2. 石家庄天泉良种奶牛有限公司, 石家庄 050200
2. Shijiazhuang Tianquan Elite Dairy Ltd., Shijiazhuang 050200, China
卵母细胞活体采卵(ovum pick-up,OPU)是一项能够获得优良种母牛卵母细胞的新型技术,该技术在动物胚胎生产和发育机理研究及人类辅助生殖技术临床实践中有着广泛的应用前景。OPU可以提高种母畜和珍稀野生动物的资源保护率,提高优良母畜的遗传力,为牛生物技术发展提供必要的支撑[1]。
OPU与体外受精(in vitro fertilization, IVF)技术相结合能够加快优良品种的繁育进程,让具有高遗传价值的牛产生大量后代有助于增加选择强度和缩短世代间隔,从而显著改善其遗传背景,批量生产良种胚胎,保存优良品种使其得到扩繁[2],对于充分发挥良种牛的繁育潜力具有重要价值。体外生产体系还被用于生产大量科学研究所需的胚胎,包括通过体细胞核移植生产克隆动物、转基因动物、胚胎干细胞[3]以及拯救不可替代的遗传物质[4]。据报道,OPU-IVF的胚胎生产效率高于体内胚胎生产[5]。
近年来,养牛业对可移植胚胎的需求不断增加,全球体外生产的胚胎数量也在不断增加,并与体内生产的胚胎数量相似[6]。对性能良好的牛进行OPU获得卵母细胞并进行IVF产生体外胚胎。然而,OPU的卵母细胞发育能力仍然相对较差,大多数卵母细胞会发生退化,为了提高体外胚胎生产效率,应该正确预测卵母细胞的发育能力,并以合适的方式培养它们。如果能产生高能力的卵母细胞,体外胚胎生产效率将会提高,牛的遗传改良将大大加快。研究表明,体外成熟卵母细胞的发育能力低于体内成熟卵母细胞[7]。从而需要进一步提高卵母细胞体外成熟能力,以改善牛体外胚胎生产系统。因此,本文分析了OPU卵母细胞体外成熟存在的问题,总结了提高卵母细胞体外成熟能力的方法,以期为OPU卵母细胞体外成熟体系的优化提供一定的参考。
1 OPU卵母细胞体外成熟存在的问题卵母细胞体外成熟是体外胚胎生产过程中卵母细胞获得发育能力的关键步骤之一。卵巢内原始卵泡库非常丰富,如何将这些未成熟的卵母细胞在体外成熟培养,为体细胞克隆、转基因技术以及胚胎体外生产技术提供更多的卵母细胞,是科学研究和产业化生产最关键、最基础的步骤之一。提高牛卵母细胞成熟度对于优化体外胚胎至关重要,卵母细胞的能力包括减数分裂恢复以及细胞质和分子成熟的能力。
1.1 卵母细胞胞质成熟不同步核成熟是在第一次减数分裂的恢复、过程和完成以及向第二次分裂的过渡期间实现的,在成熟的卵母细胞中,第二次分裂将在中期停止。细胞质成熟包括卵母细胞细胞质中的所有变化,包括多腺苷酸化母体RNA和受精过程中配子融合所必需的蛋白质的积累,以及在卵母细胞mRNA的控制下胚胎的有丝分裂,直到胚胎基因组逐渐被激活[8]。卵母细胞减数分裂和细胞质成熟的同步对于成功受精以及植入前后的发育非常重要[9]。然而,传统的IVM侧重于促进核成熟,无法支持细胞质成熟,它没有实现减数分裂和细胞质成熟的同步。因此,常规IVM后受精、胚胎发育、植入和妊娠都会受到影响。
哺乳动物卵母细胞在终变期停滞,直到它们完成生长。减数分裂是通过诱导体内排卵前促黄体生成素(LH) 激增,或通过将卵母细胞从其周围的卵泡细胞转移到合适的培养条件中来恢复[10]。当细胞核和细胞质准备好受精并能够启动早期胚胎发育时,就会发生体内卵母细胞成熟[11]。然而,未成熟的卵母细胞没有足够的时间进行所有必要的变化以获得完整的有能力的细胞质成熟[12]。在体外成熟过程中,细胞核和细胞质之间缺乏同步性导致体外受精胚胎效率低。
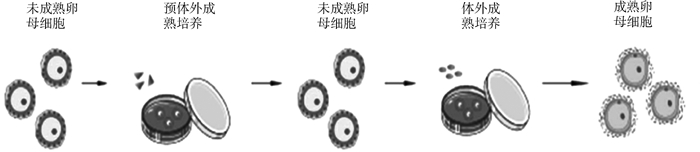
近年来已建立了尽可能接近体内发育微环境的培养体系(pre-IVM)[13-14],如图 1所示。未成熟卵母细胞先用pre-IVM系统处理,抑制卵母细胞自发减数分裂恢复,维持卵母细胞-卵丘细胞间隙连接通讯,并促进获得卵母细胞发育。然后再放入IVM液中培养,诱导卵母细胞减数分裂恢复和成熟,这种双相培养体系在过去十年中显着提高了IVM的效率[15]。
|
图 1 包括Pre-IVM培养和IVM培养的双相培养体系[14] Fig. 1 Biphasic IVM system of two steps, pre-IVM culture and IVM culture [14] |
氧化应激可能是选择性闭锁卵泡的起始因素之一[16],卵巢中抗氧化基因的缺失会导致卵泡异常生长。与体内环境相比,IVM期间的卵母细胞受到由活性氧(ROS) 引起的更高的氧化应激[17],ROS是一种生理调节卵母细胞功能的信号,而其积累会导致氧化应激并损害卵母细胞和胚胎发育[18]。
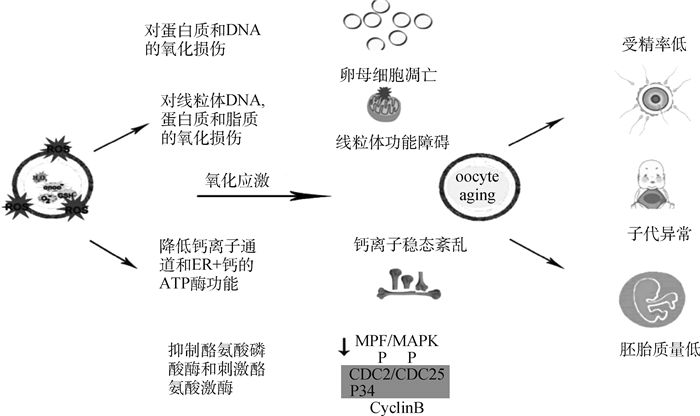
氧化应激导致卵母细胞受精率降低,胚胎质量下降,并增加了与卵母细胞衰老相关的后代异常的可能性。如图 2所示,氧化应激有可能通过多种途径损害卵母细胞:1)通过抑制酪氨酸磷酸酶和刺激酪氨酸激酶导致MPF和MAPK水平下降,最终导致减数分裂异常;2)通过质膜中的脂质过氧化,降低膜的流动性并可能影响卵母细胞与精子融合的能力;3)通过对蛋白质、DNA和线粒体的氧化损伤导致随后的功能障碍[19]。
|
图 2 氧化应激对卵母细胞和雌性生殖的影响[19] Fig. 2 Effects of oxidative stress on the oocyte and the female reproduction [19] |
利钠肽(natriuretic peptides,NPs)是具有相似序列和构象的肽家族,包括心钠肽(atrial natriuretic peptide,ANP)、脑利钠肽(brain natriuretic peptide,BNP)、石斛利钠肽(dendroaspis natriuretic peptide,DNP)和C型利钠肽(C-type natriuretic peptide,CNP)。1990年在猪脑提取物中发现了由利钠肽前体C(natriuretic peptide precursor C,NPPC)基因编码的CNP[20]。CNP是一种在多种组织中表达的旁分泌和内分泌因子[21],CNP的生物学效应是由细胞内环磷酸鸟苷(cGMP) 积累介导的,特别是通过膜结合利钠肽受体2 (membrane-bound natriuretic peptide receptor,NPR2)[22]。CNP参与多种生理过程,如维持软骨和骨功能[23]、调节心和血管功能[24]、调节血脑屏障通透性[25]、保护肾功能[26]和维持减数分裂[27]。
2.2 C型钠肽维持减数分裂停滞CNP可作为优化体外成熟胚胎体系的有效因素,从而促进体外成熟卵母细胞的发育能力。CNP改善牛卵母细胞胞质成熟的机制是由于体外减数分裂停滞期间母体mRNA和蛋白质水平升高,导致胚胎发育率超过“母体-胚胎过渡”阶段[28]。
CNP在卵泡壁上的颗粒细胞中表达,而卵母细胞周围的卵丘细胞表达CNP受体NPR2的mRNA。如图 3所示,CNP通过激活NPR2来刺激卵丘细胞中cGMP的产生,环状cGMP通过间隙连接转移到卵母细胞,通过抑制磷酸二酯酶3A (PDE3A) 的水解活性来调节cAMP水平,cAMP由腺苷酸环化酶(ADCY)产生,该酶由GPR3和GPR12通过Gs蛋白的组成作用控制[29-30],从而维持卵母细胞减数分裂停滞。
2.3 C型钠肽在卵母细胞成熟上的应用最近的研究表明,CNP可以通过维持足够水平的cAMP暂时维持体外培养的牛卵母细胞减数分裂停滞6~8 h[31-32],对卵母细胞发育能力产生积极影响,增加牛卵母细胞胞质的成熟[33]和卵母细胞体外发育能力[34],增加囊胚细胞数[31]。
研究表明,在IVM前用CNP预处理可显著增加牛[33]和绵羊[35]卵母细胞线粒体发育和抗氧化防御机制相关的mRNA转录物和蛋白质的相对丰度,并降低卵母细胞中的ROS含量,改善线粒体功能和抗氧化防御机制,有助于增强卵母细胞的胞质成熟。在IVM之前用CNP处理未成熟牛卵母细胞时,当线粒体膜电位较大时,培养基中的ROS积累较少,这表明CNP可能不仅调节线粒体发育,而且还诱导抗氧化机制,据报道SIRT1通过增加细胞抗氧化酶的浓度来保护细胞免受ROS诱导的氧化应激,包括锰超氧化物歧化酶(MnSOD)、过氧化氢酶(CAT)和谷胱甘肽过氧化物酶(GPX)[36-37]。越来越多的研究表明,抗氧化蛋白的丰度与卵母细胞IVM期间的ROS浓度相关[38]。在IVM之前用CNP处理未成熟卵母细胞导致抗氧化酶GPX1、CAT和MnSOD mRNA转录物的相对丰度更高[37, 39]。
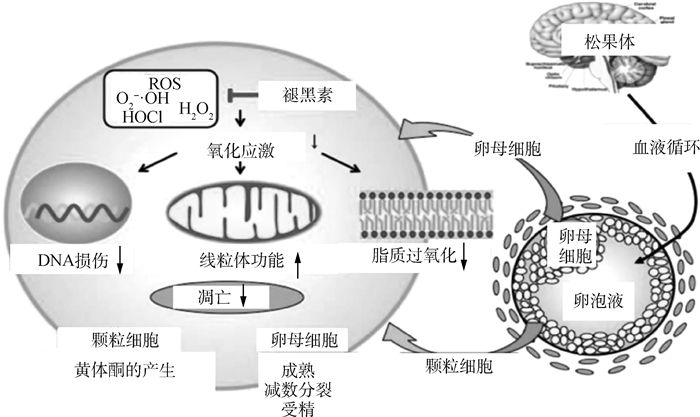
3 褪黑素对卵母细胞成熟的影响 3.1 褪黑素的简介褪黑素主要由脊椎动物的松果体分泌,具有免疫调节和细胞保护作用,同时也是一种抗凋亡剂[40]。作为一种有效的抗氧化剂,褪黑素可防止ROS的氧化应激,这是辅助生殖技术(ART) 中配子缺陷或胚胎发育不良的主要原因之一[41]。体外成熟环境下较高的氧气浓度会导致产生高浓度的ROS,这可能对发育中的胚胎产生不利影响,褪黑素的抗氧化性提高了劣质卵母细胞的质量[42],褪黑素减少了小鼠卵母细胞的减数分裂缺陷[43],并减少了牛卵巢颗粒细胞的凋亡和氧化应激[44]。
3.2 褪黑素的抗氧化作用褪黑素是一种直接的自由基清除剂,具有比维生素C和E、甘露醇和谷胱甘肽等传统抗氧化剂更强大的抗氧化能力[45]。褪黑素通过直接清除和间接抗氧化作用,降低细胞的氧化应激,保护DNA和其他成分免受损害[46]。褪黑素及其代谢物的抗氧化作用极其广泛,包括中和超氧阴离子(O2 ·-)、羟基自由基(·OH)、单氧(O2)、过氧化氢(H2O2)、次氯酸(HOCl)、一氧化氮(NO) 和过氧亚硝酸根阴离子(ONOO-)[47]。当褪黑素消除自由基时,它会转化为代谢物,包括环状3-羟基褪黑素(C3OHM)、N1-乙酰基-N2-甲酰基-5-甲氧基犬尿胺(AFMK)和N1-乙酰基-5-甲氧基犬尿胺(AMK),这些代谢物具有有效的抗氧化作用[48]。褪黑素还间接增加抗氧化酶活性,包括超氧化物歧化酶(SOD) 和谷胱甘肽过氧化物酶(GPx),并通过褪黑素的膜受体(MT1、MT2) 增加抗氧化酶活性和mRNA表达[49]。
Tanabe等[50]报道,褪黑素通过减少细胞成分(包括细胞核、线粒体和细胞膜)的氧化应激来保护颗粒细胞免受ROS的侵害。将小鼠颗粒细胞与H2O2一起孵育,H2O2处理后DNA损伤、线粒体功能障碍和膜脂质过氧化升高,而补充褪黑素可减轻ROS对细胞成分的有害影响。此外,H2O2处理增加了凋亡细胞的数量和半胱天冬酶3/7 (Csp3/7) 活性,这些活性在褪黑素处理后受到抑制。如图 4所示,褪黑素可减少氧化应激诱导的DNA损伤、线粒体功能障碍、脂质过氧化和颗粒细胞的凋亡,表明褪黑素通过减少细胞成分的自由基损伤来保护这些细胞[51]。
3.3 褪黑素在卵母细胞成熟上的应用据报道,褪黑素能改善卵母细胞的质量,在猪[52]和牛[53]卵母细胞的体外成熟液中添加褪黑素会降低卵母细胞ROS水平,促进卵母细胞成熟,氧化应激会导致线粒体分布异常和损伤。
研究表明,褪黑素会影响卵巢功能,在人[54]和猪[55]的卵泡液中已经报道了它的存在。褪黑素能够改善线粒体功能、降低活性氧的产生、减少氧化应激、增加三磷酸腺苷(ATP) 的产生、降低细胞凋亡和提高抗氧化能力[56]。一些研究表明,通过在IVM培养液中添加褪黑素可以提高核成熟率[52, 57],褪黑素还加速了鲤鱼卵母细胞的成熟[58]。同时,褪黑素提高了牛OPU卵丘卵母细胞能力,体外受精胚胎的囊胚细胞数[59]。
4 生长因子对卵母细胞成熟的影响生长因子也可能对卵母细胞发育产生积极影响,最近,Yuan等[60]探索了添加3种细胞因子(IGF1+FGF2+LIF,FLI)的改良IVM培养基,可促进猪卵母细胞的核成熟,促进体外受精后胚胎发育。
4.1 胰岛素样生长因子1 (insulin-like growth factor 1,IGF1)胰岛素样生长因子1(IGF1) 是一种重要的生长激素,是细胞存活、生长和分化的重要物质,它还作为一种监测信号调节哺乳动物的卵母细胞和胚胎发育[61-62]。IGF1在与其受体IGF1R结合后发挥其生理作用,从而触发多个信号通路,IGF1介导的激活PI3K/Akt信号通路在调节细胞增殖和存活中起关键作用[63]。此外,IGF1在卵巢功能、排卵、卵母细胞胞质成熟以及类固醇的产生中具有调节作用[64]。
众所周知,IGF1影响卵母细胞的成熟和发育能力,它可能会刺激猪和兔卵母细胞减数分裂的恢复[65]。在马中,在IVM培养基中添加IGF1不会增加核成熟到MII阶段的速率,但会对细胞质成熟产生积极影响[66]。此外,它还具有在卵母细胞成熟过程中作为抗凋亡因子的作用,Wasielak和Bogacki[67]提出体外培养期间卵丘细胞产生的IGF1量不足以使其成熟,因此补充IGF1是成熟所必需的。
IGF1参与卵泡发生,增加卵泡直径并促进卵母细胞成熟[68]。研究表明,在IVM培养基中添加IGF1显著增加了牛卵母细胞的成熟率[69],同时刺激了体外桑椹胚向囊胚转变过程中内细胞团的增殖[70]。在IVM期间用IGF1处理猪[71]和羊[72]卵母细胞显著增加了卵裂率和胚胎发育率。
4.2 成纤维细胞生长因子2(fibroblast growth factor 2,FGF2)成纤维细胞生长因子2(FGF2)是FGF家族的成员[73],能促进山羊[74]、绵羊[75]和猕猴[76]等多种物种的卵泡发育。FGF2具有抑制颗粒细胞凋亡[77],调节卵泡发育和促进卵母细胞成熟的能力,FGF2可以提高小鼠[78]、猪[60]、牛[77]、绵羊[79]、山羊[74]的卵母细胞成熟率和牛囊胚形成[80]。Li等[81]研究表明,添加FGF2显著促进了小鼠卵母细胞的体外成熟,外源性FGF2刺激卵母细胞中FGFR1的表达,通过使用选择性抑制剂FGFR揭示了FGF信号传导的重要性,它显著降低了卵母细胞成熟率和卵丘扩张。
4.3 白血病抑制因子(leukemia inhibitory factor,LIF)白血病抑制因子(LIF) 通过与LIF特异性受体(LIFR) 和白细胞介素6受体亚基β (IL6ST) 的信号转导表面蛋白受体形成的异二聚体膜受体复合物结合发出信号。受体复合物通过多种途径介导下游LIF信号传导,包括JAK/STAT、MAPK和PI3K/AKT[82]。LIF通过激活JAK/STAT3通路驱动DNMT3A、DNMT3B、DNMT3L的表达,并在小鼠体细胞重编程过程中抑制组蛋白去乙酰化酶(HDAC) 的表达[83]。研究表明,LIF处理的卵母细胞体外受精后胚胎中DNMT3A水平增加,说明LIF增加了胚胎的甲基化水平[84]。
LIF在卵泡生长发育过程中发挥关键作用,添加到体外成熟培养基中可诱导人和小鼠卵母细胞的卵丘扩张,并促进人类[85]、小鼠[86]、猪[87]、牛[88]、绵羊[89]、山羊[90]的卵母细胞成熟和卵母细胞受精后的胚胎发育能力。同时,LIF能够提高细胞对玻璃化冷冻的耐受性[91],虽然玻璃化卵母细胞的卵裂率和囊胚率显著降低,但在添加LIF的IVM培养液中成熟的牛卵母细胞经玻璃化冷冻后与新鲜卵母细胞具有相似的卵裂率和囊胚率[84]。
4.4 FLI(IGF1+FGF2+LIF)在卵母细胞成熟上的应用研究表明,在体外成熟培养基中添加FLI (IGF1+FGF2+LIF)可以提高猪[60, 92]、牛[93]和羔羊[94]卵母细胞的发育能力,这些细胞因子可能通过激活不同的信号通路对卵母细胞发挥有益作用。LIF可以激活猪和牛卵母细胞中的丝裂原活化蛋白激酶(MAPK3/1) 和转录激活因子3 (STAT3) 通路[88]。研究表明,在小鼠和猪中,卵丘细胞中的MAPK3/1活性对促性腺激素诱导的卵母细胞减数分裂恢复、刺激卵丘扩张相关基因(HAS2和PTGS2)的表达是必不可少的[95-97]。MAPK3/1信号传导参与哺乳动物卵母细胞中减数分裂纺锤体的形成[98],研究表明,在猪体外成熟培养基中添加FLI可以激活COC中的MAPK3/1通路,从而影响卵母细胞发育相关基因的差异表达,提高卵母细胞减数分裂和发育能力[99]。FGF2可以调节细胞外基质(ECM)信号通路[77],IGF1促进细胞生长和分化,可能通过卵丘和卵母细胞之间的双向通讯参与颗粒细胞的代谢,提高卵母细胞的质量[100]。虽然这些细胞因子可以单独促进卵母细胞的核成熟,但只有它们的组合才能有效提高卵母细胞发育和囊胚发育的能力。
5 小结卵母细胞的成熟是决定卵母细胞发育到囊胚的关键因素,随着牛OPU-IVF技术的发展,牛卵母细胞体外成熟越来越重要,但大多数成熟卵母细胞通常不能发育到囊胚阶段,卵母细胞的成熟是决定卵母细胞发育到囊胚的关键因素。因此,提高卵母细胞体外成熟效率对于优化牛的体外胚胎生产至关重要。目前的研究通过使用CNP预成熟体系,在卵母细胞体外成熟培养基中添加抗氧化物质褪黑素和细胞因子FLI等方法获得稳定、有较高成功率的培养体系,提高卵母细胞的体外成熟能力,进一步提高卵母细胞受精率和卵裂率,从而提高牛IVP体系和繁殖效率。
| [1] |
BALBOULA A Z, ABOELENAIN M, SAKATANI M, et al. Effect of E-64 supplementation during in vitro maturation on the developmental competence of bovine OPU-derived oocytes[J]. Genes (Basel), 2022, 13(2): 324. DOI:10.3390/genes13020324 |
| [2] |
FERRÉ L B, KJELLAND M E, TAIYEB A M, et al. Recent progress in bovine in vitro-derived embryo cryotolerance: Impact of in vitro culture systems, advances in cryopreservation and future considerations[J]. Reprod Domest Anim, 2020, 55(6): 659-676. DOI:10.1111/rda.13667 |
| [3] |
NIEMANN H, KUES W A. Application of transgenesis in livestock for agriculture and biomedicine[J]. Anim Reprod Sci, 2003, 79(3-4): 291-317. DOI:10.1016/S0378-4320(03)00169-6 |
| [4] |
HASLER J F. The current status and future of commercial embryo transfer in cattle[J]. Anim Reprod Sci, 2003, 79(3-4): 245-264. DOI:10.1016/S0378-4320(03)00167-2 |
| [5] |
BLONDIN P. Logistics of large scale commercial IVF embryo production[J]. Reprod Fertil Dev, 2017, 29(1): 32-36. DOI:10.1071/RD16317 |
| [6] |
PERRY G. Statistics of embryo collection and transfer in domestic farm animals[J]. ET Newsletter, 2017, 35: 8-23. |
| [7] |
MATOBA S, YOSHIOKA H, MATSUDA H, et al. Optimizing production of in vivo-matured oocytes from superstimulated Holstein cows for in vitro production of embryos using X-sorted sperm[J]. J Dairy Sci, 2014, 97(2): 743-753. DOI:10.3168/jds.2013-6838 |
| [8] |
JAFFE L A, EGBERT J R. Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle[J]. Annu Rev Physiol, 2017, 79: 237-260. DOI:10.1146/annurev-physiol-022516-034102 |
| [9] |
COTICCHIO G, DAL CANTO M, MIGNINI RENZINI M, et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization[J]. Hum Reprod Update, 2015, 21(4): 427-454. DOI:10.1093/humupd/dmv011 |
| [10] |
PINCUS G, ENZMANN E V. The comparative behavior of mammalian eggs in vivo and in vitro: I.The activation of ovarian eggs[J]. J Exp Med, 1935, 62(5): 655-675. |
| [11] |
ASSEY R J, HYTTEL P, GREVE T, et al. Oocyte morphology in dominant and subordinate follicles[J]. Mol Reprod Dev, 1994, 37(3): 335-344. DOI:10.1002/mrd.1080370313 |
| [12] |
MACHATKOVA M, KRAUSOVA K, JOKESOVA E, et al. Developmental competence of bovine oocytes: effects of follicle size and the phase of follicular wave on in vitro embryo production[J]. Theriogenology, 2004, 61(2-3): 329-335. DOI:10.1016/S0093-691X(03)00216-4 |
| [13] |
FRANCIOSI F, COTICCHIO G, LODDE V, et al. Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes[J]. Biol Reprod, 2014, 91(3): 61. |
| [14] |
GONG X Q, LI H M, ZHAO Y Q. The improvement and clinical application of human oocyte in vitro maturation (IVM)[J]. Reprod Sci, 2022, 29(8): 2127-2135. DOI:10.1007/s43032-021-00613-3 |
| [15] |
GILCHRIST R B, LUCIANO A M, RICHANI D, et al. Oocyte maturation and quality: role of cyclic nucleotides[J]. Reproduction, 2016, 152(5): R143-R157. DOI:10.1530/REP-15-0606 |
| [16] |
MENG L, WU Z F, ZHAO K, et al. Transcriptome analysis of porcine granulosa cells in healthy and atretic follicles: Role of steroidogenesis and oxidative stress[J]. Antioxidants (Basel), 2020, 10(1): 22. DOI:10.3390/antiox10010022 |
| [17] |
TAKAHASHI M. Oxidative stress and redox regulation on in vitro development of mammalian embryos[J]. J Reprod Dev, 2012, 58(1): 1-9. DOI:10.1262/jrd.11-138N |
| [18] |
CAJAS Y N, CAÑÓN-BELTRÁN K, DE GUEVARA M L, et al. Antioxidant nobiletin enhances oocyte maturation and subsequent embryo development and quality[J]. Int J Mol Sci, 2020, 21(15): 5340. DOI:10.3390/ijms21155340 |
| [19] |
WANG L, TANG J H, WANG L, et al. Oxidative stress in oocyte aging and female reproduction[J]. J Cell Physiol, 2021, 236(12): 7966-7983. DOI:10.1002/jcp.30468 |
| [20] |
SUDOH T, MINAMINO N, KANGAWA K, et al. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain[J]. Biochem Biophys Res Commun, 1990, 168(2): 863-870. DOI:10.1016/0006-291X(90)92401-K |
| [21] |
SELLITTI D F, KOLES N, MENDONÇA M C. Regulation of C-type natriuretic peptide expression[J]. Peptides, 2011, 32(9): 1964-1971. DOI:10.1016/j.peptides.2011.07.013 |
| [22] |
STR Ą CZYŃSKA P, PAPIS K, MORAWIEC E, et al. Signaling mechanisms and their regulation during in vivo or in vitro maturation of mammalian oocytes[J]. Reprod Biol Endocrinol, 2022, 20(1): 37. DOI:10.1186/s12958-022-00906-5 |
| [23] |
UEDA Y, YASODA A, YAMASHITA Y, et al. C-type natriuretic peptide restores impaired skeletal growth in a murine model of glucocorticoid-induced growth retardation[J]. Bone, 2016, 92: 157-167. DOI:10.1016/j.bone.2016.08.026 |
| [24] |
NAKAGAWA H, SAITO Y. Roles of natriuretic peptides and the significance of neprilysin in cardiovascular diseases[J]. Biology (Basel), 2022, 11(7): 1017. |
| [25] |
BOHARA M, KAMBE Y, NAGAYAMA T, et al. C-type natriuretic peptide modulates permeability of the blood-brain barrier[J]. J Cereb Blood Flow Metab, 2014, 34(4): 589-596. DOI:10.1038/jcbfm.2013.234 |
| [26] |
HU P, LIU S Y, ZHANG D D, et al. Urinary c-type natriuretic peptide excretion: a promising biomarker to detect underlying renal injury and remodeling both acutely and chronically[J]. Biomark Med, 2016, 10(9): 999-1008. DOI:10.2217/bmm-2016-0089 |
| [27] |
ZHANG M J, SU Y Q, SUGIURA K, et al. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes[J]. Science, 2010, 330(6002): 366-369. DOI:10.1126/science.1193573 |
| [28] |
DE SOUSA P A, WATSON A J, SCHULTZ G A, et al. Oogenetic and zygotic gene expression directing early bovine embryogenesis: a review[J]. Mol Reprod Dev, 1998, 51(1): 112-121. DOI:10.1002/(SICI)1098-2795(199809)51:1<112::AID-MRD14>3.0.CO;2-9 |
| [29] |
MEHLMANN L M, SAEKI Y, TANAKA S, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes[J]. Science, 2004, 306(5703): 1947-1950. DOI:10.1126/science.1103974 |
| [30] |
HINCKLEY M, VACCARI S, HORNER K, et al. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes[J]. Dev Biol, 2005, 287(2): 249-261. DOI:10.1016/j.ydbio.2005.08.019 |
| [31] |
FRANCIOSI F, COTICCHIO G, LODDE V, et al. Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes[J]. Biol Reprod, 2014, 91(3): 61. |
| [32] |
SOTO-HERAS S, PARAMIO M T, THOMPSON J G. Effect of pre-maturation with C-type natriuretic peptide and 3-isobutyl-1-methylxanthine on cumulus-oocyte communication and oocyte developmental competence in cattle[J]. Anim Reprod Sci, 2019, 202: 49-57. DOI:10.1016/j.anireprosci.2019.01.007 |
| [33] |
JIA Z W, YANG X Y, LIU K. Treatment of cattle oocytes with C-type natriuretic peptide before in vitro maturation enhances oocyte mitochondrial function[J]. Anim Reprod Sci, 2021, 225: 106685. DOI:10.1016/j.anireprosci.2020.106685 |
| [34] |
XI G Y, AN L, JIA Z W, et al. Natriuretic peptide receptor 2 (NPR2) localized in bovine oocyte underlies a unique mechanism for C-type natriuretic peptide (CNP)-induced meiotic arrest[J]. Theriogenology, 2018, 106: 198-209. DOI:10.1016/j.theriogenology.2017.09.003 |
| [35] |
ZHANG T, FAN X M, LI R L, et al. Effects of pre-incubation with C-type natriuretic peptide on nuclear maturation, mitochondrial behavior, and developmental competence of sheep oocytes[J]. Biochem Biophys Res Commun, 2018, 497(1): 200-206. DOI:10.1016/j.bbrc.2018.02.054 |
| [36] |
HORI Y S, KUNO A, HOSODA R, et al. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress[J]. PLoS One, 2013, 8(9): e73875. DOI:10.1371/journal.pone.0073875 |
| [37] |
DI EMIDIO G, FALONE S, VITTI M, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging[J]. Hum Reprod, 2014, 29(9): 2006-2017. DOI:10.1093/humrep/deu160 |
| [38] |
COMBELLES C M H, GUPTA S, AGARWAL A. Could oxidative stress influence the in-vitro maturation of oocytes?[J]. Reprod Biomed Online, 2009, 18(6): 864-980. DOI:10.1016/S1472-6483(10)60038-7 |
| [39] |
NIE J Y, SUI L, ZHANG H T, et al. Mogroside V protects porcine oocytes from in vitro ageing by reducing oxidative stress through SIRT1 upregulation[J]. Aging (Albany NY), 2019, 11(19): 8362-8373. |
| [40] |
YONG W, MA H Y, NA M, et al. Roles of melatonin in the field of reproductive medicine[J]. Biomed Pharmacother, 2021, 144: 112001. DOI:10.1016/j.biopha.2021.112001 |
| [41] |
MESALAM A, KHAN I, LEE K L, et al. 2-Methoxystypandrone improves in vitro-produced bovine embryo quality through inhibition of IKBKB[J]. Theriogenology, 2017, 99: 10-20. DOI:10.1016/j.theriogenology.2017.05.012 |
| [42] |
YANG M H, TAO J L, CHAI M L, et al. Melatonin improves the quality of inferior bovine oocytes and promoted their subsequent IVF embryo development: Mechanisms and results[J]. Molecules, 2017, 22(12): 2059. DOI:10.3390/molecules22122059 |
| [43] |
SUN Z Y, ZHANG P, WANG J J, et al. Melatonin alleviates meiotic defects in fetal mouse oocytes induced by Di (2-ethylhexyl) phthalate in vitro[J]. Aging (Albany NY), 2018, 10(12): 4175-4187. |
| [44] |
YANG F X, LI L, CHEN K L, et al. Melatonin alleviates β-zearalenol and HT-2 toxin-induced apoptosis and oxidative stress in bovine ovarian granulosa cells[J]. Environ Toxicol Pharmacol, 2019, 68: 52-60. DOI:10.1016/j.etap.2019.03.005 |
| [45] |
REITER R J. Functional pleiotropy of the neurohormone melatonin: Antioxidant protection and neuroendocrine regulation[J]. Front Neuroendocrinol, 1995, 16(4): 383-415. DOI:10.1006/frne.1995.1014 |
| [46] |
TAN D X, REITER R J. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells[J]. Melatonin Res, 2019, 2(1): 44-66. DOI:10.32794/mr11250011 |
| [47] |
REITER R J, TAN D X, MANCHESTER L C, et al. Melatonin: Detoxification of oxygen and nitrogen-based toxic reactants[J]. Adv Exp Med Biol, 2003, 527: 539-548. |
| [48] |
TAN D X, MANCHESTER L C, TERRON M P, et al. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species?[J]. J Pineal Res, 2007, 42(1): 28-42. DOI:10.1111/j.1600-079X.2006.00407.x |
| [49] |
MONIRUZZAMAN M, GHOSAL I, DAS D, et al. Melatonin ameliorates H2O2-induced oxidative stress through modulation of Erk/Akt/NFkB pathway[J]. Biol Res, 2018, 51(1): 17. DOI:10.1186/s40659-018-0168-5 |
| [50] |
TANABE M, TAMURA H, TAKETANI T, et al. Melatonin protects the integrity of granulosa cells by reducing oxidative stress in nuclei, mitochondria, and plasma membranes in mice[J]. J Reprod Dev, 2015, 61(1): 35-41. DOI:10.1262/jrd.2014-105 |
| [51] |
TAMURA H, JOZAKI M, TANABE M, et al. Importance of melatonin in assisted reproductive technology and ovarian aging[J]. Int J Mol Sci, 2020, 21(3): 1135. DOI:10.3390/ijms21031135 |
| [52] |
NIU Y J, ZHOU W J, NIE Z W, et al. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos[J]. J Pineal Res, 2020, 68(2): e12627. |
| [53] |
El-RAEY M, GESHI M, SOMFAI T, et al. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle[J]. Mol Reprod Dev, 2011, 78(4): 250-262. DOI:10.1002/mrd.21295 |
| [54] |
KHAN H L, BHATTI S, ABBAS S, et al. Melatonin levels and microRNA (miRNA) relative expression profile in the follicular ambient microenvironment in patients undergoing in vitro fertilization process[J]. J Assist Reprod Genet, 2021, 38(2): 443-459. DOI:10.1007/s10815-020-02010-2 |
| [55] |
SHI J M, TIAN X Z, ZHOU G B, et al. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes[J]. J Pineal Res, 2009, 47(4): 318-323. DOI:10.1111/j.1600-079X.2009.00717.x |
| [56] |
ZHU Q, DING D, YANG H, et al. Melatonin protects mitochondrial function and inhibits oxidative damage against the decline of human oocytes development caused by prolonged cryopreservation[J]. Cells, 2022, 11(24): 4018. DOI:10.3390/cells11244018 |
| [57] |
MANJUNATHA B M, DEVARAJ M, GUPTA P S P, et al. Effect of taurine and melatonin in the culture medium on buffalo in vitro embryo development[J]. Reprod Domest Anim, 2009, 44(1): 12-16. DOI:10.1111/j.1439-0531.2007.00982.x |
| [58] |
CHATTORAJ A, SETH M, MAITRA S K. Influence of serotonin on the action of melatonin in MIH-induced meiotic resumption in the oocytes of carp Catla catla[J]. Comp Biochem Physiol A Mol Integr Physiol, 2008, 150(3): 301-306. DOI:10.1016/j.cbpa.2008.03.014 |
| [59] |
GUTIÉRREZ-AÑEZ J C, LUCAS-HAHN A, HADELER K G, et al. Melatonin enhances in vitro developmental competence of cumulus-oocyte complexes collected by ovum pick-up in prepubertal and adult dairy cattle[J]. Theriogenology, 2021, 161: 285-293. DOI:10.1016/j.theriogenology.2020.12.011 |
| [60] |
YUAN Y, SPATE L D, REDEL B K, et al. Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation[J]. Proc Natl Acad Sci U S A, 2017, 114(29): E5796-E5804. |
| [61] |
DELAFONTAINE P, SONG Y H, LI Y X. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels[J]. Arterioscler Thromb Vasc Biol, 2004, 24(3): 435-444. DOI:10.1161/01.ATV.0000105902.89459.09 |
| [62] |
VELAZQUEZ M A, SPICER L J, WATHES D C. The role of endocrine insulin-like growth factor-I (IGF-I) in female bovine reproduction[J]. Domest Anim Endocrinol, 2008, 35(4): 325-342. DOI:10.1016/j.domaniend.2008.07.002 |
| [63] |
YU J S L, CUI W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination[J]. Development, 2016, 143(17): 3050-3060. DOI:10.1242/dev.137075 |
| [64] |
LI X H, DAI Y F, ALLEN W R. Influence of insulin-like growth factor-I on cytoplasmic maturation of horse oocytes in vitro and organization of the first cell cycle following nuclear transfer and parthenogenesis[J]. Biol Reprod, 2004, 71(4): 1391-1396. DOI:10.1095/biolreprod.104.029066 |
| [65] |
SIROTKIN A V, DUKESOVÁ J, MAKAREVICH A V, et al. Evidence that growth factors IGF-I, IGF-Ⅱ and EGF can stimulate nuclear maturation of porcine oocytes via intracellular protein kinase A[J]. Reprod Nutr Dev, 2000, 40(6): 559-569. DOI:10.1051/rnd:2000137 |
| [66] |
CARNEIRO G, LORENZO P, PIMENTEL C, et al. Influence of insulin-like growth factor-I and its interaction with gonadotropins, estradiol, and fetal calf serum on in vitro maturation and parthenogenic development in equine oocytes[J]. Biol Reprod, 2001, 65(3): 899-905. DOI:10.1095/biolreprod65.3.899 |
| [67] |
WASIELAK M, BOGACKI M. Apoptosis inhibition by insulin-like growth factor (IGF)-I during in vitro maturation of bovine oocytes[J]. J Reprod Dev, 2007, 53(2): 419-426. DOI:10.1262/jrd.18076 |
| [68] |
DEMEESTERE I, GERVY C, CENTNER J, et al. Effect of insulin-like growth factor-I during preantral follicular culture on steroidogenesis, in vitro oocyte maturation, and embryo development in mice[J]. Biol Reprod, 2004, 70(6): 1664-1669. DOI:10.1095/biolreprod.103.023317 |
| [69] |
YANG S, YANG Y Z, HAO H S, et al. Supplementation of EGF, IGF-1, and Connexin 37 in IVM medium significantly improved the maturation of bovine oocytes and vitrification of their IVF blastocysts[J]. Genes (Basel), 2022, 13(5): 805. DOI:10.3390/genes13050805 |
| [70] |
VELAZQUEZ M A, HADELER K G, HERRMANN D, et al. In vivo oocyte IGF-1 priming increases inner cell mass proliferation of in vitro-formed bovine blastocysts[J]. Theriogenology, 2012, 78(3): 517-527. DOI:10.1016/j.theriogenology.2012.02.034 |
| [71] |
CURRIN L, GLANZNER W G, GUTIERREZ K, et al. Optimizing swine in vitro embryo production with growth factor and antioxidant supplementation during oocyte maturation[J]. Theriogenology, 2022, 194: 133-143. DOI:10.1016/j.theriogenology.2022.10.005 |
| [72] |
SHABANKAREH H K, ZANDI M. Developmental potential of sheep oocytes cultured in different maturation media: effects of epidermal growth factor, insulin-like growth factor I, and cysteamine[J]. Fertil Steril, 2010, 94(1): 335-340. DOI:10.1016/j.fertnstert.2009.01.160 |
| [73] |
ORNITZ D M, ITOH N. The fibroblast growth factor signaling pathway[J]. Wiley Interdiscip Rev Dev Biol, 2015, 4(3): 215-266. DOI:10.1002/wdev.176 |
| [74] |
ALMEIDA A P, SARAIVA M V A, ALVES FILHO J G, et al. Gene expression and immunolocalization of fibroblast growth factor 2 in the ovary and its effect on the in vitro culture of caprine preantral ovarian follicles[J]. Reprod Domest Anim, 2012, 47(1): 20-25. DOI:10.1111/j.1439-0531.2011.01793.x |
| [75] |
SANTOS J M, MENEZES V G, BARBERINO R S, et al. Immunohistochemical localization of fibroblast growth factor-2 in the sheep ovary and its effects on pre-antral follicle apoptosis and development in vitro[J]. Reprod Domest Anim, 2014, 49(3): 522-528. DOI:10.1111/rda.12322 |
| [76] |
LU C L, YAN J, ZHI X, et al. Basic fibroblast growth factor promotes macaque follicle development in vitro[J]. Reproduction, 2015, 149(5): 425-433. DOI:10.1530/REP-14-0557 |
| [77] |
BARROS R G, LIMA P F, SOARES A C S, et al. Fibroblast growth factor 2 regulates cumulus differentiation under the control of the oocyte[J]. J Assist Reprod Genet, 2019, 36(5): 905-913. DOI:10.1007/s10815-019-01436-7 |
| [78] |
DU C, DAVIS J S, CHEN C, et al. FGF2/FGFR signaling promotes cumulus-oocyte complex maturation in vitro[J]. Reproduction, 2021, 161(2): 205-214. DOI:10.1530/REP-20-0264 |
| [79] |
MONDAL S, MOR A, REDDY I J, et al. Effect of fibroblast growth factor 2 (FGF2) and insulin transferrin selenium (ITS) on in vitro maturation, fertilization and embryo development in sheep[J]. Braz Arch Biol Technol, 2015, 58(4): 521-525. DOI:10.1590/S1516-8913201500059 |
| [80] |
FIELDS S D, HANSEN P J, EALY A D. Fibroblast growth factor requirements for in vitro development of bovine embryos[J]. Theriogenology, 2011, 75(8): 1466-1475. DOI:10.1016/j.theriogenology.2010.12.007 |
| [81] |
LI S H, HWU Y M, LU C H, et al. VEGF and FGF2 improve revascularization, survival, and oocyte quality of cryopreserved, subcutaneously-transplanted mouse ovarian tissues[J]. Int J Mol Sci, 2016, 17(8): 1237. DOI:10.3390/ijms17081237 |
| [82] |
FISCHER P, HILFIKER-KLEINER D. Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects[J]. Br J Pharmacol, 2008, 153(S1): S414-S427. DOI:10.1038/bjp.2008.1 |
| [83] |
TANG Y, LUO Y, JIANG Z L, et al. Jak/Stat3 signaling promotes somatic cell reprogramming by epigenetic regulation[J]. Stem Cells, 2012, 30(12): 2645-2656. DOI:10.1002/stem.1225 |
| [84] |
VENDRELL-FLOTATS M, GARCÍA-MARTÍNEZ T, MARTÍNEZ-RODERO I, et al. In vitro maturation in the presence of leukemia inhibitory factor modulates gene and miRNA expression in bovine oocytes and embryos[J]. Sci Rep, 2020, 10(1): 17777. DOI:10.1038/s41598-020-74961-6 |
| [85] |
SPATE L D, MURPHY S L, BENNE J A, et al. In vitro-matured gilt oocytes can have equal or better developmental competence than sow oocytes with new maturation media[J]. Reprod Fertil Dev, 2016, 29(1): 150. |
| [86] |
DE MATOS D G, MILLER K, SCOTT R, et al. Leukemia inhibitory factor induces cumulus expansion in immature human and mouse oocytes and improves mouse two-cell rate and delivery rates when it is present during mouse in vitro oocyte maturation[J]. Fertil Steril, 2008, 90(6): 2367-2375. DOI:10.1016/j.fertnstert.2007.10.061 |
| [87] |
DANG-NGUYEN T Q, HARAGUCHI S, KIKUCHI K, et al. Leukemia inhibitory factor promotes porcine oocyte maturation and is accompanied by activation of signal transducer and activator of transcription 3[J]. Mol Reprod Dev, 2014, 81(3): 230-239. DOI:10.1002/mrd.22289 |
| [88] |
MO X H, WU G Q, YUAN D S, et al. Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development[J]. Mol Reprod Dev, 2014, 81(7): 608-618. DOI:10.1002/mrd.22327 |
| [89] |
PTAK G, LOPES F, MATSUKAWA K, et al. Leukaemia inhibitory factor enhances sheep fertilization in vitro via an influence on the oocyte[J]. Theriogenology, 2006, 65(9): 1891-1899. DOI:10.1016/j.theriogenology.2005.10.018 |
| [90] |
AN L Y, LIU J, DU Y Y, et al. Synergistic effect of cysteamine, leukemia inhibitory factor, and Y27632 on goat oocyte maturation and embryo development in vitro[J]. Theriogenology, 2018, 108: 56-62. DOI:10.1016/j.theriogenology.2017.11.028 |
| [91] |
KOCYIGIT A, CEVIK M. Effects of leukemia inhibitory factor and insulin-like growth factor-I on the cell allocation and cryotolerance of bovine blastocysts[J]. Cryobiology, 2015, 71(1): 64-69. DOI:10.1016/j.cryobiol.2015.05.068 |
| [92] |
SERRANO ALBAL M, SILVESTRI G, KIAZIM L G, et al. Supplementation of porcine in vitro maturation medium with FGF2, LIF, and IGF1 enhances cytoplasmic maturation in prepubertal gilts oocytes and improves embryo quality[J]. Zygote, 2022, 30(6): 801-808. DOI:10.1017/S0967199422000284 |
| [93] |
STOECKLEIN K S, ORTEGA M S, SPATE L D, et al. Improved cryopreservation of in vitro produced bovine embryos using FGF2, LIF, and IGF1[J]. PLoS One, 2021, 16(2): e0243727. DOI:10.1371/journal.pone.0243727 |
| [94] |
TIAN H, QI Q, YAN F X, et al. Enhancing the developmental competence of prepubertal lamb oocytes by supplementing the in vitro maturation medium with sericin and the fibroblast growth factor 2-leukemia inhibitory factor-Insulin-like growth factor 1 combination[J]. Theriogenology, 2021, 159: 13-19. DOI:10.1016/j.theriogenology.2020.10.019 |
| [95] |
SU Y Q, DENEGRE J M, WIGGLESWORTH K, et al. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex[J]. Dev Biol, 2003, 263(1): 126-138. DOI:10.1016/S0012-1606(03)00437-8 |
| [96] |
MEINECKE B, KRISCHEK C. MAPK/ERK kinase (MEK) signalling is required for resumption of meiosis in cultured cumulus-enclosed pig oocytes[J]. Zygote, 2003, 11(1): 7-16. DOI:10.1017/S0967199403001023 |
| [97] |
PROCHÁZKA R, PETLACH M, NAGYOVÁ E, et al. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: Comparison with gonadotropins[J]. Reproduction, 2011, 141(4): 425-435. DOI:10.1530/REP-10-0418 |
| [98] |
TONG C, FAN H Y, CHEN D Y, et al. Effects of MEK inhibitor U0126 on meiotic progression in mouse oocytes: Microtuble organization, asymmetric division and metaphase Ⅱ arrest[J]. Cell Res, 2003, 13(5): 375-383. DOI:10.1038/sj.cr.7290183 |
| [99] |
PROCHÁZKA R, BARTKOVÁ A, NĚMCOVÁ L, et al. The role of MAPK3/1 and AKT in the acquisition of high meiotic and developmental competence of porcine oocytes cultured in vitro in FLI medium[J]. Int J Mol Sci, 2021, 22(20): 11148. DOI:10.3390/ijms222011148 |
| [100] |
STEFANELLO J R, BARRETA M H, PORCIUNCULA P M, et al. Effect of angiotensin Ⅱ with follicle cells and insulin-like growth factor-I or insulin on bovine oocyte maturation and embryo development[J]. Theriogenology, 2006, 66(9): 2068-2076. DOI:10.1016/j.theriogenology.2006.06.005 |
(编辑 郭云雁)