.1007/s00018-017-2480-6 |
| [2] |
DUMESIC D A, MELDRUM D R, KATZ-JAFFE M G, et al. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health[J]. Fertil Steril, 2015, 103(2): 303-316. DOI:10.1016/j.fertnstert.2014.11.015 |
| [3] |
TIAN C L, LIU L L, YE X Y, et al. Functional oocytes derived from granulosa cells[J]. Cell Rep, 2019, 29(13): 4256-4267. DOI:10.1016/j.celrep.2019.11.080 |
| [4] |
ELGEBALY M M, HAZAA A B M, AMER H A, et al. L-Cysteine improves bovine oocyte developmental competence in vitro via activation of oocyte-derived growth factors BMP-15 and GDF-9[J]. Reprod Domest Anim, 2022, 57(7): 734-742. DOI:10.1111/rda.14113 |
| [5] |
SUN L C, ZHANG H F, GAO P. Metabolic reprogramming and epigenetic modifications on the path to cancer[J]. Protein Cell, 2022, 13(12): 877-919. DOI:10.1007/s13238-021-00846-7 |
| [6] |
SHA Q Q, ZHANG J, FAN H Y. Function and regulation of histone H3 lysine-4 methylation during oocyte meiosis and maternal-to-zygotic transition[J]. Front Cell Dev Biol, 2020, 8: 597498. DOI:10.3389/fcell.2020.597498 |
| [7] |
SHA Q Q, ZHU Y Z, XIANG Y L, et al. Role of CxxC-finger protein 1 in establishing mouse oocyte epigenetic landscapes[J]. Nucleic Acids Res, 2021, 49(5): 2569-2582. DOI:10.1093/nar/gkab107 |
| [8] |
SHEARN A, HERSPERGER E, HERSPERGER G. Genetic studies of mutations at two loci of Drosophila melanogaster which cause a wide variety of homeotic transformations[J]. Rouxs Arch Dev Biol, 1987, 196(4): 231-242. DOI:10.1007/BF00376347 |
| [9] |
MIYAZAKI H, HIGASHIMOTO K, YADA Y, et al. Ash1l methylates Lys36 of histone H3 independently of transcriptional elongation to counteract polycomb silencing[J]. PLoS Genet, 2013, 9(11): e1003897. DOI:10.1371/journal.pgen.1003897 |
| [10] |
ROGAWSKI D S, NDOJ J, CHO H J, et al. Two loops undergoing concerted dynamics regulate the activity of the ASH1L histone methyltransferase[J]. Biochemistry, 2015, 54(35): 5401-5413. DOI:10.1021/acs.biochem.5b00697 |
| [11] |
JONES M, CHASE J, BRINKMEIER M, et al. Ash1l controls quiescence and self-renewal potential in hematopoietic stem cells[J]. J Clin Invest, 2015, 125(5): 2007-2020. DOI:10.1172/JCI78124 |
| [12] |
STEWART K R, VESELOVSKA L, KIM J, et al. Dynamic changes in histone modifications precede de novo DNA methylation in oocytes[J]. Genes Dev, 2015, 29(23): 2449-2462. DOI:10.1101/gad.271353.115 |
| [13] |
BRINKMEIER M L, GEISTER K A, JONES M, et al. The histone methyltransferase gene Absent, Small, or homeotic discs-1 like is required for normal Hox gene expression and fertility in mice[J]. Biol Reprod, 2015, 93(5): 121. |
| [14] |
CUI L X, TIAN Y Q, HAO H S, et al. Knockdown of ASH1L methyltransferase induced apoptosis inhibiting proliferation and H3K36 methylation in bovine cumulus cells[J]. Theriogenology, 2021, 161: 65-73. DOI:10.1016/j.theriogenology.2020.11.007 |
| [15] |
ZHANG W, CHEN E M, CHEN M, et al. IGFBP7 regulates the osteogenic differentiation of bone marrow-derived mesenchymal stem cells via Wnt/β-catenin signaling pathway[J]. FASEB J, 2018, 32(4): 2280-2291. DOI:10.1096/fj.201700998RR |
| [16] |
ZHANG T, REN T H, LIN H, et al. ASH1L contributes to oocyte apoptosis by regulating DNA damage[J]. Am J Physiol Cell Physiol, 2022, 323(4): C1264-C1273. DOI:10.1152/ajpcell.00196.2022 |
| [17] |
XIA M, LIU J, WU X H, et al. Histone methyltransferase Ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme A20[J]. Immunity, 2013, 39(3): 470-481. DOI:10.1016/j.immuni.2013.08.016 |
| [18] |
WANG W J, ZOU H Y, CHEN N Z, et al. Knockdown of CLAUDIN-6 inhibited apoptosis and induced proliferation of bovine cumulus cells[J]. Int J Mol Sci, 2022, 23(21): 13222. DOI:10.3390/ijms232113222 |
| [19] |
TIAN Y Q, CUI L X, WANG W J, et al. Knockdown of bone morphogenetic protein 4 gene induces apoptosis and inhibits proliferation of bovine cumulus cells[J]. Theriogenology, 2022, 188: 28-36. DOI:10.1016/j.theriogenology.2022.05.015 |
| [20] |
CHENG Q, XIE H, ZHANG X Y, et al. An essential role for PTIP in mediating Hox gene regulation along PcG and trxG pathways[J]. FEBS J, 2022, 289(20): 6324-6341. DOI:10.1111/febs.16541 |
| [21] |
CASTIGLIONI I, CACCIA R, GARCIA-MANTEIGA J M, et al. The Trithorax protein Ash1L promotes myoblast fusion by activating Cdon expression[J]. Nat Commun, 2018, 9(1): 5026. DOI:10.1038/s41467-018-07313-8 |
| [22] |
YIN B, YU F Y, WANG C L, et al. Epigenetic control of mesenchymal stem cell fate decision via histone methyltransferase Ash1l[J]. Stem Cells, 2019, 37(1): 115-127. DOI:10.1002/stem.2918 |
| [23] |
ZHANG K, HAVERSAT J M, MAGER J. CTR9/PAF1c regulates molecular lineage identity, histone H3K36 trimethylation and genomic imprinting during preimplantation development[J]. Dev Biol, 2013, 383(1): 15-27. DOI:10.1016/j.ydbio.2013.09.005 |
| [24] |
YU M M, JIA Y J, MA Z C, et al. Structural insight into ASH1L PHD finger recognizing methylated histone H3K4 and promoting cell growth in prostate cancer[J]. Front Oncol, 2022, 12: 906807. DOI:10.3389/fonc.2022.906807 |
| [25] |
LIU W M, XU L L, ZHANG C, et al. ASH1L may contribute to the risk of Tourette syndrome: combination of family-based analysis and case-control study[J]. Brain Behav, 2022, 12(4): e2539. |
| [26] |
ROGAWSKI D S, DENG J, LI H, et al. Discovery of first-in-class inhibitors of ASH1L histone methyltransferase with anti-leukemic activity[J]. Nat Commun, 2021, 12(1): 2792. DOI:10.1038/s41467-021-23152-6 |
| [27] |
YUAN W, XU M, HUANG C, et al. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation[J]. J Biol Chem, 2011, 286(10): 7983-7989. DOI:10.1074/jbc.M110.194027 |
| [28] |
ZHU L, LI Q, WONG S H K, et al. ASH1L links histone H3 lysine 36 dimethylation to MLL leukemia[J]. Cancer Discov, 2016, 6(7): 770-783. DOI:10.1158/2159-8290.CD-16-0058 |
| [29] |
BALBO POGLIANO C, GATTI M, RVTHEMANN P, et al. ASH1L histone methyltransferase regulates the handoff between damage recognition factors in global-genome nucleotide excision repair[J]. Nat Commun, 2017, 8(1): 1333. DOI:10.1038/s41467-017-01080-8 |
| [30] |
PARMAR N, CHANDRAKAR P, KAR S. Leishmania donovani Subverts Host Immune response by epigenetic reprogramming of Macrophage M(Lipopolysaccharides+IFN-γ)/M(IL-10) polarization[J]. J Immunol, 2020, 204(10): 2762-2778. DOI:10.4049/jimmunol.1900251 |
| [31] |
ALJAZI M B, GAO Y E, WU Y, et al. Histone H3K36me2-Specific methyltransferase ASH1L promotes MLL-AF9-Induced leukemogenesis[J]. Front Oncol, 2021, 11: 754093. DOI:10.3389/fonc.2021.754093 |
| [32] |
崔立欣, 田雅晴, 郝海生, 等. ASH1L甲基转移酶在牛卵丘细胞中的表达与功能研究[J]. 畜牧兽医学报, 2020, 51(8): 1866-1877. CUI L X, TIAN Y Q, HAO H S, et al. Expression and function of ASH1L methyltransferase in bovine cumulus cells[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(8): 1866-1877. (in Chinese) |
| [33] |
QIN L Y, WILLIAMS J B, TAN T, et al. Deficiency of autism risk factor ASH1L in prefrontal cortex induces epigenetic aberrations and seizures[J]. Nat Commun, 2021, 12(1): 6589. DOI:10.1038/s41467-021-26972-8 |
| [34] |
FUJIMOTO A, FURUTA M, TOTOKI Y, et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer[J]. Nat Genet, 2016, 48(6): 500-509. |
| [35] |
ABBASTABAR M, KHEYROLLAH M, AZIZIAN K, et al. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: a double-edged sword protein[J]. DNA Repair (Amst), 2018, 69: 63-72. DOI:10.1016/j.dnarep.2018.07.008 |
| [36] |
MU W P, STARMER J, SHIBATA Y, et al. EZH1 in germ cells safeguards the function of PRC2 during spermatogenesis[J]. Dev Biol, 2017, 424(2): 198-207. DOI:10.1016/j.ydbio.2017.02.017 |
| [37] |
MJELLE R, HEGRE S A, AAS P A, et al. Cell cycle regulation of human DNA repair and chromatin remodeling genes[J]. DNA Repair (Amst), 2015, 30: 53-67. DOI:10.1016/j.dnarep.2015.03.007 |
| [38] |
XU R F, QIN N, XU X X, et al. Inhibitory effect of SLIT2 on granulosa cell proliferation mediated by the CDC42-PAKs-ERK1/2 MAPK pathway in the prehierarchical follicles of the chicken ovary[J]. Sci Rep, 2018, 8(1): 9168. DOI:10.1038/s41598-018-27601-z |
| [39] |
LIU L X, KIMBALL S, LIU H, et al. Genetic alterations of histone lysine methyltransferases and their significance in breast cancer[J]. Oncotarget, 2015, 6: 2466-2482. DOI:10.18632/oncotarget.2967 |
| [40] |
LI G, YE Z S, SHI C, et al. The Histone methyltransferase Ash1l is required for epidermal homeostasis in mice[J]. Sci Rep, 2017, 7: 45401. DOI:10.1038/srep45401 |
(编辑 郭云雁)



