2. 河南省岐伯实业有限公司, 鹤壁 456250
2. Henan Qibo Industrial Co. Ltd., Hebi 456250, China
裂殖壶菌(Schizochytrium),是一种类藻海洋真菌,和微藻较为类似,但不进行光合作用,通常又将其称为裂壶藻。在培养一段时间后,细胞中油脂含量在20%以上,且主要以中性油脂形式存在[1]。裂殖壶菌所产油脂成分简单,主要有二十二碳六烯(DHA C22:6)、二十二碳五烯酸(DPA C22:5)、棕榈酸(C16:0)和豆蔻酸(C14:0)等,是工业上理想的产油微生物[2-3]。
DHA(docosahexaenic Acid,C22:6)在裂殖壶菌藻油中含量超过30%,是动物机体中不可或缺的多不饱和脂肪酸(PUFAs)。DHA作为细胞膜的结构成分,通过生成脂质信号来调节细胞免疫反应[4],具有预防癌症[5-6]、抗炎和抗氧化应激等作用[7-8]。此外,DHA还参与肠道菌群调节、瘦素代谢等通路[9];Janczyk等[10]在蛋鸡饲粮中添加微藻,发现蛋鸡嗉囊和肠道中有益微生物数量和丰度显著升高。在畜禽养殖中,裂殖壶菌发酵物中的ω-3 PUFAs具有改善畜禽繁殖性能,提高免疫力,改善糖、脂代谢等作用[11]。在蛋鸡饲粮中添加微藻裂殖壶菌能够显著增加DHA在蛋黄中的沉积[12]。实践中,常用鱼油、亚麻籽、藻粉或藻油作为饲粮中DHA的来源,但鱼油的使用会影响鸡蛋风味,藻粉或藻油则在无形中增加生产成本;而采用发酵的方法可以利用廉价农副产品如裂殖壶菌开发富含DHA的新型饲料原料。
本试验以裂殖壶菌发酵物作为饲料添加剂,研究其对海兰褐壳蛋鸡生产性能、蛋品质及肠道菌群结构的影响,以期为生产实践提供参考。
1 材料与方法 1.1 试验材料试验所需裂殖壶菌以实验室保存的突变株为菌种,发酵条件为:麸皮和菜籽粕比例为1∶1、固液比1∶7、培养时间84 h、初始发酵pH 8.5、海水晶浓度35 g·L-1、接种量为人工海水10%。对其发酵物料和脂肪酸组成进行测定,结果见表 1和2。
|
|
表 1 发酵物料组成(风干基础) Table 1 Fermentation material composition(air-dry basis) |
|
|
表 2 发酵物料脂肪酸组成及含量 Table 2 Fatty acid composition and content of fermentation materials |
选取960只健康且体重均匀的40周龄海兰褐蛋鸡,随机分成4组,每组6个重复,每个重复40只。对照组饲喂基础饲粮,试验Ⅰ、Ⅱ、Ⅲ组在基础饲粮的基础上分别添加0.25%、0.5%、1%的裂殖壶菌发酵物。预试验7 d,正试期30 d。预试期各组蛋鸡均饲喂基础饲粮。基础饲粮以玉米、豆粕为主要原料,依据NRC(1994)、《鸡饲养标准》(NY/T33—2004)及《海兰褐蛋鸡饲养手册》配制基础饲粮,基础饲粮组成及营养水平见表 3。
|
|
表 3 基础饲粮组成及营养水平(风干基础) Table 3 Composition and nutrient levels of the basal diet (air-dry basis) |
试验蛋鸡采用三层全阶梯式笼养,每笼3只鸡。鸡舍光照时间为16 h·d-1;试验全期自由采食和饮水,每天08:00、16:00各喂料1次,每天匀料2次,清粪1次。
1.4 样品采集与处理试验结束后,分别采集蛋样和血样,以重复为单位,每个重复收集12枚蛋样,用于蛋品质和蛋黄中脂肪酸组分的测定。每个重复随机抽取1只鸡(禁食12 h,但不禁水),进行翅下静脉采血。促凝真空采血管采集5 mL血样,35 ℃水浴静置1 h后,3 000 r·min-1离心10 min得到上层血清,分装后于-20 ℃冰箱中保存待测。每组屠宰1只蛋鸡取盲肠内容物分装后于-86 ℃冰箱中保存待测。
1.5 指标测定与方法1.5.1 生产性能的测定 试验全期以重复为单位,记录每天产蛋数、蛋重及破软蛋数,每周清料1次。计算试验全期各组日均采食量、料蛋比、产蛋率和平均蛋重等生产性能指标。
1.5.2 蛋品质的测定 蛋壳颜色、哈氏单位、蛋白高度和蛋黄颜色用蛋品质分析仪(QCH,英国)测定;蛋壳强度用强度测定仪(In-spec 2 200,美国)测定;蛋形指数用游标卡尺测量纵径和横径后计算(纵径/横径);蛋壳厚度用螺旋测微器测定蛋壳钝端、中部及锐端的厚度(已去除蛋壳内外壳膜)。
1.5.3 血清生化指标的测定 采用AVDIA2400全自动生化分析仪(西门子,美国)测定血清总胆固醇(T-CHO)、甘油三酯(TG)、低密度脂蛋白胆固醇(LDL-C)含量及总抗氧化能力(T-AOC)、超氧化物歧化酶(SOD)活性、谷胱甘肽过氧化物酶(GSH-Px)活性、过氧化氢酶(CAT)活性及丙二醛(MDA)含量共8项血清生化指标,所用试剂盒均购于南京建成生物工程研究所。
1.5.4 脂肪酸组成的测定1.5.4.1 样品前处理 采用GB 5009.168—2016[13],试样水解→脂肪的提取→脂肪的皂化和脂肪酸甲酯化。
1.5.4.2 检测方法[14]色谱柱:TG-5MS(30 m×0.25 mm×0.25 μm);升温程序:80 ℃保持1 min,以10 ℃·min-1的速率升温至200 ℃,继续以5 ℃·min-1的速率升温至250 ℃,最后以2 ℃·min-1的速率升到270 ℃,保持3 min;进样口温度:290 ℃;载气流速:1.2 mL·min-1;分流比:不分流。
质谱条件:离子源温度:280 ℃;传输线温度:280 ℃;溶剂延迟时间:5.00 min;扫描范围:30~400 amu;离子源:EI源70 eV。
1.5.4.3 计算公式 试样中各脂肪酸的含量按公式计算:
| $ W = C \times V \times N \times k \times {10^4}/m。$ |
式中:W——试样中各脂肪酸的含量,单位为百分含量(%);
C——试样测定液中脂肪酸甲酯的浓度,单位mg·L-1;
V——定容体积,单位mL;
k——各脂肪酸甲酯转化为脂肪酸的换算系数;
N——稀释倍数;
m——试样的称样质量,单位为克(g)。
1.5.5 肠道菌群结构测定 称取200~500 mg的样品,放入灭菌的2 mL离心管中,加入1×PBS溶液,震荡混匀,10 000 r·min-1室温离心3 min,弃置上层液体。倒置2 mL管于吸水纸上1 min,直至没有液体流出。经OMEGA试剂盒(E.Z.N.ATM Mag-Bind Soil DNA Kit)提取,经PCR扩增和文库比对。
1.6 数据处理与分析利用origin 2021进行数据绘图和SPSS 20.0对试验数据进行单因素方差分析(one-way ANOVA),并用最小显著差异法(LSD)进行多重比较、回归剖分线性和二次曲线P值,P < 0.05表示差异显著,所有数据均以“平均值±标准差”表示。
2 结果 2.1 裂殖壶菌发酵物对蛋鸡生产性能的影响由表 4可知,与对照组相比,试验Ⅰ、Ⅱ、Ⅲ组蛋鸡的日均采食量显著高于对照组(P < 0.05),各试验组间差异不显著(P>0.05);试验Ⅱ、Ⅲ的蛋重显著低于对照组和试验Ⅰ组(P < 0.05),试验Ⅰ、Ⅲ的只日产蛋量显著低于试验Ⅱ组和对照组(P < 0.05),但试验Ⅱ组与对照组相比差异不显著(P>0.05)。各组间蛋鸡产蛋率、料蛋比和破软蛋率均无显著差异(P>0.05)。
|
|
表 4 裂殖壶菌发酵物对蛋鸡生产性能的影响 Table 4 Effects of Schizochytrium fermentation products on production performance of laying hens |
由表 5可知,各试验组间鸡蛋的蛋形指数、蛋壳强度、蛋白高度、哈氏单位、蛋黄颜色、蛋壳颜色及蛋壳厚度均无显著差异(P>0.05)。
|
|
表 5 裂殖壶菌发酵物对蛋鸡蛋品质的影响 Table 5 Effects of Schizochytrium fermentation products on egg quality of laying hens |
由表 6可知,试验Ⅱ总抗氧化能力显著高于对照组(P < 0.05),且试验Ⅱ组总抗氧化能力显著高于试验Ⅲ组(P < 0.05);试验Ⅰ、Ⅱ组过氧化氢酶活力显著高于对照组(P < 0.05);试验Ⅱ、Ⅲ组谷胱甘肽过氧化物酶活力显著高于对照组(P < 0.05)。各组间总胆固醇、甘油三酯、低密度脂蛋白胆固醇、丙二醛含量及总超氧化物歧化酶活力差异不显著(P>0.05)。
|
|
表 6 裂殖壶菌发酵物对蛋鸡血清生化指标的影响 Table 6 Effects of Schizochytrium fermentation products on serum biochemical indexes of laying hens |
由表 7可知,与对照组相比,试验Ⅱ、Ⅲ组蛋黄中SFA与MUFA含量均显著下降(P < 0.05);试验Ⅰ组蛋黄中SFA与MUFA含量与对照组相比有所降低,但差异不显著(P>0.05),并显著高于试验Ⅱ、Ⅲ组(P < 0.05)。
|
|
表 7 裂殖壶菌发酵物对蛋黄脂肪酸组成的影响 Table 7 Effect of Schizochytrium fermentation products on fatty acid composition of egg yolk |
试验Ⅰ、Ⅱ、Ⅲ组蛋黄中PUFAs与ω-3 PUFAs含量均显著高于对照组(P < 0.05);试验Ⅱ、Ⅲ组蛋黄中PUFAs与ω-3 PUFAs含量无显著差异(P>0.05),但均显著高于试验Ⅰ组(P < 0.05)。
试验Ⅰ、Ⅱ、Ⅲ组蛋黄中ω-6 PUFAs含量均显著高于对照组(P < 0.05);试验Ⅱ组中ω-6 PUFAs含量显著高于试验Ⅰ组(P < 0.05),但与试验Ⅲ组相比差异不显著(P>0.05)。
试验Ⅰ、Ⅱ、Ⅲ组蛋黄中ω-6/ω-3均显著低于对照组(P < 0.05);试验Ⅰ组蛋黄中ω-6/ω-3显著高于试验Ⅱ、Ⅲ组(P < 0.05);试验Ⅱ、Ⅲ组蛋黄中ω-6/ω-3无显著差异(P>0.05)。
2.5 裂殖壶菌发酵物对蛋鸡盲肠微生物的影响试验共获得420 852条高质量序列,平均长度为418.70 nt,样品覆盖率均在0.99以上。经97%相似性回归,共得到5 434个OTUs。
由表 8可知,在丰富度上,试验Ⅱ、Ⅲ组的OTUs、Ace、Chao指数高于对照组,说明饲喂裂殖壶菌发酵物可以增加蛋鸡盲肠微生物种群丰富度。
|
|
表 8 盲肠菌群高通量测序结果 Table 8 High-throughput sequencing data summary of cecal microflora |
2.5.1 β多样性分析 如图 1所示,基于欧式距离主坐标分析(PCA),主成分PCA1和PCA2对样品差异的贡献值为51.78%和28.63%,结果显示,添加裂殖壶菌发酵物后,盲肠菌群丰富度增加。

|
图 1 根据欧式距离主坐标分析 Fig. 1 Analysis according to Euclidean distance principal coordinates |
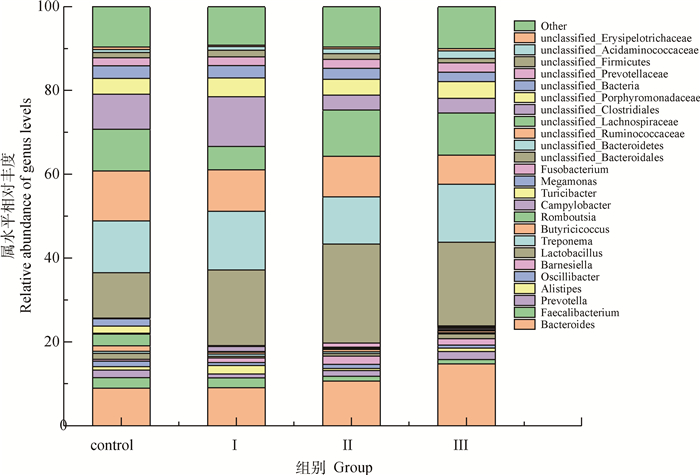
2.5.2 蛋鸡盲肠内容物菌群结构分析 由图 2可知,试验组蛋鸡盲肠菌群丰度均有增加。由图 3可知,在属水平上,菌群结构均发生显著性变化,主要引起乳酸杆菌属、未分类的拟杆菌门升高。

|
图 2 蛋鸡盲肠菌群OTUs差异韦恩图 Fig. 2 Venn diagram of differences in OTUs in cecal microflora of laying hens |
|
Bacteroides:细菌;Faecalibacterium:普拉梭菌;Prevotella:普雷沃氏菌;Alistipes:另枝菌属;Oscillibacter:李斯特菌;Barnesiella:肠道巴尼斯菌;Lactobacillus:乳酸杆菌属;Treponema:密螺旋体属;Butyricicoccus:梭菌科;Romboutsia:罗姆布茨菌;Campylobacter:弯曲杆菌属;Turicibacter:血尿杆菌;Megamonas:巨单胞菌属;Fusobacterium:梭杆菌属;unclassified_Bacteroidetes:未分类-拟杆菌属;unclassified_Ruminococcaceae:未分类瘤胃菌属;unclassified_Lachnospiraceae:未分类毛螺菌属;unclassified_Clostridiales:未分类梭菌属;unclassified_Porphyromonadaceae:未分类紫单胞菌科;unclassified_Bacteria:未分类细菌;unclassified_Prevotellaceae:未分类普雷沃氏菌;unclassified_Firmicutes:未分类厚壁菌门;unclassified_Acidaminococcaceae:未分类酸氨基菌科;unclassified_Erysipelotrichaceae: 未分类丹毒丝菌科;other: 其他 图 3 蛋鸡盲肠菌群在属水平相对丰度柱形图 Fig. 3 Histogram of relative abundance of cecum microbiota in laying hens at genus level |
本试验结果表明,在蛋鸡饲粮中添加裂殖壶菌发酵物对蛋鸡产蛋率、料蛋比和破损蛋率均无显著影响。Liu等[15]在蛋鸡饲粮中添加不同水平的商品化裂殖壶菌菌粉,发现对蛋鸡的料蛋比、平均蛋重、产蛋率无显著性影响,与本试验结果一致。Ao等[16]研究认为,在产蛋高峰期海兰褐蛋鸡饲粮中添加不同水平的富DHA微藻对蛋鸡的产蛋率、料蛋比和蛋重等生产性能无显著影响,也与本试验相吻合,但采食量没有显著变化,这可能与蛋鸡饲养环境和生理阶段有关。邓兴照等[17]在蛋鸡饲粮中分别添加ω-3 PUFAs和ω-6 PUFAs油脂后发现,添加ω-3 PUFAs组和添加ω-6 PUFAs组的采食量均显著高于对照组。在蛋鸡饲粮中添加PUFAs除了改善饲粮适口性、诱导蛋鸡进行采食外,也与血液中较高的PUFAs可以调节脂肪细胞分泌瘦素,增加机体运动,降低了血液中的血糖浓度有关[18],进而增加了平均日采食量。
在蛋品质方面,对照组与各试验组之间均没有显著性影响,说明裂殖壶菌发酵物不会影响鸡蛋的蛋品质感官评价。Wang等[19]研究认为,在饲粮中添加裂殖壶菌菌粉对母鸡生产性能和蛋品质均无不良影响,与本试验结果一致。有报道认为,在蛋鸡饲粮中添加裂殖壶菌可以显著增加蛋黄颜色,而对蛋形指数、蛋壳强度等感官特征无不良影响[20],这可能与裂殖壶菌在低碳下产生类胡萝卜素有关[21-22]。
3.2 裂殖壶菌发酵物对蛋鸡血清生化指标的影响胆固醇在动物机体中具有广泛的生理功能,但过高时容易产生动脉粥样硬化等障碍,降低动物的生理功能和生产性能。随着动物机体的衰老,体内抗氧化能力下降,动物生产性能也会降低。ω-3 PUFAs可通过促进脂质代谢的上游调控基因Fra1/Fra2表达,进而抑制PPARγ基因表达,达到对脂质代谢的调控[23]。在高脂饲粮下,微藻中ω-3 PUFAs能够抑制肝和脂肪组织中脂肪酸合成酶的生成、降低元件结合蛋白-1C和乙酰CoA羧化酶的基因表达,但促进了激素敏感脂肪酶和脂蛋白脂肪酶生成,进而降低肝和脂肪组织中脂质积累[24]。杨蕊等[25]在蛋鸡饲粮中分别添加微藻DHA和ALA发现,微藻DHA组和ALA组均显著降低蛋黄中的总胆固醇,且微藻DHA的添加能显著降低血清中总三酰甘油含量,与本试验添加裂殖壶菌发酵物组一致。这表明在饲粮中添加富含ω-3PUFAs裂殖壶菌发酵物能保护肝损伤,改善动物肝的健康,提高产蛋后期的生产性能和避免脂质代谢异常造成的慢性疾病。
细胞的氧化应激在诱导机体衰老的过程中发挥着重要作用[26]。随着蛋鸡产蛋日龄的推移,体内抗氧化酶活性会逐渐降低,同时自由基逐渐积累会加速细胞功能的氧化损伤,而细胞内源性抗氧化酶能保护机体免受氧化损伤,增加抗氧化能力和卵巢的卵泡数目等作用[27]。本研究发现,在饲粮中添加裂殖壶菌发酵物对蛋鸡血清中的总抗氧化能力、谷胱甘肽过氧化物酶活力和过氧化氢酶活力显著高于对照组,这表明裂殖壶菌发酵物,蛋鸡起着积极调节作用。有报道发现,在反刍动物日粮中添加富含DHA的微藻同样显著增加血液中的抗氧化能力,显著增加总抗氧化物酶活力和超氧化物歧化酶活力[28-29]。这可能与裂殖壶菌发酵产物中含有丰富的β胡萝卜素、类黄酮和维生素E有关[30]。
3.3 裂殖壶菌发酵物对蛋黄脂肪酸组成的影响饲粮是蛋鸡沉积ω-3 PUFAs的主要来源[31]。本研究发现,在蛋鸡饲粮中添加裂殖壶菌发酵物可以显著增加蛋黄中ω-3 PUFAs和ω-6 PUFAs含量,降低蛋黄中ω-6/ω-3 PUFAs,与前人研究相一致[32]。在动物机体中,ω-3和ω-6PUFAs共用一套酶系统,蛋黄中ω-6/ω-3 PUFAs下降可能有两方面原因:1)ω-3 PUFAs参与体内的脂代谢,Yang等[33]研究发现,高ω-3 PUFAs的摄入量能够提高脂联素和瘦素,降低胰岛素等含量,改变脂质代谢;2)机体对饲粮中不同脂肪酸沉积的顺序和效率不同[34]。
蛋黄脂肪酸的组成和水平受饲粮脂肪酸的影响[35]。本试验中,与对照组相比,试验组鸡蛋中SFA含量均有降低,试验Ⅱ、Ⅲ组显著低于对照组。在蛋鸡饲粮中添加裂殖壶菌发酵物能够显著增加鸡蛋中DHA浓度,且对感官评价无不良影响,这与前人研究相吻合[36]。蛋鸡饲粮中裂殖壶菌添加量超过一定量时,鸡蛋中的DHA不会增加;有报道认为,鸡蛋中DHA饱和后,机体C18:3n-3转化为C22:6n-3沉积效率降低。
3.4 裂殖壶菌发酵物对蛋鸡盲肠微生物的影响肠道菌群与动物机体代谢及健康间存在密切关[37],其数量和组成能够间接反映动物机体代谢及健康[38]。本研究发现,裂殖壶菌发酵物可以增加蛋鸡盲肠微生物的数量和丰度,与对照组相比,裂殖壶菌发酵物组直接影响物种丰度OTUs和α多样性,且与添加比例呈正相关。
EPA和DHA在肠道内发挥着重要作用[39],如参与机体脂质代谢和肠道菌群调节[40]、缓解肺部炎症[41]和抑制肝中的糖异生[42]等作用。本研究发现,饲粮中添加裂殖壶菌发酵物主要引起盲肠中unclassified_Bacteroidetes(未分类-拟杆菌属)、Barnesiella(肠道巴尼斯菌)、Lactobacillus(乳酸杆菌属)、unclassified_Ruminococcaceae(未分类瘤胃菌属)、unclassified_Lachnospiraceae(未分类毛螺菌属)和unclassified_Clostridiales(未分类梭菌属)变化。有研究认为,拟杆菌属参与脂质代谢,通过调节炎症通路改善脂肪肝[43];本试验中,裂殖壶菌中的DHA引起盲肠unclassified_Bacteroidetes丰度增加对肝的保护具有一定的潜力。Barnesiella(肠道巴尼斯菌)属于益生菌,具有塑造肠道健康,增强蛋鸡免疫的作用[44]。本试验结果显示,添加裂殖壶菌发酵物血清中丙二醛含量变化并不显著,但总抗氧化能力、过氧化氢酶活力和谷胱甘肽过氧化氢酶活力均显著升高,表明其对氧化应激造成的机体损伤具有一定缓解作用。瘤胃菌属是蛋鸡肠道中一种常见的菌属,属于厚壁菌门,通过短链脂肪酸介导α脂肪酸来促进肠道干细胞增殖[45],提高蛋鸡对营养物质的吸收。本试验结果显示,试验组蛋鸡料蛋比并未显著变化降低,这可能与蛋鸡日龄有关。Clostridiales在水牛发情期最丰富[46],与蛋鸡生产性能的关系需要进一步研究。
4 结论 4.1饲粮添加裂殖壶菌发酵物对蛋鸡生产性能及蛋品质无不利影响。
4.2饲粮中添加裂殖壶菌发酵物可显著提高蛋鸡血清总抗氧化能力、过氧化氢酶和谷胱甘肽过氧化物酶活力。
4.3饲粮中添加裂殖壶菌发酵物能够显著增加蛋黄中ω-3 PUFAs和DHA含量,降低ω-6/ω-3。
4.4饲粮中添加裂殖壶菌发酵物增加蛋鸡盲肠微生物的数量和丰度。
综上,在本试验条件下,添加0.5%裂殖壶菌发酵物可提高蛋黄中DHA含量,并对蛋鸡生产性能和蛋品质无不良影响。
| [1] |
FAN K W, JIANG Y, FAAN Y W, et al. Lipid characterization of mangrove thraustochytrid-Schizochytrium mangrovei[J]. J Agric Food Chem, 2007, 55(8): 2906-2910. DOI:10.1021/jf070058y |
| [2] |
刘源. 碳源和磷源优化裂殖壶菌发酵合成类胡萝卜素和DHA的研究[D]. 无锡: 江南大学, 2016. LIU Y. Research on the optimization of carbon and phosphorus resources for carotenoids and DHA production by Schizochytrium sp. S31[D]. Wuxi: Jiangnan University, 2016. (in Chinese) |
| [3] |
JIANG Y, FAN K W, WONG R T Y, et al. Fatty acid composition and squalene content of the marine microalga Schizochytrium mangrovei[J]. J Agric Food Chem, 2004, 52(5): 1196-1200. DOI:10.1021/jf035004c |
| [4] |
SUNDARAM T S, GIROMINI C, REBUCCI R, et al. Omega-3 polyunsaturated fatty acids counteract inflammatory and oxidative damage of non-transformed porcine enterocytes[J]. Animals (Basel), 2020, 10(6): 956. |
| [5] |
HORIA E, WATKINS B A. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells[J]. Carcinogenesis, 2007, 28(4): 809-815. |
| [6] |
ZHANG W K, LONG Y P, ZHANG J H, et al. Modulatory effects of EPA and DHA on proliferation and apoptosis of pancreatic cancer cells[J]. J Huazhong Univ Sci Technol, 2007, 27(5): 547-550. DOI:10.1007/s11596-007-0518-y |
| [7] |
ANDERSON E J, THAYNE K A, HARRIS M, et al. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARγ activation?[J]. Antioxid Redox Signal, 2014, 21(8): 1156-1163. DOI:10.1089/ars.2014.5888 |
| [8] |
ZHANG J N, FREUND M A, CULLER M D, et al. How to stabilize ω-3 polyunsaturated fatty acids (PUFAs) in an animal feeding study?-Effects of the temperature, oxygen level, and antioxidant on oxidative stability of ω-3 PUFAs in a mouse diet[J]. J Agric Food Chem, 2020, 68(46): 13146-13153. DOI:10.1021/acs.jafc.9b08298 |
| [9] |
RIMM E B, APPEL L J, CHIUVE S E, et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: A science advisory from the American heart association[J]. Circulation, 2018, 138(1): e35-e47. |
| [10] |
JANCZYK P, HALLE B, SOUFFRANT W B. Microbial community composition of the crop and ceca contents of laying hens fed diets supplemented with Chlorella vulgaris[J]. Poult Sci, 2009, 88(11): 2324-2332. DOI:10.3382/ps.2009-00250 |
| [11] |
薛永强, 余苗, 马永喜, 等. ω-3多不饱和脂肪酸对畜禽生理功能的影响及其应用的研究进展[J]. 动物营养学报, 2021, 33(9): 4870-4881. XUE Y Q, YU M, MA Y X, et al. Research progress on effects of ω-3 polyunsaturated fatty acids on physiological function of livestock and poultry and their application[J]. Chinese Journal of Animal Nutrition, 2021, 33(9): 4870-4881. DOI:10.3969/j.issn.1006-267x.2021.09.008 (in Chinese) |
| [12] |
KRALIK Z, KRALIK G, GRČEVIĆ M, et al. Microalgae Schizochytrium limacinum as an alternative to fish oil in enriching table eggs with n-3 polyunsaturated fatty acids[J]. J Sci Food Agric, 2020, 100(2): 587-594. DOI:10.1002/jsfa.10052 |
| [13] |
中华人民共和国国家卫生和计划生育委员会, 国家食品药品监督管理总局. GB 5009.168-2016食品安全国家标准食品中脂肪酸的测定[S]. 北京: 中国标准出版社, 2017. National Health and Family Planning Commission of the People's Republic of China, State Food and Drug Administration. GB 5009.168-2016 National food safety standard for determination of fatty acids in food[S]. Beijing: China Standards Press, 2017. (in Chinese) |
| [14] |
孙翠霞, 张正尧, 鹿尘. 气相色谱-质谱法测定食品中的37种脂肪酸含量[J]. 中国卫生检验杂志, 2019, 29(3): 275-277. SUN C X, ZHANG Z Y, LU C. Determination of 37 fatty acids in food by gas chromatography-mass spectrometry[J]. Chinese Journal of Health Laboratory Technology, 2019, 29(3): 275-277. (in Chinese) |
| [15] |
LIU B, ZHOU Q, ZHU J M, et al. Time course of nutritional and functional property changes in egg yolk from laying hens fed docosahexaenoic acid-rich microalgae[J]. Poult Sci, 2020, 99(9): 4616-4625. DOI:10.1016/j.psj.2020.06.007 |
| [16] |
AO T, MACALINTAL L M, PAUL M A, et al. Effects of supplementing microalgae in laying hen diets on productive performance, fatty acid profile, and oxidative stability of eggs[J]. J Appl Poult Res, 2015, 24(3): 394-400. DOI:10.3382/japr/pfv042 |
| [17] |
邓兴照, 齐广海, 刘福柱, 等. 日粮多不饱和脂肪酸类型对蛋鸡生产性能和蛋黄脂肪酸富集的影响[J]. 中国畜牧杂志, 2006, 42(3): 31-34. DENG X Z, QI G H, LIU F Z, et al. Effect of dietary polyunsaturated fatty acids on performance and composition of yolk lipids in layer hens[J]. Chinese Journal of Animal Science, 2006, 42(3): 31-34. DOI:10.3969/j.issn.0258-7033.2006.03.011 (in Chinese) |
| [18] |
LEPRETTI M, MARTUCCIELLO S, ACEVES M A B, et al. Omega-3 fatty acids and insulin resistance: focus on the regulation of mitochondria and endoplasmic reticulum stress[J]. Nutrients, 2018, 10(3): 350. DOI:10.3390/nu10030350 |
| [19] |
WANG H, ZHANG H J, WANG X C, et al. Dietary choline and phospholipid supplementation enhanced docosahexaenoic acid enrichment in egg yolk of laying hens fed a 2% Schizochytrium powder-added diet[J]. Poult Sci, 2017, 96(8): 2786-2794. DOI:10.3382/ps/pex095 |
| [20] |
陈秀丽, 李连彬, 岳洪源, 等. 裂殖壶菌粉对鸡蛋品质与蛋黄n-3PUFA含量的影响[J]. 中国畜牧杂志, 2014, 50(23): 66-70. CHEN X L, LI L B, YUE H Y, et al. Effect of Schizochytrium limacinum on egg quality and content of n-3PUFA in egg yolk[J]. Chinese Journal of Animal Science, 2014, 50(23): 66-70. DOI:10.3969/j.issn.0258-7033.2014.23.016 (in Chinese) |
| [21] |
HPANGESTUTI R, KIM S K. Biological activities and health benefit effects of natural pigments derived from marine algae[J]. J Funct Foods, 2011, 3(4): 255-266. DOI:10.1016/j.jff.2011.07.001 |
| [22] |
程启坤, 王儒昕, 王攀攀, 等. 葡萄糖亚适量补加胁迫裂殖壶菌胞内类胡萝卜素积累[J]. 中国油脂, 2018, 43(7): 102-107, 134. CHENG Q K, WANG R X, WANG P P, et al. Insufficient supplement of glucose enforcing a large accumulation of carotenoids by Schizochytrium sp.S31[J]. China Oils and Fats, 2018, 43(7): 102-107, 134. DOI:10.3969/j.issn.1003-7969.2018.07.023 (in Chinese) |
| [23] |
周佳. ω-3 PUFAs通过Fra1/Fra2调控C57BL/6 J小鼠脂质代谢作用[D]. 武汉: 武汉轻工大学, 2015. ZHOU J. Effects of ω-3 PUFAs on lipid metabolism of C57BL/6 J mice by Fra1/Fra2[D]. Wuhan: Wuhan Polytechnic University, 2015. (in Chinese) |
| [24] |
YU J H, MA Y, SUN J, et al. Microalgal oil from Schizochytrium sp.prevents HFD-induced abdominal fat accumulation in mice[J]. J Am Coll Nutr, 2017, 36(5): 347-356. DOI:10.1080/07315724.2017.1302366 |
| [25] |
杨蕊, SHIN J S, 刘玉海, 等. 饲粮中添加微藻DHA和ALA对蛋黄脂肪酸构成及蛋黄胆固醇、三酰甘油的影响[J]. 饲料研究, 2014(21): 11-14, 64. YANG R, SHIN J S, LIU Y H, et al. Effects of adding microalgae DHA and ALA to the diet on the fatty acid composition of egg yolk and egg yolk cholesterol and triacylglycerol[J]. Feed Research, 2014(21): 11-14, 64. DOI:10.13557/j.cnki.issn1002-2813.2014.21.003 (in Chinese) |
| [26] |
YANG L Q, CHEN Y, LIU Y, et al. The role of oxidative stress and natural antioxidants in ovarian aging[J]. Front Pharmacol, 2021, 11: 617843. DOI:10.3389/fphar.2020.617843 |
| [27] |
贺维朝, 王浩, 张会艳, 等. 饲粮添加虾青素对69~74周龄蛋鸡生产性能、蛋品质、抗氧化能力、生殖激素和卵泡数量的影响[J]. 动物营养学报, 2022, 34(4): 2383-2392. HE W C, WANG H, ZHANG H Y, et al. Effects of dietary astaxanthin on performance, egg quality, antioxidant capacity, reproductive hormone and follicle number of laying hens from 69 to 74 weeks of age[J]. Chinese Journal of Animal Nutrition, 2022, 34(4): 2383-2392. DOI:10.3969/j.issn.1006-267x.2022.04.033 (in Chinese) |
| [28] |
XU C C, ZHANG S, SUN B Z, et al. Dietary supplementation with microalgae (Schizochytrium sp.) improves the antioxidant status, fatty acids profiles and volatile compounds of beef[J]. Animals, 2021, 11(12): 3517. DOI:10.3390/ani11123517 |
| [29] |
徐晨晨, 李慧, 张寿, 等. 富含DHA微藻对柴达木福牛生长性能和血清抗氧化指标的影响[J]. 中国畜牧兽医, 2021, 48(6): 2074-2081. XU C C, LI H, ZHANG S, et al. Effect of dietary DHA-rich microalgae on growth performance and serum antioxidant indices of qaidamford cattle[J]. China Animal Husbandry & Veterinary Medicine, 2021, 48(6): 2074-2081. DOI:10.16431/j.cnki.1671-7236.2021.06.020 (in Chinese) |
| [30] |
LV J W, YANG X Q, MA H X, et al. The oxidative stability of microalgae oil (Schizochytrium aggregatum) and its antioxidant activity after simulated gastrointestinal digestion: relationship with constituents[J]. European J Lipid Sci Technol, 2015, 117(12): 1928-1939. DOI:10.1002/ejlt.201400588 |
| [31] |
NIETO G, ROS G. Modification of fatty acid composition in meat through diet: Effect on lipid peroxidation and relationship to nutritional quality-a review[J]. InTech, 2012, 12: 239-258. |
| [32] |
PETROVIĆ M, GAČIĆ M, KARAČIĆ V, et al. Enrichment of eggs in n-3 polyunsaturated fatty acids by feeding hens with different amount of linseed oil in diet[J]. Food Chem, 2012, 135(3): 1563-1568. DOI:10.1016/j.foodchem.2012.06.020 |
| [33] |
YANG L G, SONG Z X, YIN H, et al. Low n-6/n-3 PUFA ratio improves lipid metabolism, inflammation, oxidative stress and endothelial function in rats using plant oils as n-3 fatty acid source[J]. Lipids, 2016, 51(1): 49-59. DOI:10.1007/s11745-015-4091-z |
| [34] |
赵丹阳. 亚麻籽对北京油鸡生长性能、肉品质和n-3PUFA沉积规律的影响研究[D]. 北京: 中国农业科学院, 2020. ZHAO D Y. Effect of flaxseed on growth performance, meat quality and n-3PUFAs deposition of Beijing You chicken[D]. Beijing: Chinese Academy of Agricultural Sciences, 2020. (in Chinese) |
| [35] |
NGO NJEMBE M T, DEJONGHE L, VERSTRAELEN E, et al. The egg yolk content in ω-3 and conjugated fatty acids can be sustainably increased upon long-term feeding of laying hens with a diet containing flaxseeds and pomegranate seed oil[J]. Foods, 2021, 10(5): 1134. DOI:10.3390/foods10051134 |
| [36] |
KEEGAN J D, CURRIE D, KNOX A, et al. Heterotrophic Aurantiochytrium sp.supplementation to layer diets sustainably increases the omega-3 concentration of eggs[J]. Br Poult Sci, 2019, 60(5): 570-578. DOI:10.1080/00071668.2019.1622079 |
| [37] |
SONNENBURG J L, BÄCKHED F. Diet-microbiota interactions as moderators of human metabolism[J]. Nature, 2016, 535(7610): 56-64. DOI:10.1038/nature18846 |
| [38] |
吴娟娟. 肠道菌群对仔鸡肠道黏膜结构、免疫功能及脂肪代谢的影响[D]. 南昌: 江西农业大学, 2015. WU J J. Effects of gut microbiota on the structure of intestinal mucosa and immune function and fat metabolism of chicks[D]. Nanchang: Jiangxi Agricultural University, 2015. (in Chinese) |
| [39] |
MAGALHÃES R, GUERREIRO I, SANTOS R A, et al. Oxidative status and intestinal health of gilthead sea bream (Sparus aurata) juveniles fed diets with different ARA/EPA/DHA ratios[J]. Sci Rep, 2020, 10(1): 13824. DOI:10.1038/s41598-020-70716-5 |
| [40] |
LU X D, ZHONG R B, HU L, et al. DHA-enriched phospholipids from large yellow croaker roe regulate lipid metabolic disorders and gut microbiota imbalance in SD rats with a high-fat diet[J]. Food Funct, 2021, 12(11): 4825-4841. DOI:10.1039/D1FO00747E |
| [41] |
CHEN J, YI C M, LU C Y, et al. High DHA tuna oil alleviated cigarette smoking exposure induced lung inflammation via the regulation of gut microbiota and serum metabolites[J]. J Funct Foods, 2021, 82: 104505. DOI:10.1016/j.jff.2021.104505 |
| [42] |
ZHANG P, LI H Y, JIA W, et al. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice[J]. Microbiome, 2021, 9(1): 185. DOI:10.1186/s40168-021-01126-6 |
| [43] |
LEE N Y, YOON S J, HAN D H, et al. Lactobacillus and Pediococcus ameliorate progression of non-alcoholic fatty liver disease through modulation of the gut microbiome[J]. Gut Microbes, 2020, 11(4): 882-899. DOI:10.1080/19490976.2020.1712984 |
| [44] |
DAILLÈRE R, VÉTIZOU M, WALDSCHMITT N, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects[J]. Immunity, 2016, 45(4): 931-943. DOI:10.1016/j.immuni.2016.09.009 |
| [45] |
XIE J, LI L F, DAI T Y, et al. Short-chain fatty acids produced by Ruminococcaceae mediates α-Linolenic acid promote intestinal stem cells proliferation[J]. Mol Nutr Food Res, 2022, 66(1): e2100408. DOI:10.1002/mnfr.202100408 |
| [46] |
SHARMA R, SINGH P K, ONTERU S K, et al. Faecal microbiome analysis reveals Clostridiales and Bacteroidales as signature gut microbes during estrus of buffalo[J]. Reprod Biol, 2021, 21(2): 100509. DOI:10.1016/j.repbio.2021.100509 |
(编辑 范子娟)



