畜牧业规模化发展带来的环境污染以及“中美贸易战”、“新冠疫情”和“俄乌战争”引起的蛋白质饲料资源短缺等问题一直备受关注。因此,如何通过“开源、节流、提质、增效”来缓解这一现状已经成为新时代生态文明建设过程中不可或缺的部分。日粮蛋白质水平能够影响畜禽生长和生产性能,高蛋白日粮通常会降低机体氮的利用效率,引发动物疾病。例如,粗蛋白(CP)水平23%的高蛋白日粮饲喂雏鹅后诱发了高尿酸血症[1]。仔猪在采食高蛋白日粮后,肠道微生物区系发生改变,蛋白质分解代谢加快,最终引起腹泻[2]。因此,有效利用低蛋白日粮并补充必需氨基酸(essential amino acid,EAA)是缓解蛋白质饲料资源短缺问题和避免高蛋白日粮对畜禽产生不利影响的重要途径。缬氨酸(valine, Val)是动物机体的EAA之一,具有调控蛋白质合成[3]、脂质代谢[4]、葡萄糖代谢、抗氧化防御[5]和免疫等生物学功能。Val作为饲料添加剂应用于低蛋白日粮可以平衡氨基酸营养,提高畜禽生产性能。本文通过总结国内外最新研究现状,阐述了Val的来源、分类、代谢途径、与其他氨基酸之间的相互作用、生物学功能及其在单胃和反刍动物日粮中的应用研究进展,为缬氨酸在畜禽日粮中的科学、高效和广泛应用提供参考。
1 Val概述 1.1 Val的来源Val是一种EAA,动物本身不能合成,必须从日粮中获取。通过查阅《中国饲料成分及营养价值表》(2021年第32版)[6](表 1)可知:饲料原料中Val的主要来源是血粉、大豆等一些蛋白质饲料,少部分Val来源于高粱、稻谷、玉米和大麦皮等一些能量饲料及粗饲料。目前,Val可以通过物理、化学和生物技术等方法进行人工合成,由于微生物发酵技术具有绿色高效且不受原料限制的优点,被广泛应用于Val的工业化生产[7-8]。
1.2 Val的分类Val主要有L和D型两种异构体,它们在调控蛋白质合成、抗炎和免疫等方面存在一定的差异[9-14](表 2)。L-Val主要通过培养诱变选育的大肠杆菌发酵产生,可作为安全性饲料添加剂应用于畜禽日粮中[15-16]。有研究发现,在感染肺炎克雷伯菌的小鼠尾静脉中注射L-Val(0.5 g ·kg-1)可提高巨噬细胞吞噬病原体的能力,进而增强免疫力,其过程可通过抑制精氨酸酶的活性来实现[11]。D-Val同样具有增强巨噬细胞吞噬病原体的能力,但与L-Val不同的是,D-Val主要通过抑制RAW264.7巨噬细胞M1的极化来提高机体的免疫力[17]。此外,相较于L-Val,D-Val能够阻滞牙龈卟啉单胞菌生物膜的形成,因此D-Val可以作为潜在的生物制剂用于菌斑生物膜相关疾病的研究[18];在培养人子宫肌层平滑肌细胞时,D-Val替代培养基中的L-Val更能够减轻成纤维细胞对平滑肌细胞的污染[19]。
|
|
表 2 D/L-缬氨酸在动物机体和细菌中的作用 Table 2 The roles of D/L-valine in animals and bacteria |
Val、异亮氨酸(isoleucine,Ile)和亮氨酸(leucine,Leu)具有相似的支链结构,统称为支链氨基酸(branched-chain amino acids,BCAA),BCAA的代谢主要发生在肌肉中,在代谢过程中共同竞争支链氨基酸转移酶(branched amino acid transferase,BCAT)和支链α-酮酸脱氢酶(branched chain alpha keto acid dehy-drogenase,BCKDH)[20]。Val、Ile和Leu在肌肉中首先被BCAT分解生成对应的α-酮酸,随后在BCKDH的作用下产生对应的酰基辅酶A,最后,分解代谢生成的终产物进入三羧酸循环(tricarboxylic acid cycle,TCA cycle)途径,参与能量和物质代谢(糖类、脂质和氨基酸)[21](图 1)。
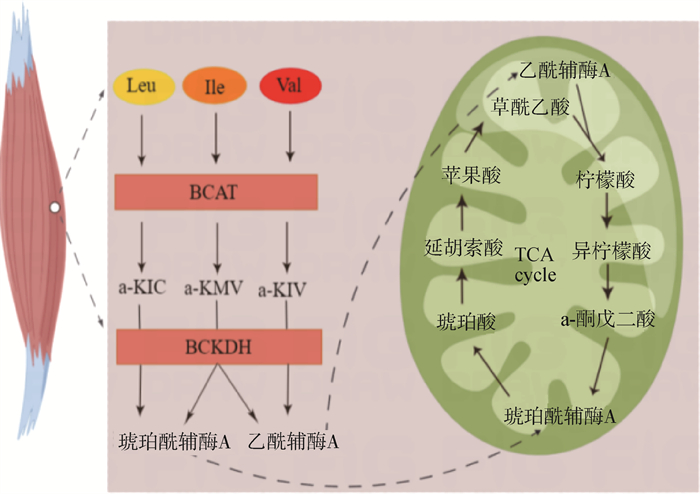
|
Leu. 亮氨酸;Ile. 异亮氨酸;Val. 缬氨酸;BCAT. 支链氨基酸转移酶;α-KIC. α-酮戊己酸;α-KMV. α-酮-β-甲基戊酸;α-KIV. α-酮异戊酸;BCKDH. 支链α-酮酸脱氢酶;TCA cycle. 三羧酸循环。下同 Leu. Leucine; Ile. Isoleucine; Val. Valine; BCAT. Branched amino acid transferase; α-KIC. α-ketoisocaproic acid; α-KMV. α-keto-β-methylvaleric acid; α-KIV. α-ketoisovaleric acid; BCKDH. Branched chain alpha keto acid dehy-drogenase; TCA cycle. Tricarboxylic acid cycle. The same as below 图 1 支链氨基酸代谢途径[21] Fig. 1 Metabolic pathway of BCAA[21] |
Val属于生糖氨基酸,其在一系列分解代谢酶如BCAT、BCKDH、异丁酰辅酶A脱氢酶(isobutyryl CoA dehydrogenase,IBDD)、短链烯酰辅酶A水合酶(short-chain enoyl-CoA hydratase,ECHS)和3-羟异丁酰辅酶A水解酶(3-hydroxyisobutyryl coenzyme A hydrolase,HIBCH)的作用下生成琥珀酰辅酶A,最终进入TCA cycle途径参与葡萄糖代谢[22](图 2)。
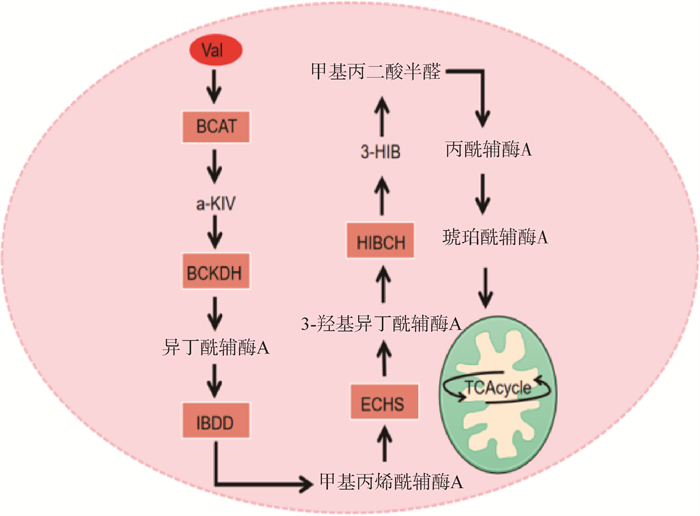
|
IBDD. 异丁酰辅酶A脱氢酶;ECHS. 短链烯酰辅酶A水合酶;HIBCH. 3-羟异丁酰辅酶A水解酶;3-HIB: 3-羟基异丁酸 IBDD. Isobutyryl CoA dehydrogenase; ECHS. Short-chain enoyl-CoA hydratase; HIBCH. 3-hydroxyisobutyryl coenzyme A hydrolase; 3-HIB. 3-hydroxyisobutyryl 图 2 缬氨酸代谢途径[22] Fig. 2 Metabolic pathway of valine[22] |
Val、Ile和Leu在代谢过程中共同竞争BCAT和BCKDH,因此3种氨基酸之间可能存在拮抗作用[20]。Kwon等[23]报道称,当猪日粮中标准化回肠可消化(SID)Leu过量(1.71%)时,BCAT和BCKDH会加速Leu的分解,导致体内可利用的Val含量降低,最终影响生产性能,此时补充0.1% SID Val可减轻因Leu过量造成的负面影响。此外,在比目鱼低Leu水平日粮(1.6%日粮水平)中补充Val(2.5%日粮水平)同样能够维持鱼的正常生长[24]。在猪的低蛋白日粮(14%CP)中按一定比例添加Val和Ile(Val ∶Ile=0.77 ∶0.68),能够通过提高肠道表皮生长因子受体(epidermal growth factor receptor,EGFR)和半胱天冬酶9(caspase 9,CASP9)的表达水平,促进肠道发育,从而改善生长性能[25]。同样地,在肉鸡低蛋白日粮(16%CP)中补充Val、苏氨酸和色氨酸(缬氨酸∶苏氨酸∶色氨酸=0.76 ∶0.55 ∶0.18)也能够提高免疫因子白细胞介素-4(interleukin-4,IL-4)和干扰素-γ(interferon-γ,IFN-γ)的mRNA表达水平,增强免疫力,提高肉鸡生产性能[26]。在畜禽低蛋白日粮中添加适宜水平的Val对提高氮的利用效率,节约蛋白质饲料资源,降低养殖成本具有重要意义。根据Val与其他氨基酸之间的相互作用关系,在饲喂畜禽低蛋白日粮时应充分考虑Val与其他氨基酸之间的配比。
2 Val的生物学功能及其作用机制 2.1 调控蛋白质合成Val可以通过激活哺乳动物雷帕酶素靶蛋白复合物1(mammalian target of rapamycin complex 1,mTORC1)信号通路来调控蛋白质合成,具体代谢机制如图 3所示[27-28]。Zhang等[9]研究发现,在猪乳腺上皮细胞(porcine mammary epithelial cells,PMEC)培养基中加入不同浓度的Val(0.05、0.25、0.5、1 mmol ·L-1)均可影响蛋白质的合成,尤其是添加量为0.5 mmol ·L-1时,能显著提高大鼠肉瘤(rat sarcoma,Ras)、p70核糖体S6蛋白激酶(p70 ribosomal protein S6 kinase, p70S6K)和细胞外调节蛋白激酶1/2(extracellular-regulated kinase 1/2, ERK1/2)的mRNA表达水平。在仔猪低蛋白日粮(16.7%CP)中添加BCAA(Val ∶Leu ∶Ile=0.95 ∶1.83 ∶0.81)同样能够调节蛋白质的合成,提高仔猪生长性能[29]。在肉鸡日粮中补充Val能够通过激活哺乳动物的雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)信号通路,增加下游调节因子核糖体蛋白S6激酶1(ribosomal protein S6 kinase,S6k1)的表达水平来促进蛋白质合成,从而提高肉鸡的胴体品质[30-31]。
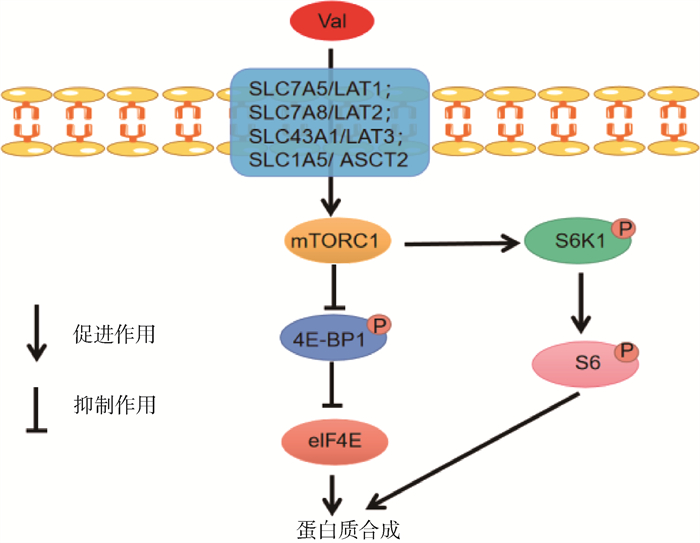
|
SLC7A5/LAT1. 溶质载体家族7成员5/L型氨基酸转运载体1;SLC7A8/LAT2. 溶质载体家族7成员8/L型氨基酸转运载体2;SLC43A1/LAT3. 溶质载体家族43成员1/L型氨基酸转运载体3;SLC1A5/ASCT2. 溶质载体家族1成员5/氨基酸转运载体2;P. 磷酸化作用;mTORC1. 哺乳动物雷帕酶素靶蛋白复合物1;4E-BP1. 真核mRNA翻译起始因子4E结合蛋白1;eIF4E. 真核mRNA翻译起始因子4E;S6K1. 核糖体蛋白S6激酶;S6. 核糖体蛋白。下同 SLC7A5/LAT1. Solute carrier family 7 member 5/large amino acid transporter 1; SLC7A8/LAT2. Solute carrier family 7 member 8/large amino acid transporter 2; SLC43A1/LAT3. Solute carrier family 43 member 1/large amino acid transporter 3; SLC1A5/ASCT2. Solute carrier family 1 member 5/amino acid transporter 2; P. Phosphorylation; mTORC1. Mammalian target of rapamycin complex 1; 4E-BP1. Eukaryotic mRNA translation initiation factor 4E-binding protein 1; eIF4E. Eukaryotic mRNA translation initiation factor 4E; S6K1. Ribosomal protein S6 kinase; S6. Ribosomal protein S6. The same as below 图 3 缬氨酸调控蛋白质合成机制[27-28] Fig. 3 Mechanism of protein synthesis regulated by valine[27-28] |
Val及其代谢产物丙酰辅酶A可通过激活一磷酸腺苷活化的蛋白激酶-mTOR(adenosine monophosphate-activated protein kinase-mammalian target of rapamycin,AMPK-mTOR)信号通路来调控脂质代谢,具体代谢机制如图 4所示[32-34]。Gart等[35]在肥胖小鼠的高脂日粮中添加Val降低了肝的脂肪变性,抑制脂质氧化应激,减少肝损伤。然而,在蛋鸡日粮中添加过量的Val(0.79%日粮水平),抑制了成纤维细胞生长因子19-雷帕酶素靶蛋白复合物1(fibroblast growth factor 19-target of rapamycin complex 1,FGF19-TORC1)信号通路,加速了脂肪沉积,最终导致了非酒精性脂肪性肝病(non-alcoholic fatty liver disease,NAFLD)和脂肪肝出血综合征(fatty liver hemorrhagic syndrome,FLHS)的发生[36]。
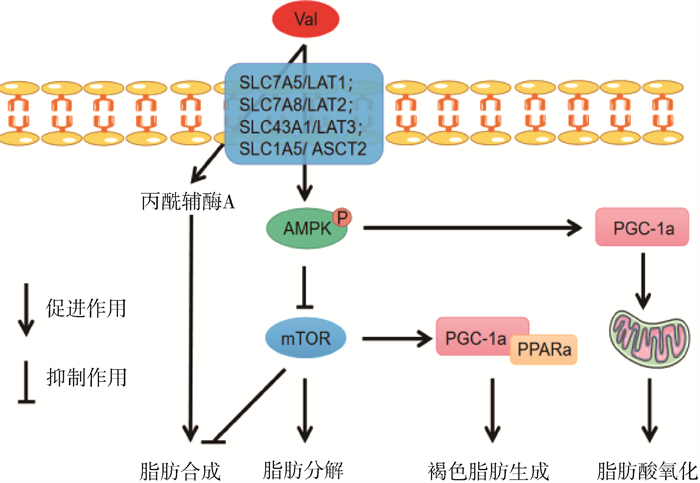
|
AMPK. 一磷酸腺苷活化的蛋白激酶;mTOR. 哺乳动物雷帕酶素靶蛋白;PPARα. 过氧化物酶体增殖物激活受体α;PGC-1α. 过氧化物酶体增殖物激活受体γ共激活剂-1α AMPK. Adenosine monophosphate-activated protein kinase; mTOR. Mammalian target of rapamycin; PPARα. Peroxisome proliferatoractivated receptor α; PGC-1α. Peroxisome proliferator-activated receptor gamma co-activator-1α 图 4 缬氨酸调控脂质代谢机制[32-34] Fig. 4 Mechanism of lipid metabolism regulated by valine[32-34] |
Val能够通过参与葡萄糖转运蛋白的转运调控葡萄糖代谢[37]。Xiao等[38]通过小鼠试验探究Val对葡萄糖代谢的影响,结果表明,饲喂不含Val日粮的小鼠肝中胰岛素敏感性增强,葡萄糖-6-磷酸酶(glucose-6-phosphatase,G6Pase)水平降低,进而促进胰岛素的信号传导,降低了因肥胖引起的胰岛素抵抗。然而,在小鼠高脂日粮中添加Val(50 g ·kg-1) 会产生过量的3-HIB,导致葡萄糖耐量降低,阻碍了胰岛素的信号传导,进而引发高血糖和2型糖尿病[39]。此外,Val分解代谢产生的3-HIB在人白色和棕色脂肪细胞中会以时间依赖性方式刺激葡萄糖摄取,降低胰岛素的敏感性,引发2型糖尿病[40]。
2.4 调控抗氧化防御Val可通过增强机体抗氧化酶和溶菌酶活性、促进转录因子类似核转录因子红系2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)的表达以及增加血清还原型谷胱甘肽(glutathione,GSH)的含量,提高机体抗氧化能力,具体代谢机制如图 5所示[41-43]。Bernal等[44]研究发现,在鸡精液稀释剂中加入10 mmol Val,能够抑制活性氧(reactive oxygen species,ROS)对精子的损害作用,进而减少解冻后精子DNA的片段化。有研究表明,饲喂围产期母猪平衡氨基酸模式的日粮(缬氨酸∶赖氨酸∶苏氨酸∶亮氨酸∶精氨酸=0.71 ∶1 ∶0.72 ∶0.95 ∶1.1)可提高血清中GSH水平和总超氧化物歧化酶(total superoxide dismutase,T-SOD)的活性,进而增强母猪抗氧化能力[45]。在猪的低蛋白日粮(18%CP)中添加BCAA(Val ∶Leu ∶Ile=0.83 ∶1.56 ∶0.70)可提高血清中抗氧化酶活性,同时降低血清中丙二醛(malondialdehyde,MDA)的含量,进而增强机体抗氧化能力[46]。
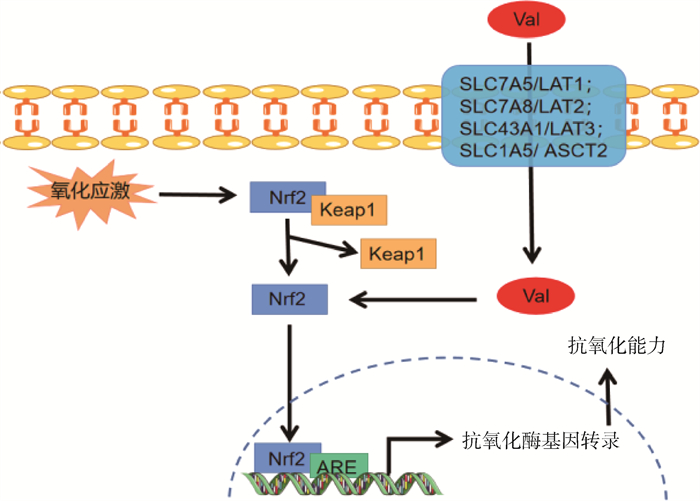
|
Nrf2. 核因子E2相关因子2;Keap1. kelch样ECH关联蛋白1;ARE. 抗氧化反应元件 Nrf2. Nuclear factor erythroid 2-related factor 2; Keap1. Kelch-like-ECH-associated protein 1; ARE. Antioxidant response elements 图 5 缬氨酸调控抗氧化机制[41-43] Fig. 5 Mechanism of antioxidant metabolism regulated by valine[41-43] |
Val能够通过调控免疫细胞和肠道免疫球蛋白(immunoglobulin,Ig)来提高机体的免疫水平。研究发现,Val可以通过调控人肿瘤细胞和单核细胞衍生树突状细胞(monocyte-derivedmodendritic cells,MoDC)来增强免疫力[47-48];另外,Val还可以促进间充质干细胞(mesenchymal stem cell,MSC)的增殖来提高巨噬细胞吞噬病原体的能力[49]。Luo等[50]在幼草鱼日粮中添加不同水平的Val(0.43%~ 1.91%日粮水平)探究其对机体肠道免疫反应的影响,结果表明,日粮中缺乏Val(0.43%日粮水平) 会引发幼草鱼的肠道炎症反应,导致生长性能下降。Val调控机体免疫还与肠道Ig水平有关。在断奶仔猪日粮中添加0.27%Val能够提高空肠和回肠的IgA水平,改善肠道免疫功能,从而促进仔猪生长[51]。
3 Val在畜禽日粮中的应用 3.1 Val在猪日粮中的应用Val对猪的影响在各个生产阶段具有不同的作用效果[52-61](表 3)。生长肥育猪、仔猪和母猪日粮中Val水平分别在0.59%~0.68%、0.68%~1.01%和1.07%~1.67%范围内能够满足猪在特定生产阶段的营养需要并提高生长和生产性能。研究发现,在育肥猪日粮中添加0.24%Val可以提高平均日增重(average daily gain,ADG)[53]。然而,日粮中添加0.32%Val对育肥猪的ADG无显著影响,但减少了肌肉滴水损失[52]。此外,在仔猪日粮中添加0.24%Val增加了小肠的绒毛高度,提高了营养物质的吸收速率[54]。李方方等[59]研究发现,在哺乳母猪日粮中添加0.45%Val可提高乳脂和乳蛋白含量,改善泌乳性能。日粮中添加Val(0.93%日粮水平)能够调节妊娠后期母猪乳腺中脂肪酸代谢,增加初乳中脂肪含量[62]。Waguespack等[63]发现,在生长肥育猪低蛋白日粮(14.59%CP)中添加Val(0.51%~0.59%日粮水平)可提高猪的生长性能, 但当日粮Val水平达到0.61%时生长性能反而下降。这与易孟霞等[53](表 3)的结果不一致,原因可能是受猪品种、日粮蛋白水平等条件影响。
|
|
表 3 缬氨酸在猪日粮中的应用 Table 3 Applications of valine in pig diets |
Val在提高家禽生长和生产性能方面具有重要作用[64-70](表 4)。由表中数据可知,蛋鸡和肉鸡日粮中Val水平分别在0.79%~0.81%和0.80%~0.88%范围内能够满足特定生产阶段鸡的营养需要并提高生长和生产性能。在以玉米和豆粕为基础的肉鸡日粮中,Val属于第四限制性氨基酸,且在日粮中添加适宜水平的Val能够促进骨骼和肠道发育[67],有利于肉鸡的生长和肠道内营养物质的吸收,从而改善肉鸡生产性能[71-72]。Jian等[64]研究表明,蛋鸡日粮中添加0.20%Val可以提高蛋品质和产蛋量,从而获得最佳饲料转化率(feed conversion rate, FCR)。此外,日粮中Val水平为1.0%时,蛋鸡的生产性能受到影响,主要表现为平均日采食量(average daily feed intake, ADFI)和蛋品质下降[65]。
|
|
表 4 缬氨酸在家禽日粮中的应用 Table 4 Applications of valine in poultry diets |
在日粮中添加Val可以改善奶牛泌乳性能并促进犊牛生长,增强机体免疫力。研究发现,Val能够通过上调β-酪蛋白水平刺激牛乳腺上皮细胞(bovine mammary epithelial cells,BMEC)蛋白质的合成[73-74],因此在日粮中添加Val能够提高奶牛泌乳性能[73-75]。高酮血症和脂肪肝是奶牛在妊娠期最为常见的两类疾病,尤其在高产奶牛中最为常见,研究发现在日粮中添加BCAA(Val ∶Leu ∶Ile=1.49 ∶2.48 ∶1)可以减少奶牛产后高酮血症和脂肪肝的发生[76-77];同时,饲喂犊牛BCAA(Val ∶Leu ∶Ile=1 ∶1 ∶1)可改善肠道健康,增强免疫力,进而提高生长性能[78]。目前,有关Val在反刍动物日粮中的应用主要集中在奶牛方面。然而,在实际生产中,尤其是在低蛋白日粮条件下,Val促进奶牛泌乳和肉牛生长的作用机制仍待进一步探究。
4 小结与展望Val是动物在生长发育过程中不可或缺的EAA之一,在畜禽日粮中添加适宜水平的Val能够增强机体免疫和抗氧化能力,并且通过调节蛋白质和葡萄糖代谢,改善畜禽生产性能。尤其在蛋白质资源供应不足的情况下,合理添加Val不仅能够促进畜禽生长发育,还可以有效缓解我国畜禽养殖过程中蛋白质饲料不足的现状。目前,有关Val在畜禽日粮上的应用研究还存在一些问题:1)Val在畜禽低蛋白日粮中最佳添加水平和使用范围亟需建立相应的标准;2)D-Val主要应用于医药、食品和农药方面,其能否用于畜禽生产以及和L-Val存在怎样的关系有待进一步确认;3)Val对机体抗氧化能力、免疫功能调节和葡萄糖代谢作用机制的探究主要集中在实验动物和水产动物类模型上,关于Val在养殖动物上的作用机制有待进一步探究;4)Val在反刍动物日粮中的研究较少,尤其是Val对肉牛免疫水平、肠道健康和生长性能方面的影响有待进一步探究;5)尽管Val在蛋鸡和肉鸡日粮中研究较多,但在鹅、鸭日粮中的作用效果和需要量还需进一步探索;6)Val作为一种BCAA,在后续的研究中应当充分考虑其与另外两种BCAA之间的拮抗作用,同时也要注意BCAA在畜禽日粮中的合理配比。因此,今后可从Val对单胃动物营养调控机制和对反刍动物生产性能的作用效果及分子机制方面着手,研究Val在低蛋白日粮体系中的应用,为Val在畜禽日粮中的科学、高效和广泛应用提供参考。
| [1] |
王志, 胡仲皓, 李思婷, 等. 高蛋白日粮诱发雏鹅高尿酸血症的血清代谢组学研究[J]. 南京农业大学学报, 2021, 44(6): 1169-1176. WANG Z, HU Z H, LI S T, et al. Study on serum metabonomics of goslings with hyperuricemia induced by high protein diet[J]. Journal of Nanjing Agricultural University, 2021, 44(6): 1169-1176. (in Chinese) |
| [2] |
ZHANG H L, VAN DER WIELEN N, VAN DER HEE B, et al. Impact of fermentable protein, by feeding high protein diets, on microbial composition, microbial catabolic activity, gut health and beyond in pigs[J]. Microorganisms, 2020, 8(11): 1735. DOI:10.3390/microorganisms8111735 |
| [3] |
WALEJKO J M, CHRISTOPHER B A, CROWN S B, et al. Branched-chain α-ketoacids are preferentially reaminated and activate protein synthesis in the heart[J]. Nat Commun, 2021, 12(1): 1680. DOI:10.1038/s41467-021-21962-2 |
| [4] |
LEE J H, CHO Y R, KIM J H, et al. Branched-chain amino acids sustain pancreatic cancer growth by regulating lipid metabolism[J]. Exp Mol Med, 2019, 51(11): 1-11. |
| [5] |
ALQARALEH M, KASABRI V, AL-MAJALI I, et al. Branched chain amino acids as in vitro and in vivo anti-oxidation compounds[J]. Res J Pharm Technol, 2021, 14(7): 3899-3904. |
| [6] |
熊本海, 罗清尧, 赵峰, 等. 中国饲料成分及营养价值表(2021年第32版)制订说明[J]. 中国饲料, 2021(23): 97-107. XIONG B H, LUO Q Y, ZHAO F, et al. China feed composition and nutritional value table (2021 32nd edition)(to be continued)[J]. China Feed, 2021(23): 97-107. DOI:10.15906/j.cnki.cn11-2975/s.20212319 (in Chinese) |
| [7] |
D'ESTE M, ALVARADO-MORALES M, ANGELIDAKI I. Amino acids production focusing on fermentation technologies-a review[J]. Biotechnol Adv, 2018, 36(1): 14-25. DOI:10.1016/j.biotechadv.2017.09.001 |
| [8] |
HAO Y N, MA Q, LIU X Q, et al. High-yield production of L-valine in engineered Escherichia coli by a novel two-stage fermentation[J]. Metab Eng, 2020, 62: 198-206. DOI:10.1016/j.ymben.2020.09.007 |
| [9] |
ZHANG J M, HE W L, YI D, et al. Regulation of protein synthesis in porcine mammary epithelial cells by L-valine[J]. Amino Acids, 2019, 51(4): 717-726. DOI:10.1007/s00726-019-02709-2 |
| [10] |
KOO B, NYACHOTI M. Effect of dietary L-valine supplementation on growth performance, immune response, and microbial metabolites in weaned pigs raised in varying sanitary conditions[J]. J Anim Sci, 2021, 99(S3): 113-114. |
| [11] |
CHEN X H, LIU S R, PENG B, et al. Exogenous L-valine promotes phagocytosis to kill multidrugresistant bacterial pathogens[J]. Front Immunol, 2017, 8: 207. |
| [12] |
JAVRUSHYAN H, NADIRYAN E, GRIGORYAN A, et al. Antihyperglycemic activity of L-norvaline and L-arginine in high-fat diet and streptozotocin-treated male rats[J]. Exp Mol Pathol, 2022, 126: 104763. DOI:10.1016/j.yexmp.2022.104763 |
| [13] |
王昆于, 孙毓言, 张慧彦, 等. 负载D-缬氨酸水凝胶对大鼠实验性牙周炎的影响[J]. 口腔医学研究, 2020, 36(7): 658-663. WANG K Y, SUN Y Y, ZHANG H Y, et al. Effects of D-valine loaded hydrogel on rat periodontitis[J]. Journal of Oral Science Research, 2020, 36(7): 658-663. (in Chinese) |
| [14] |
QI H, LI B S, WANG H L, et al. Effects of D-valine on periodontal or periimplant pathogens: Porphyromonas gingivalis biofilm[J]. J Periodontol, 2018, 89(3): 303-314. DOI:10.1002/JPER.17-0405 |
| [15] |
EFSA Panel on Additives, Products or Substances Used in Animal Feed (FEEDAP), BAMPIDIS V, AZIMONTI G, et al. Safety and efficacy of a feed additive consisting of L-valine produced by Escherichia coli CCTCC M2020321 for all animal species (Kempex Holland BV)[J]. EFSA J, 2022, 20(2): e07163. |
| [16] |
HAO Y N, PAN X W, XING R F, et al. High-level production of L-valine in Escherichia coli using multi-modular engineering[J]. Bioresour Technol, 2022, 359: 127461. DOI:10.1016/j.biortech.2022.127461 |
| [17] |
倪宇昕, 王勇, 邓艳芳. D-缬氨酸抑制巨噬细胞M1极化的体外研究[J]. 中国实验诊断学, 2021, 25(8): 1221-1224. NI Y X, WANG Y, DENG Y F. Inhibitory effect of D-valine on M1 polarization of macrophages in vitro[J]. Chinese Journal of Experimental Diagnostics, 2021, 25(8): 1221-1224. DOI:10.3969/j.issn.1007-4287.2021.08.034 (in Chinese) |
| [18] |
张慧彦, 李保胜, 张震阳, 等. D/L-缬氨酸对牙龈卟啉单胞菌及其生物膜的作用[J]. 口腔医学研究, 2020, 36(7): 648-653. ZHANG H Y, LI B S, ZHANG Z Y, et al. Effects of D/L-valine on Porphyromonas gingivalis and porphyromonas gingivalis biofilm[J]. Journal of Stomatology, 2020, 36(7): 648-653. (in Chinese) |
| [19] |
HONGPAISAN J. Inhibition of proliferation of contaminating fibroblasts by D-valine in cultures of smooth muscle cells from human myometrium[J]. Cell Biol Int, 2000, 24(1): 1-7. DOI:10.1006/cbir.1999.0448 |
| [20] |
WILTAFSKY M K, PFAFFL M W, ROTH F X. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs[J]. Br J Nutr, 2010, 103(7): 964-976. DOI:10.1017/S0007114509992212 |
| [21] |
邵静. 支链氨基酸及其代谢产物调控糖脂代谢的新机制[D]. 上海: 上海交通大学, 2019. SHAO J. Novel roles of branched-chain amino acids and their catabolic intermediates in glucose and lipid metabolic regulation[D]. Shanghai: Shanghai Jiao Tong University, 2019. (in Chinese) |
| [22] |
ADEVA-ANDANY M M, LÓPEZ-MASIDE L, DONAPETRY-GARCÍA C, et al. Enzymes involved in branched-chain amino acid metabolism in humans[J]. Amino Acids, 2017, 49(6): 1005-1028. DOI:10.1007/s00726-017-2412-7 |
| [23] |
KWON W B, SOTO J A, STEIN H H. Effects of dietary isoleucine and valine supplementation to excess or low leucine diets on nitrogen balance and metabolism of branched-chained amino acids in growing pigs[J]. J Anim Sci, 2020, 98(S3): 33. |
| [24] |
HAN Y Z, HAN R Z, KOSHIO S, et al. Interactive effects of dietary valine and leucine on two sizes of Japanese flounder Paralichthys olivaceus[J]. Aquaculture, 2014, 432: 130-138. DOI:10.1016/j.aquaculture.2014.05.004 |
| [25] |
HABIBI M, GOODARZI P, SHILI C N, et al. A mixture of valine and isoleucine restores the growth of protein-restricted pigs likely through improved gut development, hepatic IGF-1 pathway, and plasma metabolomic profile[J]. Int J Mol Sci, 2022, 23(6): 3300. DOI:10.3390/ijms23063300 |
| [26] |
ABOU-ELKHAIR R, AHMED H, KETKAT S, et al. Supplementation of a low-protein diet with tryptophan, threonine, and valine and its impact on growth performance, blood biochemical constituents, immune parameters, and carcass traits in broiler chickens[J]. Vet World, 2020, 13(6): 1234-1244. DOI:10.14202/vetworld.2020.1234-1244 |
| [27] |
REZAEI R, WU G Y. Branched-chain amino acids regulate intracellular protein turnover in porcine mammary epithelial cells[J]. Amino Acids, 2022, 54(11): 1491-1504. DOI:10.1007/s00726-022-03203-y |
| [28] |
MANN G, MORA S, MADU G, et al. Branched-chain amino acids: catabolism in skeletal muscle and implications for muscle and whole-body metabolism[J]. Front Physiol, 2021, 12: 702826. DOI:10.3389/fphys.2021.702826 |
| [29] |
ZHENG L F, WEI H K, HE P L, et al. Effects of supplementation of branched-chain amino acids to reduced protein diet on skeletal muscle protein synthesis and degradation in the fed and fasted states in a piglet model[J]. Nutrients, 2016, 9(1): 17. DOI:10.3390/nu9010017 |
| [30] |
OSPINA-ROJAS I C, POZZA P C, RODRIGUEIRO R J B, et al. High leucine levels affecting valine and isoleucine recommendations in low-protein diets for broiler chickens[J]. Poult Sci, 2020, 99(11): 5946-5959. DOI:10.1016/j.psj.2020.08.053 |
| [31] |
SADEGHZADEH S, DANESHYAR M, FARHOOMAND P, et al. Effects of different levels of valine amino acid on performance, carcas traits, meat quality and insulin like growth factor-1 and insulin genes expression in male ross 308 broiler chickens[J]. Anim Sci, 2020, 32(125): 89-108. |
| [32] |
DUAN Y H, LI F N, GUO Q P, et al. Branched-chain amino acid ratios modulate lipid metabolism in adipose tissues of growing pigs[J]. J Funct Foods, 2018, 40: 614-624. DOI:10.1016/j.jff.2017.12.004 |
| [33] |
ARAKAWA M, MASAKI T, NISHIMURA J, et al. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice[J]. Endocr J, 2011, 58(3): 161-170. DOI:10.1507/endocrj.K10E-221 |
| [34] |
BISHOP C A, SCHULZE M B, KLAUS S, et al. The branched-chain amino acids valine and leucine have differential effects on hepatic lipid metabolism[J]. FASEB J, 2020, 34(7): 9727-9739. DOI:10.1096/fj.202000195R |
| [35] |
GART E, VAN DUYVENVOORDE W, CASPERS M P M, et al. Intervention with isoleucine or valine corrects hyperinsulinemia and reduces intrahepatic diacylglycerols, liver steatosis, and inflammation in Ldlr-/-.Leiden mice with manifest obesity-associated NASH[J]. FASEB J, 2022, 36(8): e22435. |
| [36] |
JIAN H F, XU Q Q, WANG X M, et al. Amino acid and fatty acid metabolism disorders trigger oxidative stress and inflammatory response in excessive dietary valine-induced NAFLD of laying hens[J]. Front Nutr, 2022, 9: 849767. DOI:10.3389/fnut.2022.849767 |
| [37] |
IWAI S, HASEGAWA T, IKEDA H O, et al. Branched chain amino acids promote ATP production via translocation of glucose transporters[J]. Invest Ophthalmol Vis Sci, 2022, 63(9): 7. DOI:10.1167/iovs.63.9.7 |
| [38] |
XIAO F, YU J, GUO Y, et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice[J]. Metabolism, 2014, 63(6): 841-850. DOI:10.1016/j.metabol.2014.03.006 |
| [39] |
BISHOP C A, MACHATE T, HENNING T, et al. Detrimental effects of branched-chain amino acids in glucose tolerance can be attributed to valine induced glucotoxicity in skeletal muscle[J]. Nutr Diabetes, 2022, 12(1): 20. DOI:10.1038/s41387-022-00200-8 |
| [40] |
NILSEN M S, JERSIN R Å, ULVIK A, et al. 3-Hydroxyisobutyrate, a strong marker of insulin resistance in type 2 diabetes and obesity that modulates white and brown adipocyte metabolism[J]. Diabetes, 2020, 69(9): 1903-1916. DOI:10.2337/db19-1174 |
| [41] |
ZHOU Z Y, WU X Y, GATLIN D M, et al. Dietary valine levels affect growth, protein utilisation, immunity and antioxidant status in juvenile hybrid grouper (Epinephelus fuscoguttatus♀×Epinephelus lanceolatus♂)[J]. Br J Nutr, 2021, 125(4): 408-419. DOI:10.1017/S0007114520002858 |
| [42] |
HE F, ANTONUCCI L, YAMACHIKA S, et al. NRF2 activates growth factor genes and downstream AKT signaling to induce mouse and human hepatomegaly[J]. J Hepatol, 2020, 72(6): 1182-1195. DOI:10.1016/j.jhep.2020.01.023 |
| [43] |
XU W, KENÉZ Á, MANN S, et al. Effects of dietary branched-chain amino acid supplementation on serum and milk metabolome profiles in dairy cows during early lactation[J]. J Dairy Sci, 2022, 105(10): 8497-8508. DOI:10.3168/jds.2022-21892 |
| [44] |
BERNAL B, IGLESIAS-CABEZA N, SÁNCHEZ-RIVERA U, et al. Effect of supplementation of valine to chicken extender on sperm cryoresistance and post-thaw fertilization capacity[J]. Poult Sci, 2020, 99(12): 7133-7141. DOI:10.1016/j.psj.2020.09.060 |
| [45] |
刘霜, 张健文, 肖俊峰, 等. 围产期母猪饲粮中平衡氨基酸模式对母仔猪生产性能、免疫功能及抗氧化功能的影响[J]. 中国饲料, 2020(7): 48-54. LIU S, ZHANG J W, XIAO J F, et al. Effects of dietary balance amino acid patterns for perinatal sows on performance, immune function and antioxidative function of sows and newborn piglets[J]. China Feed, 2020(7): 48-54. (in Chinese) |
| [46] |
魏立民, 刘圈炜, 晁哲, 等. 低蛋白质饲粮添加支链氨基酸对生长前期海南猪体尺指标和抗氧化指标的影响[J]. 养猪, 2022, 182(3): 7-11. WEI L M, LIU Q W, CHAO Z, et al. Effects of low protein diet supplemented with BCAAs on body size and antioxidant index of weaned Hainan pigs[J]. Pig Breeding, 2022, 182(3): 7-11. (in Chinese) |
| [47] |
GANDHIRAJAN R K, MEYER D, SAGWAL S K, et al. The amino acid metabolism is essential for evading physical plasma-induced tumour cell death[J]. Br J Cancer, 2021, 124(11): 1854-1863. DOI:10.1038/s41416-021-01335-8 |
| [48] |
KAKAZU E, KANNO N, UENO Y, et al. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells[J]. J Immunol, 2007, 179(10): 7137-7146. DOI:10.4049/jimmunol.179.10.7137 |
| [49] |
SARTORI T, SANTOS A C A, DA SILVA R O, et al. Branched chain amino acids improve mesenchymal stem cell proliferation, reducing nuclear factor kappa B expression and modulating some inflammatory properties[J]. Nutrition, 2020, 78: 110935. DOI:10.1016/j.nut.2020.110935 |
| [50] |
LUO J B, FENG L, JIANG W D, et al. The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine[J]. Fish Shellfish Immunol, 2014, 40(1): 197-207. DOI:10.1016/j.fsi.2014.07.003 |
| [51] |
REN M, ZHANG S H, ZENG X F, et al. Branched-chain amino acids are beneficial to maintain growth performance and intestinal immune-related function in weaned piglets fed protein restricted diet[J]. Asian-Australas J Anim Sci, 2015, 28(12): 1742-1750. DOI:10.5713/ajas.14.0131 |
| [52] |
王宇波, 许豆豆, 何鑫, 等. 低蛋白饲粮缬氨酸水平对肥育猪生长性能、胴体性状和肉品质的影响[J]. 畜牧兽医学报, 2019, 50(9): 1832-1840. WANG Y B, XU D D, HE X, et al. Effects of valine level in low protein diets on growth performance, carcass traits and meat quality of finishing pigs[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(9): 1832-1840. (in Chinese) |
| [53] |
易孟霞, 易学武, 贺喜, 等. 饲粮标准回肠可消化缬氨酸水平对育肥猪生长性能、血浆氨基酸和尿素氮含量的影响[J]. 动物营养学报, 2015, 27(7): 2027-2037. YI M X, YI X W, HE X, et al. Effects of dietary standardized ileal digestible valine level on growth performance, plasma amino acid and urea nitrogen contents of finishing pigs[J]. Chinese Journal of Animal Nutrition, 2015, 27(7): 2027-2037. DOI:10.3969/j.issn.1006-267x.2015.07.007 (in Chinese) |
| [54] |
XU Y T, MA X K, WANG C L, et al. Effects of dietary valine: lysine ratio on the performance, amino acid composition of tissues and mRNA expression of genes involved in branched-chain amino acid metabolism of weaned piglets[J]. Asian-Australas J Anim Sci, 2018, 31(1): 106-115. DOI:10.5713/ajas.17.0148 |
| [55] |
ZHAO L Y, LI Y S, LI Z J, et al. Effect of the valine-to-lysine ratio on the performance of sows and piglets in a hot, humid environment[J]. J Therm Biol, 2019, 81: 89-97. DOI:10.1016/j.jtherbio.2019.02.021 |
| [56] |
SIEBERT D, KHAN D R, TORRALLARDONA D. The optimal valine to lysine ratio for performance parameters in weaned piglets[J]. Animals (Basel), 2021, 11(5): 1255. |
| [57] |
CHE L, XU M M, GAO K G, et al. Valine supplementation during late pregnancy in gilts increases colostral protein synthesis through stimulating mTOR signaling pathway in mammary cells[J]. Amino Acids, 2019, 51(10): 1547-1559. |
| [58] |
GAO K G, LI G, ZHU C, et al. Effect of optimizing dietary valine-to-lysine ratio in late gestation or lactation on biochemical indices and performance of lactating primiparous sows[J]. Anim Feed Sci Technol, 2019, 253: 13-21. |
| [59] |
李方方, 王军, 林燕, 等. 饲粮缬氨酸与赖氨酸比对初产母猪繁殖性能及血清生化指标的影响[J]. 动物营养学报, 2013, 25(4): 720-728. LI F F, WANG J, LIN Y, et al. Effects of dietary valine/lysine on reproductive performance and serum biochemical indices of gilts[J]. Chinese Journal of Animal Nutrition, 2013, 25(4): 720-728. (in Chinese) |
| [60] |
陈熠, 彭艺, 贺建华, 等. 日粮中添加缬氨酸对泌乳母猪生产性能及其仔猪生长性能的影响[J]. 动物营养学报, 2009, 21(5): 727-733. CHEN Y, PENG Y, HE J H, et al. Effects of dietary valine supplementation on productive performance of lactating sows and growth performance of suckling piglets[J]. Chinese Journal of Animal Nutrition, 2009, 21(5): 727-733. (in Chinese) |
| [61] |
REZAEI R, GABRIEL A S, WU G. Dietary supplementation with branched-chain amino acids enhances milk production by lactating sows and the growth of suckling piglets[J]. J Anim Sci Biotechnol, 2022, 13(1): 65. |
| [62] |
CHE L, XU M M, GAO K G, et al. Mammary tissue proteomics in a pig model indicates that dietary valine supplementation increases milk fat content via increased de novo synthesis of fatty acid[J]. Food Sci Nutr, 2021, 9(11): 6213-6223. |
| [63] |
WAGUESPACK A M, BIDNER T D, PAYNE R L, et al. Valine and isoleucine requirement of 20- to 45-kilogram pigs[J]. J Anim Sci, 2012, 90(7): 2276-2284. |
| [64] |
JIAN H F, MIAO S S, LIU Y T, et al. Effects of dietary valine levels on production performance, egg quality, antioxidant capacity, immunity, and intestinal amino acid absorption of laying hens during the peak lay period[J]. Animals (Basel), 2021, 11(7): 1972. |
| [65] |
代腊, 顾林英, 朱巧明, 等. 饲粮缬氨酸水平对蛋鸡生产性能、蛋品质及血清生化指标的影响[J]. 动物营养学报, 2012, 24(4): 654-660. DAI L, GU L Y, ZHU Q M, et al. Dietary valine level affects performance, egg quality and serum biochemical indices in laying hens[J]. Chinese Journal of Animal Nutrition, 2012, 24(4): 654-660. (in Chinese) |
| [66] |
AMIRDAHRI S, JANMOHAMMADI H, TAGHIZADEH A, et al. Valine requirement of female Cobb broilers from 8 to 21 days of age[J]. J Appl Poult Res, 2020, 29(4): 775-785. |
| [67] |
ADABI S H G, CEYLAN N, ÇIFTCI, et al. Response of growing chicks to supplementation of low protein diets with leucine, valine and glycine-glutamic acid[J]. S Afr J Anim Sci, 2019, 49(6): 1047-1062. |
| [68] |
CORZO A, KIDD M T, DOZIER Ⅲ W A, et al. Marginality and needs of dietary valine for broilers fed certain all-vegetable diets[J]. J Appl Poult Res, 2007, 16(4): 546-554. |
| [69] |
陈将, 刘国华, PIRZADO S A, 等. 低蛋白质饲粮补充缬氨酸对肉鸡生长性能、屠宰性能和血清指标的影响[J]. 动物营养学报, 2019, 31(4): 1604-1612. CHEN J, LIU G H, PIRZADO S A, et al. Effects of valine supplementation in low-protein diets on growth performance, slaughter performance and serum indices of broilers[J]. Chinese Journal of Animal Nutrition, 2019, 31(4): 1604-1612. (in Chinese) |
| [70] |
黄璇, 蒋桂韬, 李闯, 等. 饲粮支链氨基酸比例对攸县麻鸭生长性能、血清生化指标和肠道发育的影响[J]. 动物营养学报, 2022, 34(9): 5759-5766. HUANG X, JIANG G T, LI C, et al. Effects of dietary branched-chain amino acid ratios on growth performance, serum biochemical indices and intestinal development of Youxian partridge ducks[J]. Chinese Journal of Animal Nutrition, 2022, 34(9): 5759-5766. (in Chinese) |
| [71] |
LIMA M B, SAKOMURA N K, SILVA E P, et al. The optimal digestible valine, isoleucine and tryptophan intakes of broiler breeder hens for rate of lay[J]. Anim Feed Sci Technol, 2018, 238: 29-38. |
| [72] |
ALLAMEH S, TOGHYANI M. Effect of dietary valine supplementation to low protein diets on performance, intestinal morphology and immune responses in broiler chickens[J]. Livest Sci, 2019, 229: 137-144. |
| [73] |
KIM J, LEE J E, LEE J S, et al. Phenylalanine and valine differentially stimulate milk protein synthetic and energy-mediated pathway in immortalized bovine mammary epithelial cells[J]. J Anim Sci Technol, 2020, 62(2): 263-275. |
| [74] |
WANG X L, XU J, ZENG H F, et al. Enhancement of BCAT2-mediated valine catabolism stimulates β-casein synthesis via the AMPK-mTOR signaling axis in bovine mammary epithelial cells[J]. J Agric Food Chem, 2022, 70(32): 9898-9907. |
| [75] |
HULTQUIST K M, CASPER D P. Effects of feeding rumen-degradable valine on milk production in late-lactating dairy cows[J]. J Dairy Sci, 2016, 99(2): 1201-1215. |
| [76] |
YEPES F A L, MANN S, OVERTON T R, et al. Effect of rumen-protected branched-chain amino acid supplementation on production- and energy-related metabolites during the first 35 days in milk in Holstein dairy cows[J]. J Dairy Sci, 2019, 102(6): 5657-5672. |
| [77] |
YEPES F A L, MANN S, OVERTON T R, et al. Hepatic effects of rumen-protected branched-chain amino acids with or without propylene glycol supplementation in dairy cows during early lactation[J]. J Dairy Sci, 2021, 104(9): 10324-10337. |
| [78] |
LI J Y, SUZUKI K, KOIKE Y, et al. Effects of dietary supplementation with branched-chain amino acids (BCAAs) during nursing on plasma BCAA levels and subsequent growth in cattle[J]. Asian-Australas J Anim Sci, 2005, 18(10): 1440-1444. |
(编辑 范子娟)



