脂多糖(lipopolysaccharide,LPS)又称内毒素(endotoxin),是革兰阴性菌细胞壁的主要成分,由多糖O抗原、核心多糖和类脂A三部分组成,仅在细菌裂解后释放,可诱发机体炎症反应和免疫应答[1-2],进一步引起一系列代谢性疾病,如糖代谢异常引起2型糖尿病,脂代谢异常引起酮病、脂肪肝和肥胖,最终导致奶牛生产性能下降[3-4]。在这个过程中,LPS作为引起炎症反应并改变机体多种糖脂代谢相关激素和脂肪因子水平的重要致病因素,进一步研究其导致糖脂代谢异常相关机制十分必要。本文综述了LPS与炎症反应和糖脂代谢相互作用关系及其导致糖脂代谢异常作用机制,为LPS致奶牛糖脂代谢异常机理研究提供参考。
1 LPS的来源LPS在环境中普遍存在,空气尘埃可作为细菌LPS传播载体,易被动物吸入机体;饲料和水中也可检测到不同浓度的LPS[5]。近年来,奶牛革兰阴性细菌病呈多发态势。乳房炎、子宫内膜炎和蹄叶炎等,以及各种原因引起的肠道菌群失调,如便秘、腹泻等,均会引起体内LPS升高,尤其是采用大剂量抗生素治疗时,革兰阴性菌裂解死亡,进而释放LPS[6-9]。此外,各种反刍动物前胃疾病,如前胃迟缓、瘤胃积食和瘤胃酸中毒等会破坏瘤胃微生态平衡,诱导革兰阴性菌崩解释放LPS,引起瘤胃内LPS浓度升高,是奶牛体内LPS的重要内源性来源[10-12]。总之,LPS来源广泛,对奶牛健康存在很大威胁。
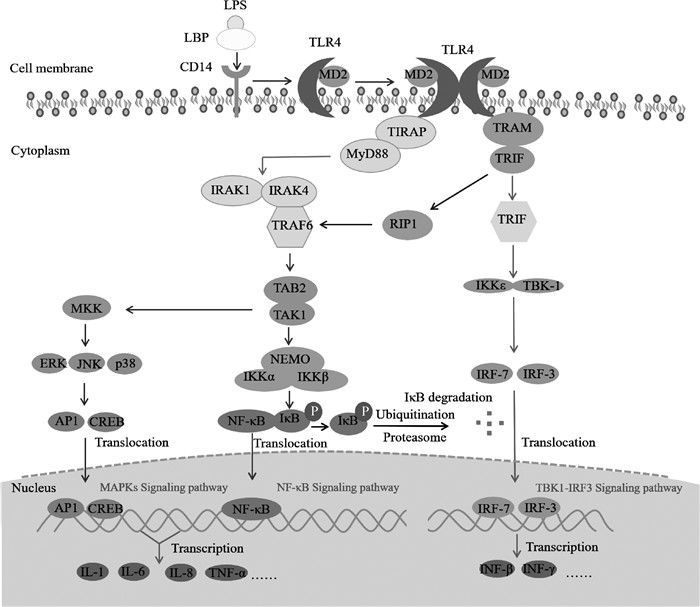
2 LPS的致炎作用及其机制由于LPS主要毒性中心和生物活性部分类脂A高度保守,且无种属差异,故不同细菌LPS毒性作用大致相同[13]。LPS侵入机体,与巨噬细胞、中性粒细胞和上皮细胞等表面Toll样受体4(toll-like receptor 4,TLR4)结合,激活下游信号通路,刺激肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素-1β(interleukin-1β,IL-1β)和白细胞介素-6(interleukin-6,IL-6)等炎症因子产生,引起炎症反应[14-15]。体外研究同样发现,LPS可激活奶牛原代肝细胞、乳腺上皮细胞和子宫内膜上皮细胞炎症通路,刺激炎症因子生成,诱导细胞和组织炎症[6, 16-17]。
LPS主要通过诱导信号级联反应最终激活核转录因子-κB(nuclear transcription factor kappa B,NF-κB)和有丝分裂原活化蛋白激酶(mitogen-activated protein kinases,MAPKs)等信号通路[18]。LPS侵入机体后,首先与血清脂多糖结合蛋白(lipopolysaccharide-binding protein,LBP)结合,形成LPS-LBP复合物,增强LPS生物活性,然后以二聚体复合物形式结合到细胞表面分化抗原簇14(cluster of differentiation 14,CD14)分子上,形成LPS-LBP-CD14三联体复合物,进一步识别并结合TLR4-髓样分化蛋白-2(myeloid differentiation protein-2,MD-2)[19-20],由此将信号由胞外传至胞内。TLR4再与其接头蛋白TIR-相关蛋白(MyD88-adaptor-like/TIR-associated protein,MAL/TIRAP)结合,通过TLR4适配蛋白髓样分化因子88(myeloid differentiation factor 88,MyD88)依赖或非依赖途径最终激活NF-κB[21-22],依赖途径包括经典NF-κB信号通路和MAPKs信号通路,主要参与炎症早期激活。
2.1 经典NF-κB信号通路正常情况下NF-κB在胞质中与NF-κB抑制蛋白(inhibitor of NF-κB,IκB)结合形成NF-κB-IκB二聚体复合物,并受其抑制而处于非活化状态,只有当IκB被IκB激酶(IκB kinase,IKK)磷酸化后才可发挥下游作用[23]。TIRAP募集MyD88后结合白介素受体相关激酶(interleukin receptor associated kinase,IRAK),包括IRAK-1和IRAK-4,启动NF-κB活化[24]。首先,IRAK-1自发磷酸化产生超磷酸化IRAK-1,结合并激活TNF受体相关因子-6(TNF-receptor associated factor 6,TRAF-6)。TRAF-6再与TAK-1结合蛋白-1(TAK-1 binding protein-1,TAB-1)和TAB-2复合物中TAB-2结合,激活转移生长因子-β激活激酶-1(transforming growth factor-β activated kinase,TAK-1),进而作为NF-κB和MAPKs通路的共同辅助激活酶。TAK-1活化后激活IKK复合物,其包括两个催化亚基:IKKα和IKKβ激酶,一个调节亚基NEMO(nuclear factor-kappa B essential modulator)/IKKγ,NEMO/IKKγ先与泛素链结合,募集IKK到达TAK-1,再磷酸化IKKβ,进而活化IKK复合物,使IκB发生磷酸化修饰,进一步脱颗粒从NF-κB-IκB复合物中解离,然后发生泛素化修饰后被蛋白酶体降解。解离后的NF-κB活化并转移至细胞核,结合DNA中同源位点并启动编码IL-6、IL-1、TNF-α的基因转录,最终诱导炎症因子释放[25-27]。
2.2 MAPKs信号通路目前发现MAPKs信号通路有三条下游通路:胞外信号调节激酶1/2(extracellular signal-regulated kinase,ERK1/2)、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)和p38 MAPK信号通路。TAK-1亦能磷酸化MAPK激酶(mitogen-activated protein kinase kinase,MKK)家族成员,激活下游通路。在ERK1/2信号激活过程中,RAS作为上游激活蛋白,通过三级激酶级联激活方式依次激活RAF/MEK/ERK,引起细胞分泌大量IL-1β、TNF-α、IL-17等炎症因子;同样,JNK和p38 MAPK信号通路激活后,也会引起大量炎症因子释放,如IL-1β、IL-6和TNF-α等[27-28]。
2.3 MyD88非依赖途径MyD88非依赖途径通过包含Toll/白细胞介素-1受体的IFN-β诱导蛋白(TIR-domain-containing adapter inducing IFN-β,TRIF)和TRIF相关接头蛋白分子(TRIF-related adaptor molecule,TRAM) 作为衔接分子激活下游信号通路,导致NF-κB延迟激活。当信号传至胞内,TLR4与TRIF和TRAM结合,TRIF进一步与另一接头分子受体相互作用蛋白1(receptor-interacting protein,RIP1)结合并激活TRAF-6,再通过MyD88依赖途径相同过程激活NF-κB,并易位到细胞核诱导相关炎症因子产生。此外,TRIF还可激活IRF-3和IRF-7,诱导包括IFN-β在内的一系列基因的转录。活化的TRIF激活IKKε/IKKi和TANK结合蛋白-1(TANK-binding protein 1,TBK-1),两者可作为IRF-3/IRF-7激酶,并与之结合,形成TRIF、TBK-1、IKK和IRF-3/IRF-7复合体,使IRF-3/IRF-7磷酸化并活化,进而与干扰素敏感反应元件(interferon-sensitive response element,ISRE)结合,启动IFN-β基因转录,IFN-β又能激活信号转录与转录激活子1(STAT1),诱导IFN基因进一步表达[23, 29-30]。
综上所述,LPS进入机体后与巨噬细胞、中性粒细胞和上皮细胞表面TLR4作用,激活NF-κB/MAPKs/TBK1-IRF3信号通路,通过信号级联放大反应,最终诱导TNF-α、IL-1、IL-6和IFN-β等炎症因子的合成和分泌,引发炎症反应,具体调控机制见图 1。
|
图 1 LPS致炎信号通路 Fig. 1 LPS-induced inflammatory signaling pathway |
LPS是一种强烈炎症刺激物,可致机体广泛出现炎症,而炎症又与代谢性疾病存在密切联系,可引起机体发生胰岛素抵抗、2型糖尿病、酮病和肥胖等[3, 31-32]。在LPS炎症与上述代谢性疾病过程中,糖脂代谢异常发挥了重要作用。糖代谢异常是指多种原因引起的胰岛素分泌和作用障碍,导致机体葡萄糖代谢和转化功能异常,是多种疾病的病理和生理基础,且两者互为因果;脂代谢异常是指包括胆固醇(CHOL)、三酰甘油(TG)、磷脂(PL)和游离脂肪酸(FFA)等在内的脂类物质在体内合成、分解、消化、吸收和转运发生异常,使各组织中脂质改变,从而影响机体健康。两者都属于病理过程,会给机体造成严重危害,甚至导致死亡。
4 LPS炎症致糖代谢异常作用机制炎症可诱发应激反应和神经内分泌系统功能紊乱,改变激素水平,进一步影响糖代谢,这是机体对应激最基本的响应机制之一。应激所致糖代谢异常多表现为血糖快速升高后维持在高水平,持续高血糖又会诱发慢性炎症,从而构成炎症-应激-高血糖-炎症的恶性循环。而炎症因子如TNF-α、IL-1β和IL-6等可引起下丘脑-垂体-靶腺轴功能变化,导致内分泌紊乱,进而影响糖脂代谢[33]。反刍动物能量代谢主要受胰岛素和胰高血糖素调控,胰岛素通过促进细胞对葡萄糖的摄取来调节碳水化合物代谢,维持正常血糖水平。炎症引起胰岛素抵抗,胰岛素水平降低,胰高血糖素水平升高,导致机体糖代谢异常,血糖波动明显。胰岛素在脂质代谢中也发挥重要作用,可促进脂肪合成,抑制脂肪分解,减少肝摄取FFA,提高组织酮体利用及降低肉碱棕榈酰转移酶1(carnitine palmitoyl transferase 1,CPT-1)活性[34],其参与脂肪酸转运。此外,LPS会引起奶牛特别是泌乳高峰期奶牛能量负平衡[35]。在此过程中,机体分解大量体脂以合成乳脂,血液中大量FFA进入肝用于氧化供能及合成TG,致肝大量TG蓄积,超过肝转运能力导致脂肪肝,出现酮血症,并伴随外周胰岛素抵抗和胰岛素水平降低的现象[35-36]。
MAPKs信号通路在调节胰岛素分泌合成中具有重要作用。p38可加重胰岛素抵抗,其家族成员p38δ是调节葡萄糖稳态的重要因子,可抑制蛋白激酶D1(protein kinase D1,PKD1)磷酸化,影响胰岛β细胞活性和胰岛素分泌[37]。在脂肪组织中,p38激活可促进葡萄糖转运蛋白1(glucose transporter 1,GLUT1)表达并抑制GLUT4表达,GLUT1负责基础葡萄糖转运,GLUT4负责胰岛素依赖性葡萄糖转运,进而影响机体葡萄糖转运[38]。JNK通路激活引起外周胰岛素抵抗、胰岛素分泌抑制、胰岛β细胞凋亡增加[37]。ERK通路可能通过抑制胰岛素受体底物1(insulin receptor substrate 1,IRS1)酪氨酸磷酸化和mRNA转录及影响脂肪因子分泌引起胰岛素抵抗[39]。
上述研究提示,LPS诱发炎症进一步改变胰岛素和胰高血糖素水平,引起奶牛能量负平衡,激活MAPKs信号通路,抑制胰岛素分泌和GLUT4转运葡萄糖,出现血糖升高和胰岛素抵抗,进而导致糖脂代谢异常。
5 LPS炎症致脂代谢异常作用机制高谷物或高脂日粮诱导奶牛瘤胃酸中毒研究发现,LPS通过影响内源性乙酸和β-羟丁酸(BHBA)及外源性FFA和TG等乳脂合成前体物,从而降低乳脂合成和乳脂率,引起奶牛糖脂代谢紊乱[40-41]。进一步研究表明,LPS主要是通过影响乳腺中乳脂合成的关键酶来调控组织中脂质代谢过程。例如,LPS抑制脂肪酶活性,减弱对TG和极低密度脂蛋白的清除作用,进而抑制乳腺吸收脂肪酸,不利于乳脂合成[42-43]。此外,乳腺灌注LPS后,奶牛血糖升高,FFA、BHBA和甘油含量降低[44]。
肝是机体代谢LPS的重要环节,LPS可活化肝巨噬细胞,产生iNOS、IL-1、IL-6和TNF-α等细胞因子,导致肝细胞凋亡并造成肝损伤,影响机体糖脂代谢,并刺激肝细胞产生血清淀粉样蛋白A(serum amyloid A,SAA),与LPS和脂蛋白形成复合物,阻碍脂肪转运,影响乳脂合成分泌[45]。奶牛静脉注射或乳腺灌注LPS后,乳汁和血清SAA含量升高[46]。在泌乳期奶牛乳腺上皮细胞中同样发现,LPS引起SAA表达明显增加[47]。研究表明,牛奶中乳脂含量降低通常与亚急性瘤胃酸中毒(subacute ruminal acidosis,SARA)有关[48],当泌乳奶牛发生SARA时,外周血LPS含量升高,脂蛋白比降低,乳脂合成受到抑制[45]。此外,LPS还可改变乳汁脂肪酸组成,降低乳糖含量[49]。
腺苷酸活化蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK)信号通路在脂代谢中发挥重要作用,激活后可抑制脂质合成,继而影响乳汁乳脂含量,而乳脂含量对乳品质具有重要意义,乳脂主要由TG(97%)和CHOL组成。LPS激活AMPK信号通路,抑制奶牛乳腺上皮细胞脂合成代谢关键基因表达,引起TG合成能力下降,组织中葡萄糖摄取和脂肪酸氧化增加[50]。究其原因,可能是因炎症状态下,奶牛乳腺上皮细胞首先将胞内物质经转换后用于抵抗细胞应激,消耗大量乳脂前体物,进而通过AMPK信号通路影响乳脂合成。此外,随日粮精粗比提高,血浆BHBA和CHOL含量下降及乳酸含量增加与瘤胃LPS升高存在高度相关性[4]。一方面是由于血浆LPS诱导产生的急性期蛋白及乳酸、氨基酸等物质的综合影响导致CHOL含量降低;另一方面是由于外周高血糖影响肝细胞,使FFA经生酮作用产生的BHBA含量减少,从而影响脂质代谢[51]。Zebeli等[4]也发现奶牛发生SARA时,瘤胃液LPS大量进入血液,引起炎症反应并活化免疫系统,刺激CHOL衍生为胆酸盐,随胆汁排入消化道,中和胃肠道过多LPS,导致血浆CHOL含量降低。
以上研究结果表明,LPS通过改变奶牛机体FFA、BHBA、TG和CHOL水平,诱导肝细胞损伤,刺激SAA分泌和激活AMPK信号通路等途径,影响糖脂代谢过程。
6 脂肪因子与糖脂代谢异常的关系脂肪组织产生的脂联素(adiponectin)、瘦素(leptin)、抵抗素(resistin)、内脂素(visfatin)和炎症因子(TNF-α、IL-1β和IL-6)等脂肪因子同样具有调节机体糖脂代谢功能,与能量代谢性疾病存在密切联系。LPS刺激引起能量负平衡,为弥补能量缺口,奶牛动员体脂供能,并改变脂肪因子分泌模式,导致脂肪肝发生[35, 52],对患脂肪肝奶牛血清脂肪因子进行检测发现,TNF-α与IL-6水平上升,脂联素和瘦素水平下降[53-54]。此外,围产期奶牛血清脂联素、瘦素、抵抗素、IL-6和TNF-α与酮病存在相关性,可作为酮病预警指标的潜力[55-56],暗示脂肪因子与糖脂代谢存在密切相关性。
炎症因子中,TNF-α既可通过激活JNK通路使胰岛素受体底物1(insulin receptor substrate,IRS1)丝氨酸磷酸化,又可通过激活p38和IKK通路抑制IRS1酪氨酸磷酸化,阻止GLUT4转位,造成胰岛素抵抗[57]。PPARγ是一种转录因子,可调节脂质代谢相关基因表达,调控脂肪细胞中脂质储存、脂肪因子分泌和能量稳态,增加肝和骨骼肌胰岛素敏感性,抑制炎症因子释放,而TNF-α可抑制PPARγ转录,造成胰岛素抵抗[58-59]。IL-6对IRS1、GLUT4和PPARγ存在长效抑制作用,可抑制细胞因子信号转录抑制因子3(suppressor of cytokine signaling 3,SOCS3)表达。在体外,SOCS3是胰岛素受体(insulin receptor,IR)自磷酸化抑制剂;在体内,SOCS3通过泛素降解IRS1和IRS2产生胰岛素抵抗[60]。磷脂酰肌醇3激酶(phosphatidylinositol 3 kinase,PI3K)通路在糖脂代谢中具有重要作用,其激活可促进葡萄糖摄取、肝糖原合成、脂肪合成及脂联素分泌以增强胰岛素敏感性。IL-6可降低肝细胞中IRS1磷酸化水平,减少IRS1与PI3K的p58亚基结合,抑制依赖胰岛素的蛋白激酶B(protein kinase B,Akt)活性,从而抑制PI3K通路。而IL-1β处理体外培养的脂肪细胞可使IR的β亚基和IRS1磷酸化,造成胰岛素抵抗[61-62]。
脂联素是一种具有促进脂肪酸氧化,抑制脂质合成,抑制糖原异生,改善胰岛素抵抗和抑制炎症反应等一系列生理作用的脂肪因子[63],是胰岛素的增敏剂,发挥维持机体能量稳态的重要作用。研究发现,脂联素对奶牛能量负平衡敏感,其浓度与胰岛素抵抗呈正相关[64],与血清FFA、BHBA和TG含量呈负相关,而正常情况下BHBA又可作为反刍动物脂质生成过程中的能量来源和合成葡萄糖的前体物质[65],并与促炎因子存在互作效应。TNF-α和IL-6可降低脂联素mRNA表达;而脂联素可抑制LPS诱导NF-κB激活和IL-6分泌,上调过氧化物酶增殖激活受体γ2(PPARγ2)表达,发挥降血糖、血脂和增加胰岛素敏感性的作用[66]。
瘦素是一种由白色脂肪分泌的细胞因子,与糖脂代谢密切相关,可增加能量消耗,减少肝细胞葡萄糖释放,并介导MAPKs和PI3K信号通路,增加胰岛素敏感性,同时下调PPARγ表达以降低脂肪合成速率[67]。机体所产生的TNF-α和IL-6等炎症因子可诱导瘦素水平升高,刺激胰岛素生成,加重胰岛素抵抗,且间接升高血脂水平,暗示瘦素可能在糖脂代谢中发挥中间作用。体外研究发现,瘦素通过牛原代肌细胞PI3K信号通路激活SOCS3,造成胰岛素抵抗[68]。因此推测,LPS诱导胰岛素抵抗和刺激炎症因子生成,刺激瘦素分泌,引起糖脂代谢紊乱。
抵抗素是一类在脂肪细胞与M2巨噬细胞中合成且可诱导胰岛素抵抗的脂肪因子,减弱胰岛素对肝、脂肪、骨骼肌等外周组织的作用,导致葡萄糖摄取和利用效率下降[69],引起机体糖脂代谢紊乱。研究发现,奶牛血糖和胰岛素含量与抵抗素存在显著线性关系,抵抗素水平降低会抑制乳腺上皮细胞对胰岛素依赖性葡萄糖的摄取,造成胰岛素抵抗,降低葡萄糖利用率。其还可促进牛脂肪组织甘油生成和基因表达,参与脂质代谢和沉积[70],激活牛肺泡巨噬细胞TLR4/MyD88非依赖信号通路,释放大量炎症因子,包括TNF-α、IL-1β和IL-6[30],进而影响胰岛素信号通路,改变糖脂代谢[61-62]。由此推测,LPS诱导炎症并刺激抵抗素水平升高,共同促进炎症反应,诱导机体胰岛素抵抗,进一步影响糖脂代谢过程。
内脂素是由白色脂肪组织分泌的胰岛素类似物,与糖脂代谢密切相关,可促进脂肪分化、合成和聚集,增加胰岛素合成和分泌及敏感性[71],可能通过以下途径改变糖脂代谢。内脂素促进Β细胞NF-κB因子表达,CD14巨噬细胞趋化作用及细胞因子合成功能,导致炎症失衡。其异常增加可能是连接脂肪细胞与巨噬细胞相互作用的介质,进一步增强脂肪组织乃至全身炎症反应。LPS刺激下其血浆浓度升高,与血清IL-6水平显著相关[72],在胰岛素抵抗与胰岛素缺乏时,内脂素表现出了胰岛素样降血糖作用,结合胰岛素受体上的非胰岛素结合位点,激活胰岛素信号传导通路,诱导葡萄糖向脂肪、肌肉细胞转运,抑制肝糖输出,从而引起血糖降低[73],是机体的一种保护措施。
综上所述,LPS诱导炎症并影响机体炎症因子、脂联素、瘦素、抵抗素和内脂素等脂肪因子水平,改变机体糖脂代谢过程,致机体糖脂代谢异常。
7 小结与展望LPS在引起奶牛机体炎症的发生、发展和糖脂代谢紊乱过程中发挥着重要作用。LPS与巨噬细胞、中性粒细胞和上皮细胞等表面TLR4作用,通过MyD88依赖和非依赖途径,逐级激活NF-κB和MAPKs等下游信号通路,刺激炎症因子生成,引发炎症反应。炎症进一步引起奶牛机体糖脂代谢相关激素和脂肪因子水平发生改变,包括胰岛素降低,胰高血糖素升高,脂联素降低,瘦素、抵抗素和内脂素升高,导致机体出现胰岛素抵抗和高血糖症,以及血清脂类物质水平改变,包括FFA水平增加,BHBA、TG和CHOL含量下降等,最终导致机体表现为糖脂代谢异常。本综述为进一步研究LPS对奶牛脂肪因子的直接调控作用提供了理论参考,从而可针对LPS引起的奶牛糖脂代谢异常信号通路上的受体开展早期控制或阻断,防控相关性疾病的发生。
| [1] |
COCHET F, PERI F. The role of carbohydrates in the lipopolysaccharide (LPS)/toll-like receptor 4 (TLR4) signalling[J]. Int J Mol Sci, 2017, 18(11): 2318. DOI:10.3390/ijms18112318 |
| [2] |
SHERMAN D J, XIE R, TAYLOR R J, et al. Lipopolysaccharide is transported to the cell surface by a membrane-to-membrane protein bridge[J]. Science, 2018, 359(6377): 798-801. DOI:10.1126/science.aar1886 |
| [3] |
ZEBELI Q, SIVARAMAN S, DUNN S M, et al. Intermittent parenteral administration of endotoxin triggers metabolic and immunological alterations typically associated with displaced abomasum and retained placenta in periparturient dairy cows[J]. J Dairy Sci, 2011, 94(10): 4968-4983. DOI:10.3168/jds.2011-4194 |
| [4] |
ZEBELI Q, DUNN S M, AMETAJ B N. Perturbations of plasma metabolites correlated with the rise of rumen endotoxin in dairy cows fed diets rich in easily degradable carbohydrates[J]. J Dairy Sci, 2011, 94(5): 2374-2382. DOI:10.3168/jds.2010-3860 |
| [5] |
汪志, 董国忠, 吴剑波. 内毒素对猪的危害及其控制[J]. 动物营养学报, 2017, 29(2): 397-402. WANG Z, DONG G Z, WU J B. The adverse effects of endotoxin on pigs and its control[J]. Chinese Journal of Animal Nutrition, 2017, 29(2): 397-402. DOI:10.3969/j.issn.1006-267x.2017.02.005 (in Chinese) |
| [6] |
AKHTAR M, GUO S, GUO Y F, et al. Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis[J]. Acta Trop, 2020, 207: 105458. DOI:10.1016/j.actatropica.2020.105458 |
| [7] |
DOHMEN M J W, JOOP K, STURK A, et al. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta[J]. Theriogenology, 2000, 54(7): 1019-1032. DOI:10.1016/S0093-691X(00)00410-6 |
| [8] |
GOMEZ D E, RODRIGUEZ-LECOMPTE J C, LOFSTEDT J, et al. Detection of endotoxin in plasma of hospitalized diarrheic calves[J]. J Vet Emerg Crit Care (San Antonio), 2019, 29(2): 166-172. DOI:10.1111/vec.12815 |
| [9] |
KIM H S, WHON T W, SUNG H, et al. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance[J]. Nat Commun, 2021, 12(1): 161. DOI:10.1038/s41467-020-20389-5 |
| [10] |
GOZHO G N, KRAUSE D O, PLAIZIER J C. Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced subacute ruminal acidosis in dairy cows[J]. J Dairy Sci, 2007, 90(2): 856-866. DOI:10.3168/jds.S0022-0302(07)71569-2 |
| [11] |
ELMHADI M E, ALI D K, KHOGALI M K, et al. Subacute ruminal acidosis in dairy herds: microbiological and nutritional causes, consequences, and prevention strategies[J]. Anim Nutr, 2022, 10: 148-155. DOI:10.1016/j.aninu.2021.12.008 |
| [12] |
KRAUSE K M, OETZEL G R. Understanding and preventing subacute ruminal acidosis in dairy herds: a review[J]. Anim Feed Sci Technol, 2006, 126(3-4): 215-236. DOI:10.1016/j.anifeedsci.2005.08.004 |
| [13] |
张晓音, 吴旻, 李雨萌, 等. 脂多糖的效应及其机理研究进展[J]. 动物医学进展, 2015, 36(12): 133-136. ZHANG X Y, WU M, LI Y M, et al. Progress on effects and mechanisms of lipopolysaccharides[J]. Progress in Veterinary Medicine, 2015, 36(12): 133-136. DOI:10.3969/j.issn.1007-5038.2015.12.028 (in Chinese) |
| [14] |
LEE Y G, LEE J, BYEON S E, et al. Functional role of Akt in macrophage-mediated innate immunity[J]. Front Biosci (Landmark Ed), 2011, 16(2): 517-530. |
| [15] |
QURESHI N, VOGEL S N, VAN WAY Ⅲ C, et al. The proteasome: a central regulator of inflammation and macrophage function[J]. Immunol Res, 2005, 31(3): 243-260. DOI:10.1385/IR:31:3:243 |
| [16] |
DAVIES D, MEADE K G, HERATH S, et al. Toll-like receptor and antimicrobial peptide expression in the bovine endometrium[J]. Reprod Biol Endocrinol, 2008, 6: 53. DOI:10.1186/1477-7827-6-53 |
| [17] |
赵欣, 王莹, 李春亭, 等. 蒲公英提取物对LPS诱导小鼠乳腺炎的减轻效应及其机制分析[J]. 畜牧兽医学报, 2022, 53(8): 2773-2781. ZHAO X, WANG Y, LI C T, et al. Alleviating effect and mechanism of dandelion extract on LPS-induced mastitis in mice[J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(8): 2773-2781. DOI:10.11843/j.issn.0366-6964.2022.08.034 (in Chinese) |
| [18] |
GUO Y F, XU N N, SUN W J, et al. Luteolin reduces inflammation in Staphylococcus aureus-induced mastitis by inhibiting NF-kB activation and MMPs expression[J]. Oncotarget, 2017, 8(17): 28481-28493. DOI:10.18632/oncotarget.16092 |
| [19] |
FICKE L M. Role of TLR4 accessory proteins CD14 and MD-2 in the combinatorial recognition of pathogens[D]. Toledo: The University of Toledo, 2008.
|
| [20] |
GIOANNINI T L, WEISS J P. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells[J]. Immunol Res, 2007, 39(1-3): 249-260. DOI:10.1007/s12026-007-0069-0 |
| [21] |
BELHAOUANE I, HOFFMANN E, CHAMAILLARD M, et al. Paradoxical roles of the MAL/tirap adaptor in pathologies[J]. Front Immunol, 2020, 11: 569127. DOI:10.3389/fimmu.2020.569127 |
| [22] |
ANTHONEY N, FOLDI I, HIDALGO A. Toll and toll-like receptor signalling in development[J]. Development, 2018, 145(9): v156018. DOI:10.1242/dev.156018 |
| [23] |
TSUKAMOTO H, TAKEUCHI S, KUBOTA K, et al. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1-IKK∈-IRF3 axis activation[J]. J Biol Chem, 2018, 293(26): 10186-10201. DOI:10.1074/jbc.M117.796631 |
| [24] |
CRONIN J G, TURNER M L, GOETZE L, et al. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium[J]. Biol Reprod, 2012, 86(2): 51. |
| [25] |
HAYDEN M S, GHOSH S. Signaling to NF-κB[J]. Genes Dev, 2004, 18(18): 2195-2224. DOI:10.1101/gad.1228704 |
| [26] |
LOYI T, KUMAR H, NANDI S, et al. Differential expression of pro-inflammatory cytokines in endometrial tissue of buffaloes with clinical and sub-clinical endometritis[J]. Res Vet Sci, 2013, 94(2): 336-340. DOI:10.1016/j.rvsc.2012.09.008 |
| [27] |
CUI L Y, WANG H, LIN J Q, et al. Progesterone inhibits inflammatory response in E. coli-or LPS-Stimulated bovine endometrial epithelial cells by NF-κB and MAPK pathways[J]. Dev Comp Immunol,, 2020, 105: 103568. DOI:10.1016/j.dci.2019.103568 |
| [28] |
ARTHUR J S C, LEY S C. Mitogen-activated protein kinases in innate immunity[J]. Nat Rev Immunol, 2013, 13(9): 679-692. DOI:10.1038/nri3495 |
| [29] |
TAKEUCHI O. IRF3:a molecular switch in pathogen responses[J]. Nat Immunol, 2012, 13(7): 634-635. DOI:10.1038/ni.2346 |
| [30] |
TIAN M Y, LI K, LIU R N, et al. Angelica polysaccharide attenuates LPS-induced inflammation response of primary dairy cow claw dermal cells via NF-κB and MAPK signaling pathways[J]. BMC Vet Res, 2021, 17(1): 248. DOI:10.1186/s12917-021-02952-4 |
| [31] |
李林, 曹萌, 宫彬彬, 等. 丁酸钠通过AMPK通路调控LPS造成牛乳腺上皮细胞脂代谢紊乱的作用机制[J]. 畜牧兽医学报, 2022, 53(9): 3221-3230. LI L, CAO M, GONG B B, et al. The mechanism of sodium butyrate through AMPK pathway to regulate lipid metabolism disorder caused by LPS in bovine mammary epithelial cells[J]. Acta Veterinaria et Zootechnica Sinica, 2022, 53(9): 3221-3230. (in Chinese) |
| [32] |
CANI P D, AMAR J, IGLESIAS M A, et al. Metabolic endotoxemia initiates obesity and insulin resistance[J]. Diabetes, 2007, 56(7): 1761-1772. DOI:10.2337/db06-1491 |
| [33] |
GROSS J J, SCHWINN A C, BRUCKMAIER R M. Free and bound cortisol, corticosterone, and metabolic adaptations during the early inflammatory response to an intramammary lipopolysaccharide challenge in dairy cows[J]. Domest Anim Endocrinol, 2020, 74: 106554. |
| [34] |
HAYIRLI A. The role of exogenous insulin in the complex of hepatic lipidosis and ketosis associated with insulin resistance phenomenon in postpartum dairy cattle[J]. Vet Res Commun, 2006, 30(7): 749-774. DOI:10.1007/s11259-006-3320-6 |
| [35] |
AMETAJ B N. A new understanding of the causes of fatty liver in dairy cows[J]. Adv Dairy Technol, 2005, 17: 97-112. |
| [36] |
WEBER C, SCHÄFF C T, KAUTZSCH U, et al. Insulin-dependent glucose metabolism in dairy cows with variable fat mobilization around calving[J]. J Dairy Sci, 2016, 99(8): 6665-6679. DOI:10.3168/jds.2016-11022 |
| [37] |
SUMARA G, FORMENTINI I, COLLINS S, et al. Regulation of PKD by the MAPK p38δ in insulin secretion and glucose homeostasis[J]. Cell, 2009, 136(2): 235-248. DOI:10.1016/j.cell.2008.11.018 |
| [38] |
FUJISHIRO M, GOTOH Y, KATAGIRI H, et al. MKK6/3 and p38 MAPK pathway activation is not necessary for insulin-induced glucose uptake but regulates glucose transporter expression[J]. J Biol Chem, 2001, 276(23): 19800-19806. DOI:10.1074/jbc.M101087200 |
| [39] |
OZAKI K I, AWAZU M, TAMIYA M, et al. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes[J]. Am J Physiol Endocrinol Metab, 2016, 310(8): E643-E651. DOI:10.1152/ajpendo.00445.2015 |
| [40] |
ANDERSEN P H. Bovine endotoxicosis--some aspects of relevance to production diseases. A review[J]. Acta Vet Scand Suppl, 2003, 98: 141-155. |
| [41] |
AMETAJ B N, BRADFORD B J, BOBE G, et al. Strong relationships between mediators of the acute phase response and fatty liver in dairy cows[J]. Can J Anim Sci, 2005, 85(2): 165-175. DOI:10.4141/A04-043 |
| [42] |
KHOVIDHUNKIT W, KIM M S, MEMON R A, et al. Thematic review series: the pathogenesis of atherosclerosis.Effects of infection and inflammation on lipid and lipoprotein metabolism mechanisms and consequences to the host[J]. J Lipid Res, 2004, 45(7): 1169-1196. DOI:10.1194/jlr.R300019-JLR200 |
| [43] |
MERKEL M, ECKEL R H, GOLDBERG I J. Lipoprotein lipase: genetics, lipid uptake, and regulation[J]. J Lipid Res, 2002, 43(12): 1997-2006. DOI:10.1194/jlr.R200015-JLR200 |
| [44] |
WALDRON M R, KULICK A E, BELL A W, et al. Acute experimental mastitis is not causal toward the development of energy-related metabolic disorders in early postpartum dairy cows[J]. J Dairy Sci, 2006, 89(2): 596-610. DOI:10.3168/jds.S0022-0302(06)72123-3 |
| [45] |
ZEBELI Q, AMETAJ B N. Relationships between rumen lipopolysaccharide and mediators of inflammatory response with milk fat production and efficiency in dairy cows[J]. J Dairy Sci, 2009, 92(8): 3800-3809. DOI:10.3168/jds.2009-2178 |
| [46] |
VELS L, RØNTVED C M, BJERRING M, et al. Cytokine and acute phase protein gene expression in repeated liver biopsies of dairy cows with a lipopolysaccharide-induced mastitis[J]. J Dairy Sci, 2009, 92(3): 922-934. DOI:10.3168/jds.2008-1209 |
| [47] |
HISS S, MIELENZ M, BRUCKMAIER R M, et al. Haptoglobin concentrations in blood and milk after endotoxin challenge and quantification of mammary Hp mRNA expression[J]. J Dairy Sci, 2004, 87(11): 3778-3784. DOI:10.3168/jds.S0022-0302(04)73516-X |
| [48] |
KHAFIPOUR E, KRAUSE D O, PLAIZIER J C. Alfalfa pellet-induced subacute ruminal acidosis in dairy cows increases bacterial endotoxin in the rumen without causing inflammation[J]. J Dairy Sci, 2009, 92(4): 1712-1724. DOI:10.3168/jds.2008-1656 |
| [49] |
SHANGRAW E M, RODRIGUES R O, WITZKE M C, et al. Intramammary lipopolysaccharide infusion induces local and systemic effects on milk components in lactating bovine mammary glands[J]. J Dairy Sci, 2020, 103(8): 7487-7497. DOI:10.3168/jds.2019-18022 |
| [50] |
XU T L, WU X Y, LU X B, et al. Metformin activated AMPK signaling contributes to the alleviation of LPS-induced inflammatory responses in bovine mammary epithelial cells[J]. BMC Vet Res, 2021, 17(1): 97. DOI:10.1186/s12917-021-02797-x |
| [51] |
AMETAJ B N, EMMANUEL D G V, ZEBELI Q, et al. Feeding high proportions of barley grain in a total mixed ration perturbs diurnal patterns of plasma metabolites in lactating dairy cows[J]. J Dairy Sci, 2009, 92(3): 1084-1091. DOI:10.3168/jds.2008-1465 |
| [52] |
DEPREESTER E, DE KOSTER J, VAN POUCKE M, et al. Influence of adipocyte size and adipose depot on the number of adipose tissue macrophages and the expression of adipokines in dairy cows at the end of pregnancy[J]. J Dairy Sci, 2018, 101(7): 6542-6555. DOI:10.3168/jds.2017-13777 |
| [53] |
朱颍琨, 肖劲邦, 钱柏霖, 等. 泌乳初期奶牛相关脂肪因子及生理生化指标与脂肪肝的相关性[J]. 浙江农业学报, 2019, 31(5): 722-729. ZHU Y K, XIAO J B, QIAN B L, et al. Correlations between adipokine, biochemical indicators in early lactation cows with fatty liver[J]. Acta Agriculturae Zhejiangensis, 2019, 31(5): 722-729. DOI:10.3969/j.issn.1004-1524.2019.05.07 (in Chinese) |
| [54] |
沈留红, 肖劲邦, 朱颍琨, 等. 围产期奶牛相关脂肪因子及生理生化指标对脂肪肝的风险评估研究[J]. 东北农业大学学报, 2019, 50(2): 37-45. SHEN L H, XIAO J B, ZHU Y K, et al. Fatty liver risk assessment function of adipokine, biochemical, and physiological indicators in perinatal dairy cows[J]. Journal of Northeast Agricultural University, 2019, 50(2): 37-45. DOI:10.19720/j.cnki.issn.1005-9369.2019.2.0005 (in Chinese) |
| [55] |
肖劲邦, 朱颍琨, 钱柏霖, 等. 围产前期奶牛血清相关能量平衡指标和脂肪因子对酮病的预警作用及意义[J]. 中国农业大学学报, 2019, 24(9): 79-87. XIAO J B, ZHU Y K, QIAN B L, et al. Early warning function and significance of serum energy balance index and adipokine levels for ketosis in dairy cows during early prenatal[J]. Journal of China Agricultural University, 2019, 24(9): 79-87. (in Chinese) |
| [56] |
SHEN L, QIAN B, XIAO J, et al. Characterization of serum adiponectin and leptin in healthy perinatal dairy cows or cows with ketosis, and their effects on ketosis involved indices[J]. Pol J Vet Sci, 2020, 23(3): 373-381. |
| [57] |
AGUIRRE V, UCHIDA T, YENUSH L, et al. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of ser307[J]. J Biol Chem, 2000, 275(12): 9047-9054. DOI:10.1074/jbc.275.12.9047 |
| [58] |
ASTAPOVA O, LEFF T. Adiponectin and PPARγ: cooperative and interdependent actions of two key regulators of metabolism[J]. Vitam Horm, 2012, 90: 143-162. |
| [59] |
YE J P. Regulation of PPARγ function by TNF-α[J]. Biochem Biophys Res Commun, 2008, 374(3): 405-408. DOI:10.1016/j.bbrc.2008.07.068 |
| [60] |
RUI L Y, YUAN M S, FRANTZ D, et al. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2[J]. J Biol Chem, 2002, 277(44): 42394-42398. DOI:10.1074/jbc.C200444200 |
| [61] |
宁茂, 曹杰. 奶牛围产期脂肪因子变化对胰岛素信号通路的影响[J]. 黑龙江畜牧兽医, 2022(2): 32-37. NING M, CAO J. Effects of adipokine changes on insulin signaling pathway in perinatal period of dairy cows[J]. Heilongjiang Animal Science and Veterinary Medicine, 2022(2): 32-37. (in Chinese) |
| [62] |
SHEN L H, ZHU Y K, XIAO J B, et al. Serum adipokines play different roles in type I and Ⅱ ketosis[J]. Asian-Australas J Anim Sci, 2020, 33(12): 1930-1939. DOI:10.5713/ajas.19.0728 |
| [63] |
VAN ANDEL M, HEIJBOER A C, DRENT M L. Adiponectin and its isoforms in pathophysiology[J]. Adv Clin Chem, 2018, 85: 115-147. |
| [64] |
KRUMM C S, GIESY S L, CAIXETA L S, et al. Effect of hormonal and energy-related factors on plasma adiponectin in transition dairy cows[J]. J Dairy Sci, 2017, 100(11): 9418-9427. DOI:10.3168/jds.2017-13274 |
| [65] |
SUTTON J D, DHANOA M S, MORANT S V, et al. Rates of production of acetate, propionate, and butyrate in the rumen of lactating dairy cows given normal and low-roughage diets[J]. J Dairy Sci, 2003, 86(11): 3620-3633. DOI:10.3168/jds.S0022-0302(03)73968-X |
| [66] |
AJUWON K M, SPURLOCK M E. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes[J]. Am J Physiol Regul, Integr Comp Physiol, 2005, 288(5): R1220-R1225. DOI:10.1152/ajpregu.00397.2004 |
| [67] |
KIM Y B, UOTANI S, PIERROZ D D, et al. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin[J]. Endocrinology, 2000, 141(7): 2328-2339. DOI:10.1210/endo.141.7.7536 |
| [68] |
LULU S A, KOKTA T A, DODSON M V, et al. Early signaling interactions between the insulin and leptin pathways in bovine myogenic cells[J]. Biochim Biophys Acta (BBA)-Mol Cell Res, 2005, 1744(2): 164-175. DOI:10.1016/j.bbamcr.2005.03.006 |
| [69] |
PESSIN J E, SALTIEL A R. Signaling pathways in insulin action: molecular targets of insulin resistance[J]. J Clin Invest, 2000, 106(2): 165-169. |
| [70] |
刘小平, 史卓言, 孙卓, 等. 抵抗素与动物肌内脂肪沉积研究进展[J]. 现代畜牧兽医, 2021(10): 92-96. LIU X P, SHI Z Y, SUN Z, et al. Research progress of resistin and intramuscular fat deposition in animals[J]. Modern Journal of Animal Husbandry and Veterinary Medicine, 2021(10): 92-96. (in Chinese) |
| [71] |
GARTEN A, SCHUSTER S, PENKE M, et al. Physiological and pathophysiological roles of NAMPT and NAD metabolism[J]. Nat Rev Endocrinol, 2015, 11(9): 535-546. |
| [72] |
PIYA M K, MCTERNAN P G, KUMAR S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin[J]. J Endocrinol, 2013, 216(1): T1-T15. |
| [73] |
FUKUHARA A, MATSUDA M, NISHIZAWA M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin[J]. Obstet Gynecol Surv, 2005, 60(8): 523-524. |
(编辑 孟培)



