2. 江苏高校动物重要疫病和重要人兽共患病防控技术国际合作联合实验室,扬州 225009;
3. 农业与农产品安全教育部国际联合研究实验室,扬州 225009
2. International Research Laboratory of Prevention and Control of Important Animal Infectious Diseases and Zoonotic Diseases of Jiangsu Higher Education Institutions, Yangzhou 225009, China;
3. Joint International Research Laboratory of Agriculture and Agri-Product Safety, the Ministry of Education, Yangzhou 225009, China
铁死亡是由各种因素导致细胞内铁离子代谢障碍,活性氧(reactive oxygen species, ROS)和脂质过氧化物蓄积所引起的细胞程序性死亡,其主要特征为谷胱甘肽过氧化物酶4(glutathione peroxidase 4, GPX4)活性下降,细胞内游离铁水平增高,脂质过氧化物蓄积[1]。研究证实,铁死亡参与癌症[2-3]、退行性脑病[4]、缺血再灌注损伤[5]和镉致鸡的肝损伤[6]等疾病的发生和发展。由于机体在免疫防御过程中,以及细菌自身的代谢均可生成ROS[7],且细菌还可干扰宿主细胞对铁的代谢,因此,铁死亡在细菌感染诱导宿主细胞损伤的过程中发挥作用。
1 铁死亡概述2003年,铁死亡作为一种新型的细胞程序性死亡方式被首次发现和报道[8],于2012年最终命名[1]。目前研究发现3种途径可引发铁死亡:第一,用Erastin[9]、柳氮磺胺吡啶[10]、丁硫氨酸亚砜亚胺[11]等物质抑制胱氨酸/谷氨酸反向转运体(cystine/glutamate antiporter, system XC-),通过减少细胞内谷胱甘肽(glutathione, GSH)的含量,进而引发细胞氧化还原失衡导致铁死亡;第二,通过Ras选择性致死化合物(Ras-selective lethal compounds, RSLs)[12]直接抑制GPX4活性,最终导致细胞脂质过氧化物堆积,诱发铁死亡;第三,非依赖于GPX4的铁死亡通路,细胞膜上铁死亡抑制蛋白1(ferroptosis suppressor protein 1, FSP1)通过利用烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate, NADPH),形成还原型辅酶Q10(reduced coenzyme Q10),以此降解细胞膜上的脂质过氧化物,从而抑制铁死亡[13]。其中第一和第二种途径,均基于GSH的抗氧化防御系统失衡,导致脂质过氧化物大量蓄积,引发铁死亡。
在铁死亡通路中,关键调控因子system XC-由溶质载体家族7成员11(solute carrier family 7 member 11, SLC7A11)和溶质载体家族3成员2(solute carrier family 3 member 2, SLC3A2)组成[14]。在正常生理条件下,system XC-将胞外胱氨酸转运至胞内,同时将胞内的谷氨酸排出。细胞内的胱氨酸转变为半胱氨酸后,在谷氨酸半胱氨酸连接酶和谷胱甘肽合成酶的作用下生成GSH[15]。GPX4作为一种硒蛋白[16],以GSH作为底物来实现过氧化酶的作用,将细胞膜中的有毒脂质过氧化物转化为无毒脂质醇[17],以此抑制铁死亡。
作为一种细胞程序性死亡方式,早期认为铁死亡在形态学上与凋亡、坏死和自噬不同,具有自己独特的形态特征,如细胞膜破裂,细胞体积减小,线粒体膜固缩,但细胞核膜完整[1]。但随着研究的深入,发现细胞出现铁死亡后,表现出类似坏死的形态学变化[18],如细胞膜断裂或出泡、细胞质与细胞器肿胀、染色质凝集等,但线粒体形态学变化与坏死不同,可见线粒体萎缩、膜密度增加、嵴减少或缺失,以及线粒体外膜破裂等[19]。在某些情况下,铁死亡还伴随着细胞的分离与聚集,以及自噬体的增加[20]。此外,发生铁死亡的细胞可向周围未暴露于铁死亡诱导剂的细胞转移脂质过氧化物,从而导致周围细胞铁死亡[21]。
2 细菌性感染中发现的铁死亡现象在细菌感染过程中,铁代谢紊乱与脂质过氧化发挥了重要的作用。已有研究证实,在脂多糖(lipopolysaccharide, LPS)刺激或病原菌侵袭致病的过程中,铁死亡参与其中。
2.1 LPS及大肠杆菌与铁死亡LPS是革兰阴性菌细胞壁的主要成分之一,可在一定程度上模拟革兰阴性菌的感染。研究证实,LPS通过Toll样受体4(toll-like receptor 4, TLR4),激活核因子κB(nuclear factor kappa-B, NF-κB)信号通路,并促进下游包括白细胞介素1β(interleukin-1β, IL-1β)、白细胞介素6(interleukin-6, IL-6)、肿瘤坏死因子α(tumor necrosis factor α, TNF-α)等炎性细胞因子的产生[22]。目前,LPS已被证明可在多种组织中诱导铁死亡。
在肺损伤模型中,LPS与人支气管上皮细胞共孵育后,SLC7A11和GPX4蛋白水平下降,丙二醛(malondialdehyde, MDA)、4-羟基壬烯醛(4-hydroxynonenal, 4-HNE)和铁离子含量升高,使用铁死亡抑制剂——铁抑素-1(ferrostatin-1, Fer-1)后,可改善LPS造成的细胞损伤,并且在LPS诱导的小鼠肺损伤模型中,Fer-1治疗显著改善了肺部损伤[23],该研究说明,LPS可导致肺部组织发生铁死亡。在另一项小鼠肺损伤模型中,也得出了相似的结果,并且该研究还证明LPS可导致肺部前列腺素内过氧化物合酶(prostaglandin-endoperoxide synthase 2, PTGS2)表达量升高,核因子E2相关因子(nuclear factor E2 related factor 2, Nrf2)表达量降低[24],这些结果说明, 铁死亡发生时的脂质过氧化是通过上调PTGS2和抑制Nrf2通路造成的。此外,铁死亡与肺部炎症也存在密切关系,抑制LPS诱导的铁死亡后,可降低支气管上皮细胞炎性因子IL-1β、IL-6以及TNF-α的表达[25],还可减少小鼠肺部中性粒细胞浸润[26],以上研究说明,抑制铁死亡可作为治疗肺部炎症的潜在手段。
在LPS诱导脓毒症引起小鼠心组织损伤的研究中,小鼠心组织收缩和射血功能减弱,外周血中肌酸激酶同工酶MB(creatine kinase-MB, CK-MB)、乳酸脱氢酶和谷草转氨酶活性显著升高,病理组织学发现心肌中铁及脂质过氧化物水平升高,透射电镜观察到细胞内线粒体固缩,使用Fer-1治疗小鼠后,可显著减少心肌损伤,增加小鼠的存活率。此外,该研究还证明核受体共激活因子4(nuclear receptor coactivator 4, NCOA4)不仅可介导铁蛋白自噬,导致细胞内游离铁水平升高,还可通过上调siderofexin(SFXN1)线粒体锚定蛋白[27]表达,将细胞内的铁离子转运进入线粒体内,加重LPS诱导的心肌细胞损伤[28]。同时,研究也证实,LPS可导致小鼠心肌中铁转运蛋白1(ferroportin 1, FPN1)表达量降低,敲除FPN1后,可促进LPS诱导的心肌细胞内铁离子和脂质过氧水平升高,加重心肌损伤[29]。有文献表明,FPN1的表达下调,会导致细胞内过量的不稳定铁池(labile iron pool, LIP)无法从细胞内排出,形成恶性循环,进一步增加细胞内铁的含量。而过量的LIP可通过芬顿反应,产生大量的ROS,最终导致细胞脂质过氧化[30],进而导致铁死亡。以上结果证明LPS可通过铁死亡的方式造成心肌组织损伤,并且在这一过程中铁外排受阻与NCOA4介导的铁蛋白自噬发挥了重要作用。
LPS除了在以上两种组织中可导致铁死亡,在其他组织中也同样可导致铁死亡:在山羊乳腺炎模型中,LPS处理山羊乳腺上皮细胞后,细胞内Fe2+、ROS与MDA水平升高,GSH与GPX4降低,透射电镜观察发现细胞内线粒体嵴减少,膜密度升高,炎性因子IL-6与TNF-α表达升高,而使用Fer-1处理,可降低Fe2+水平与炎性因子表达,上调GPX4表达,增加细胞存活率,以上结果说明LPS可导致山羊乳腺上皮细胞铁死亡[31]。经LPS处理后,人滑膜细胞内MDA和铁含量升高,转铁蛋白受体(transferrin receptor, TFR)与NCOA4蛋白水平升高,SLC7A11、GPX4与Nrf2蛋白水平降低,这些结果说明LPS可诱导滑膜细胞发生铁死亡[32]。
大肠杆菌(Escherichia coli)为革兰阴性菌,是常见的致病菌,其在胞外感染的方式下,与草鱼红细胞共孵育,可导致血红素加氧酶1(heme oxygenase 1, HO-1)、自噬相关基因5(autophagy-related gene 5, ATG5)和铁蛋白的基因表达上调,FPN1基因表达被抑制[33]。这些结果表明,大肠杆菌可通过铁死亡的方式诱导草鱼红细胞死亡。
2.2 铜绿假单胞菌与铁死亡铜绿假单胞菌(Pseudomonas aeruginosa)为革兰阴性菌,可胞内感染细胞[34]。文献报道,LOX可选择性氧化细胞膜上的花生四烯酸磷脂酰乙醇胺(arachidonic acid phosphatidylethanolamines, AA-PE)来导致铁死亡[35]。铜绿假单胞菌感染人支气管上皮细胞时,可以合成脂氧合酶(lipoxygenase, LOX)将支气管上皮细胞膜中的AA-PE氧化为15-羟基二十碳四烯酸(15-hydroperoxyeicosatetraenoic acid, 15-HETE),导致15-HETE这种脂质过氧化物蓄积[36],使人支气管上皮细胞铁死亡。在小鼠肺部感染铜绿假单胞菌模型中也得到了相同的结论,并且,在使用铁死亡抑制剂后,可减轻细胞损伤与肺部炎症[37],以上结果说明铜绿假单胞菌感染可通过合成脂氧合酶,氧化宿主细胞多不饱和脂肪酸来引发铁死亡。
2.3 结核分枝杆菌与铁死亡结核分枝杆菌(mycobacterium tuberculosis)为胞内感染菌,在其感染人巨噬细胞时,GSH和GPX4活性降低,亚铁离子、线粒体超氧化物和脂质过氧化物水平增加,且使用Fer-1和铁螯合剂后,死亡细胞比例明显下降。在动物试验中,结核分枝杆菌感染小鼠肺部导致肺组织中GPX4活性降低,脂质过氧化物蓄积;而使用Fer-1治疗后,小鼠各器官的载菌量都明显降低,肺部损伤也减轻[38],故铁死亡在结核分枝杆菌致病的过程中起到了关键作用。
2.4 金黄色葡萄球菌与铁死亡金黄色葡萄球菌(Staphylococcus aureus)为革兰阳性胞内感染菌。目前,虽无文献表明其可通过铁死亡的方式诱导宿主细胞死亡,但其感染过程中造成的铁水平及脂质过氧化的变化可能与铁死亡密切相关[39]:金黄色葡萄球菌在感染细胞时能够进入宿主细胞中[40],并且其铁摄取调节子(ferric uptake regulator, Fur)可通过感知菌体外铁水平来调节自身毒素的释放,当铁缺乏时,Fur可通过增加溶血素的分泌,裂解红细胞,导致血红蛋白释放,从而使组织中铁水平升高[41];其次,金黄色葡萄球菌感染小鼠肺部可导致严重的氧化应激,使肺部发生脂质过氧化,降低GSH水平[42],由此可见,金黄色葡萄球菌感染可能会促进铁死亡发生。另一方面,有文献报道宿主细胞中的花生四烯酸和脂质过氧化产物可杀死金黄色葡萄球菌[43],金黄色葡萄球菌产生的Staphyloferrin A和B可螯合宿主细胞内的Fe2+[39],会减少芬顿反应的发生,从而抑制铁死亡的发生。综合以上结果表明,铁死亡可能参与了金黄色葡萄球菌致病过程,但具体机制仍需进一步研究。
2.5 细菌感染诱导铁死亡的机制基于以上报道,LPS及其他细菌诱导铁死亡的过程中,其机制如图 1所示。其中,铁的过载主要依靠以下途径:①NCOA4通过选择性自噬铁蛋白,释放其中的亚铁离子;②上调TFR的表达,增强细胞对铁的摄取;③抑制FPN1的表达,阻碍细胞铁的外排。脂质过氧化主要通过:①依靠抑制Nrf2/GPX4/FPN1轴,细胞内依赖GSH的抗氧化系统受损,导致细胞清除脂质过氧化物的能力下降;②通过损伤线粒体,产生过量的ROS,最终导致脂质过氧化物蓄积。
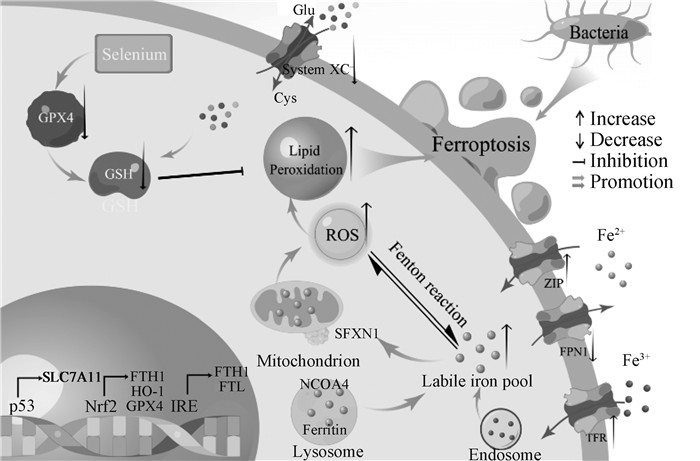
|
图 1 细菌感染诱导铁死亡机制示意图 Fig. 1 Schematic diagram of the mechanism of ferroptosis induced by bacteria |
由于在细菌感染中铁代谢紊乱促进了铁死亡的发生,故铁代谢可能参与了细菌感染的进程。正常情况下,机体循环系统中铁以转铁蛋白(transferrin, TF)和非转铁蛋白结合铁(non-transferrin bound iron, NTBI)两种形式存在。当TF与TFR结合后,TF在胞内囊泡酸化使Fe3+与TF解离,然后通过前列腺六段跨膜上皮抗原3(six-transmembrane epithelial antigen of prostate 3, STEAP3)还原为Fe2+;而NTBI通过锌转运蛋白(zinc transport, ZIP)中的ZIP8或ZIP14进入细胞[44]。铁进入细胞后以两种形式储存:一种是铁与蛋白质结合形成铁蛋白;另一种是铁离子弱结合,形成LIP[45]。当细胞内的铁超过正常水平时,多余的铁通过FPN1排出细胞[46]。
3.1 细菌感染对铁吸收的影响细菌感染可导致细胞因子的过度释放,从而上调TFR的表达,促进铁进入细胞[47]。在LPS诱导小鼠炎症模型的研究中发现,小鼠的血清铁和TF浓度显著降低,与TF结合的铁通过TFR转运进细胞内[48],肝细胞和神经细胞内铁调素表达增加,促进TFR表达[49]。神经系统的炎症还可上调二价金属转运蛋白1(divalent metal transporter 1, DMT1)的表达,使细胞外的铁通过DMT1转移到细胞内[50]。在小鼠盲肠结肠结扎穿刺导致的败血症模型[51],以及LPS刺激小鼠模型研究中发现,ZIP14表达上调[52],表明细胞内NTBI转运增强。以上结果说明细菌感染会增强细胞对铁的吸收,导致细胞内铁水平的升高,为铁死亡的发生提供基础。
3.2 细菌感染对铁储存的影响细胞内铁水平受到铁反应元件-铁调节蛋白(iron-responsive elements-iron regulatory proteins, IREs-IRPs)系统的翻译后调控[53]。细胞内铁浓度升高会加速铁蛋白mRNA的翻译,使游离铁通过铁蛋白的形式储存,从而降低细胞内的游离铁水平。当铁蛋白被降解或细胞应激而激活非选择性自噬时,细胞内游离铁水平升高[54]。研究证明,使用牙龈卟啉单胞菌产生的LPS刺激人牙周膜细胞可促进铁蛋白的表达[55],牙龈卟啉单胞菌感染牙周膜成纤维细胞12 h后,铁蛋白水平升高,但在感染24 h候后铁蛋白水平下降,且随着感染时间延长,NCOA4介导的铁蛋白自噬被激活,增加细胞内的游离铁水平,敲除NCOA4后可减少炎性因子的生成[56],该研究说明,细菌在长时间感染细胞后,铁蛋白自噬是细胞内游离铁的重要来源。铁是LOX和PTGS的辅助因子,随着铁水平的上升可增强LOX和PTGS的活性,导致脂质ROS和炎性因子释放,进而加剧脂质过氧化和铁水平的进一步升高[57],从而促进铁死亡的发生。线粒体是铁代谢的主要场所,虽然线粒体可以合成线粒体铁蛋白(mitochondrial ferritin, FTMT),但FTMT易被NCOA4介导的自噬降解[58]。并且,细菌感染导致的ROS会显著降低线粒体的铁代谢能力,使铁潴留在线粒体内,从而产生过量的ROS[44],最终导致细胞脂质过氧化,发生铁死亡。
3.3 细菌感染对铁外排的影响FPN1是唯一可将细胞内的铁运输到细胞外的蛋白[59]。当LPS与TLR结合,FPN1的转录会被下调[60],故LPS可通过抑制FPN1导致铁潴留在细胞内,加重氧化损伤,促进铁死亡的发生。研究发现,当豚鼠感染结核分枝杆菌后,Nrf2蛋白表达量升高,但并没有从细胞质进入细胞核发挥作用,且其下游抗氧化蛋白表达量也呈下降趋势,说明结核分枝杆菌可抑制Nrf2通路的激活[61],该研究虽没有检测FPN1的表达,但由于FPN1的表达受Nrf2通路的调控[62],故在结核分枝杆菌感染后,FPN1表达也可能被抑制,导致细胞内的铁在外排时受阻,使细胞内铁水平升高,促进了铁死亡的发生。
4 存在的问题与展望近些年,随着铁死亡的研究不断深入,其作用机制不断被揭露,但仍有许多问题亟待解决。脂质过氧化被认为是铁死亡发生的标志,但并非所有脂质过氧化造成的损伤都能引起铁死亡[63],故需要更深入的研究探讨脂质过氧化物在铁死亡中的作用。有文献指出,可以将多不饱和脂肪酰磷脂(polyunsaturated fatty acyl phospholipids, PUFA-PLs)和氧化还原活性铁(redox active iron)作为铁死亡的生物学标志[64],但氧化还原活性铁与PUFA-PLs具体通过怎样的机制导致细胞死亡还需更进一步阐明。
在兽医领域中,已对细菌感染疾病中的脂质过氧化进行了较多的研究,如在奶牛生产繁殖过程中,细菌感染是奶牛乳腺炎[65]和子宫内膜炎[66]的主要病因之一,患有大肠杆菌性乳腺炎的荷斯坦奶牛血液、乳汁及尿液中的脂质过氧化物呈显著上升趋势[67],而在乳房灌注铁螯合剂后可减轻乳腺损伤[68],由此可见,大肠杆菌感染奶牛乳腺过程中,铁与脂质过氧化物的蓄积发挥了重要作用。有文献表明,巴黎链球菌(Streptococcus lutetiensis)感染奶牛乳腺上皮后,可导致ROS大量生成,并能抑制Nrf2通路蛋白表达[69],使奶牛乳腺上皮细胞脂质过氧化物蓄积。LPS还能导致奶牛子宫内膜细胞发生严重的氧化应激[70],造成细胞脂质过氧化。以上结果表明,细菌感染可导致奶牛乳腺与子宫发生脂质过氧化,但铁死亡是否参与其中尚未见报道。
综上所述,靶向抑制铁死亡可能成为动物细菌性疾病的潜在治疗手段,通过更深入研究细菌与铁死亡的关系,能为动物生产养殖中预防和治疗细菌性疾病提供更多的理论支持,并最终为提高动物饲养管理水平做出科学指导。
| [1] |
DIXON S J, LEMBERG K M, LAMPRECHT M R, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death[J]. Cell, 2012, 149(5): 1060-1072. DOI:10.1016/j.cell.2012.03.042 |
| [2] |
LIN R Y, ZHANG Z H, CHEN L F, et al. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells[J]. Cancer Lett, 2016, 381(1): 165-175. DOI:10.1016/j.canlet.2016.07.033 |
| [3] |
MA S, HENSON E S, CHEN Y, et al. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells[J]. Cell Death Dis, 2016, 7(7): e2307. DOI:10.1038/cddis.2016.208 |
| [4] |
DO VAN B, GOUEL F, JONNEAUX A, et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC[J]. Neurobiol Dis, 2016, 94: 169-178. DOI:10.1016/j.nbd.2016.05.011 |
| [5] |
GAO M H, MONIAN P, QUADRI N, et al. Glutaminolysis and transferrin regulate ferroptosis[J]. Mol Cell, 2015, 59(2): 298-308. DOI:10.1016/j.molcel.2015.06.011 |
| [6] |
陈敬宜, 于淼, 张金洋, 等. 铁死亡参与镉暴露鸡肝损伤的研究[J]. 畜牧兽医学报, 2023, 54(2): 787-802. CHEN J Y, YU M, ZHANG J Y, et al. Study on the involvement of ferroptosis in liver injury of cadmium-exposed chickens[J]. Acta Veterinaria et Zootechnica Sinica, 2023, 54(2): 787-802. (in Chinese) |
| [7] |
刘武康, 吴淑燕, 陈国薇, 等. 细菌产生的活性氧及其功能[J]. 微生物学杂志, 2016, 36(1): 89-95. LIU W K, WU S Y, CHEN G W, et al. The reactive oxygen species generated by bacteria and its functions[J]. Journal of Microbiology, 2016, 36(1): 89-95. (in Chinese) |
| [8] |
DOLMA S, LESSNICK S L, HAHN W C, et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells[J]. Cancer Cell, 2003, 3(3): 285-296. DOI:10.1016/S1535-6108(03)00050-3 |
| [9] |
WANG L Y, LIU Y C, DU T T, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc-[J]. Cell Death Differ, 2020, 27(2): 662-675. DOI:10.1038/s41418-019-0380-z |
| [10] |
DIXON S J, PATEL D N, WELSCH M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis[J]. Elife, 2014, 3: e2523. |
| [11] |
XIE Y C, ZHU S, SONG X X, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity[J]. Cell Rep, 2017, 20(7): 1692-1704. DOI:10.1016/j.celrep.2017.07.055 |
| [12] |
CHEN Y, LIU Y, LAN T, et al. Quantitative profiling of protein carbonylations in ferroptosis by an aniline-derived probe[J]. J Am Chem Soc, 2018, 140(13): 4712-4720. DOI:10.1021/jacs.8b01462 |
| [13] |
BERSUKER K, HENDRICKS J M, LI Z P, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis[J]. Nature, 2019, 575(7784): 688-692. DOI:10.1038/s41586-019-1705-2 |
| [14] |
PITMAN K E, ALLURI S R, KRISTIAN A, et al. Influx rate of18F-fluoroaminosuberic acid reflects cystine/glutamate antiporter expression in tumour xenografts[J]. Eur J Nucl Med Mol Imaging, 2019, 46(10): 2190-2198. DOI:10.1007/s00259-019-04375-8 |
| [15] |
梅胜兰, 夏中元, 孟庆涛, 等. 细胞铁死亡发生机制的研究进展[J]. 医学综述, 2020, 26(21): 4207-4211, 4218. MEI S L, XIA Z Y, MENG Q T, et al. Research advances in mechanism of ferroptosis in cells[J]. Medical Recapitulate, 2020, 26(21): 4207-4211, 4218. (in Chinese) |
| [16] |
INGOLD I, BERNDT C, SCHMITT S, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis[J]. Cell, 2018, 172(3): 409-422. DOI:10.1016/j.cell.2017.11.048 |
| [17] |
CAO J Y, DIXON S J. Mechanisms of ferroptosis[J]. Cell Mol Life Sci, 2016, 73(11-12): 2195-2209. DOI:10.1007/s00018-016-2194-1 |
| [18] |
VANDEN BERGHE T, LINKERMANN A, JOUAN-LANHOUET S, et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways[J]. Nat Rev Mol Cell Biol, 2014, 15(2): 135-147. DOI:10.1038/nrm3737 |
| [19] |
RIEGMAN M, SAGIE L, GALED C, et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture[J]. Nat Cell Biol, 2020, 22(9): 1042-1048. DOI:10.1038/s41556-020-0565-1 |
| [20] |
ANGELI J P F, SCHNEIDER M, PRONETH B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice[J]. Nat Cell Biol, 2014, 16(12): 1180-1191. DOI:10.1038/ncb3064 |
| [21] |
NISHIZAWA H, MATSUMOTO M, CHEN G, et al. Lipid peroxidation and the subsequent cell death transmitting from ferroptotic cells to neighboring cells[J]. Cell Death Dis, 2021, 12(4): 332. DOI:10.1038/s41419-021-03613-y |
| [22] |
KIM H J, JOE H I, ZHANG Z Y, et al. Anti-inflammatory effect of Acalypha australis L.via suppression of NF-κB signaling in LPS-stimulated RAW 264.7 macrophages and LPS-induced septic mice[J]. Mol Immunol, 2020, 119: 123-131. DOI:10.1016/j.molimm.2020.01.010 |
| [23] |
LIU P F, FENG Y T, LI H W, et al. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis[J]. Cell Mol Biol Lett, 2020, 25(1): 10-24. DOI:10.1186/s11658-020-00205-0 |
| [24] |
HE R Y, LIU B H, XIONG R, et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury[J]. Cell Death Discov, 2022, 8(1): 43. DOI:10.1038/s41420-021-00807-3 |
| [25] |
LI J C, LU K M, SUN F L, et al. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway[J]. J Transl Med, 2021, 19(1): 96. DOI:10.1186/s12967-021-02745-1 |
| [26] |
BAO C, LIU C, LIU Q, et al. Liproxstatin-1 alleviates LPS/IL-13-induced bronchial epithelial cell injury and neutrophilic asthma in mice by inhibiting ferroptosis[J]. Int Immunopharmacol, 2022, 109: 108770. DOI:10.1016/j.intimp.2022.108770 |
| [27] |
ZHENG H R, JI C N, ZOU X Q, et al. Molecular cloning and characterization of a novel human putative transmembrane protein homologous to mouse sideroflexin associated with sideroblastic anemia[J]. DNA Seq, 2003, 14(5): 369-373. DOI:10.1080/10425170310001605491 |
| [28] |
LI N, WANG W, ZHOU H, et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury[J]. Free Radical Biol Med, 2020, 160: 303-318. DOI:10.1016/j.freeradbiomed.2020.08.009 |
| [29] |
FANG J, KONG B, SHUAI W, et al. Ferroportin-mediated ferroptosis involved in new-onset atrial fibrillation with LPS-induced endotoxemia[J]. Eur J Pharmacol, 2021, 913: 174622. DOI:10.1016/j.ejphar.2021.174622 |
| [30] |
KUANG F M, LIU J, TANG D L, et al. Oxidative damage and antioxidant defense in ferroptosis[J]. Front Cell Dev Biol, 2020, 8: 586578. DOI:10.3389/fcell.2020.586578 |
| [31] |
ZHU G Q, SUI S P, SHI F Y, et al. Inhibition of USP14 suppresses ferroptosis and inflammation in LPS-induced goat mammary epithelial cells through ubiquitylating the IL-6 protein[J]. Hereditas, 2022, 159(1): 21. DOI:10.1186/s41065-022-00235-y |
| [32] |
LUO H S, ZHANG R. Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis[J]. Exp Ther Med, 2020, 21(1): 72. DOI:10.3892/etm.2020.9504 |
| [33] |
YANG M X, LU Z J, LI F L, et al. Escherichia coli induced ferroptosis in red blood cells of grass carp (Ctenopharyngodon idella)[J]. Fish Shellfish Immun, 2021, 112: 159-167. DOI:10.1016/j.fsi.2020.09.036 |
| [34] |
PENARANDA C, CHUMBLER N M, HUNG D T. Dual transcriptional analysis reveals adaptation of host and pathogen to intracellular survival of Pseudomonas aeruginosa associated with urinary tract infection[J]. PLoS Pathog, 2021, 17(4): e1009534. DOI:10.1371/journal.ppat.1009534 |
| [35] |
ZHU H, SANTO A, JIA Z Q, et al. GPx4 in bacterial infection and polymicrobial sepsis: Involvement of ferroptosis and pyroptosis[J]. React Oxyg Species (Apex), 2019, 7(21): 154-160. |
| [36] |
DAR H H, TYURINA Y Y, MIKULSKA-RUMINSKA K, et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium[J]. J Clin Invest, 2018, 128(10): 4639-4653. DOI:10.1172/JCI99490 |
| [37] |
OUSINGSAWAT J, SCHREIBER R, GULBINS E, et al. P. aeruginosa induced lipid peroxidation causes ferroptotic cell death in airways[J]. Cell Physiol Biochem, 2021,, 55(5): 590-604. DOI:10.33594/000000437 |
| [38] |
AMARAL E P, COSTA D L, NAMASIVAYAM S, et al. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis[J]. J Exp Med, 2019, 216(3): 556-570. DOI:10.1084/jem.20181776 |
| [39] |
SOE Y M, BEDOUI S, STINEAR T P, et al. Intracellular Staphylococcus aureus and host cell death pathways[J]. Cell Microbiol, 2021, 23(5): e13317. |
| [40] |
CHRISTMAS B A F, ROLFE M D, ROSE M, et al. Staphylococcus aureus adaptation to aerobic low-redox-potential environments: Implications for an intracellular lifestyle[J]. Microbiology (Reading), 2019, 165(7): 779-791. DOI:10.1099/mic.0.000809 |
| [41] |
TORRES V J, ATTIA A S, MASON W J, et al. Staphylococcus aureus fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia[J]. Infect Immun, 2010, 78(4): 1618-1628. DOI:10.1128/IAI.01423-09 |
| [42] |
WANG F, WANG R L, LIU H F. The acute pulmonary toxicity in mice induced by Staphylococcus aureus, particulate matter, and their combination[J]. Exp Anim, 2019, 68(2): 159-168. DOI:10.1538/expanim.18-0102 |
| [43] |
BEAVERS W N, MONTEITH A J, AMARNATH V, et al. Arachidonic acid kills Staphylococcus aureus through a lipid peroxidation mechanism[J]. mBio, 2019, 10(5): e01333-19. |
| [44] |
LIU Q J, WU J, ZHANG X F, et al. Iron homeostasis and disorders revisited in the sepsis[J]. Free Radical Biol Med, 2021, 165: 1-13. |
| [45] |
COLINS A, GERDTZEN Z P, NUÑEZ M T, et al. Mathematical modeling of intestinal iron absorption using genetic programming[J]. PLoS One, 2017, 12(1): e0169601. DOI:10.1371/journal.pone.0169601 |
| [46] |
DONOVAN A, BROWNLIE A, ZHOU Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter[J]. Nature, 2000, 403(6771): 776-781. DOI:10.1038/35001596 |
| [47] |
LUDWICZEK S, AIGNER E, THEURL I, et al. Cytokine-mediated regulation of iron transport in human monocytic cells[J]. Blood, 2003, 101(10): 4148-4154. DOI:10.1182/blood-2002-08-2459 |
| [48] |
WEI S S, BI J B, YANG L F, et al. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice[J]. Clin Transl Med, 2020, 10(5): e173. |
| [49] |
WANG Q, DU F, QIAN Z M, et al. Lipopolysaccharide induces a significant increase in expression of iron regulatory hormone Hepcidin in the cortex and substantia Nigra in rat brain[J]. Endocrinology, 2008, 149(8): 3920-3925. DOI:10.1210/en.2007-1626 |
| [50] |
URRUTIA P, AGUIRRE P, ESPARZA A, et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells[J]. J Neurochem, 2013, 126(4): 541-549. DOI:10.1111/jnc.12244 |
| [51] |
WESSELS I, COUSINS R J. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation[J]. Am J Physiol Gastrointest Liver Physiol, 2015, 309(9): G768-G778. DOI:10.1152/ajpgi.00179.2015 |
| [52] |
AYDEMIR T B, COUSINS R J. The multiple faces of the metal transporter ZIP14 (SLC39A14)[J]. J Nutr, 2018, 148(2): 174-184. DOI:10.1093/jn/nxx041 |
| [53] |
SANCHEZ M, GALY B, SCHWANHAEUSSER B, et al. Iron regulatory protein-1 and -2:Transcriptome-wide definition of binding mRNAs and shaping of the cellular proteome by iron regulatory proteins[J]. Blood, 2011, 118(22): E168-E179. DOI:10.1182/blood-2011-04-343541 |
| [54] |
MANCIAS J D, VAITES L P, NISSIM S, et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis[J]. Elife, 2015, 4: e10308. DOI:10.7554/eLife.10308 |
| [55] |
HUANG W X, ZHAN Y L, ZHENG Y F, et al. Up-regulated ferritin in periodontitis promotes inflammatory cytokine expression in human periodontal ligament cells through transferrin receptor via ERK/P38 MAPK pathways[J]. Clin Sci (Lond), 2019, 133(1): 135-148. DOI:10.1042/CS20180679 |
| [56] |
GUO W, ZHAO Y H, LI H X, et al. NCOA4-mediated ferritinophagy promoted inflammatory responses in periodontitis[J]. J Periodontal Res, 2021, 56(3): 523-534. DOI:10.1111/jre.12852 |
| [57] |
MAYR L, GRABHERR F, SCHWÄRZLER J, et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn's disease[J]. Nat Commun, 2020, 11(1): 1775. DOI:10.1038/s41467-020-15646-6 |
| [58] |
FUHRMANN D C, MONDORF A, BEIFUSS J, et al. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis[J]. Redox Biol, 2020, 36: 101670. DOI:10.1016/j.redox.2020.101670 |
| [59] |
GAO G F, LI J, ZHANG Y T, et al. Cellular iron metabolism and regulation[J]. Adv Exp Med Biol, 2019, 1173: 21-32. |
| [60] |
GUIDA C, ALTAMURA S, KLEIN F A, et al. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia[J]. Blood, 2015, 125(14): 2265-2275. DOI:10.1182/blood-2014-08-595256 |
| [61] |
PALANISAMY G S, KIRK N M, ACKART D F, et al. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis[J]. PLoS One, 2011, 6(10): e26254. DOI:10.1371/journal.pone.0026254 |
| [62] |
MARRO S, CHIABRANDO D, MESSANA E, et al. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position-7007 of the FPN1 promoter[J]. Haematologica, 2010, 95(8): 1261-1268. DOI:10.3324/haematol.2009.020123 |
| [63] |
TANG D L, CHEN X, KANG R, et al. Ferroptosis: molecular mechanisms and health implications[J]. Cell Res, 2021, 31(2): 107-125. DOI:10.1038/s41422-020-00441-1 |
| [64] |
HADIAN K, STOCKWELL B R. A roadmap to creating ferroptosis-based medicines[J]. Nat Chem Biol, 2021, 17(11): 1113-1116. DOI:10.1038/s41589-021-00853-z |
| [65] |
KACZOROWSKI Ł, POWIERSKA-CZARNY J, WOLKO Ł, et al. The influence of bacteria causing subclinical mastitis on the structure of the cow's milk microbiome[J]. Molecules, 2022, 27(6): 1829. DOI:10.3390/molecules27061829 |
| [66] |
SIQUEIRA L C, FAVARETTO B, MORAES B T, et al. Bovine Endometritis and the inflammatory peripheral cholinergic system[J]. Appl Biochem Biotechnol, 2020, 190(4): 1242-1256. DOI:10.1007/s12010-019-03157-0 |
| [67] |
MAVANGIRA V, KUHN M J, ABUELO A, et al. Activity of sEH and oxidant status during systemic bovine coliform mastitis[J]. Antioxidants (Basel), 2021, 10(5): 812. DOI:10.3390/antiox10050812 |
| [68] |
LAUZON K, ZHAO X, LACASSE P. Deferoxamine reduces tissue damage during endotoxin-induced mastitis in dairy cows[J]. J Dairy Sci, 2006, 89(10): 3846-3857. DOI:10.3168/jds.S0022-0302(06)72427-4 |
| [69] |
CHEN P, YANG J Y, WU N W, et al. Streptococcus lutetiensis induces autophagy via oxidative stress in bovine mammary epithelial cells[J]. Oxid Med Cell Longev, 2022, 2022: 2549772. |
| [70] |
GUGLIANDOLO E, FUSCO R, LICATA P, et al. Protective effect of hydroxytyrosol on LPS-induced inflammation and oxidative stress in bovine endometrial epithelial cell line[J]. Vet Sci, 2020, 7(4): 161. DOI:10.3390/vetsci7040161 |
(编辑 范子娟)



