奶牛围产期通常指的是奶牛产前三周至产后三周,是奶牛的一个特殊生理时期,围产期奶牛代谢疾病高发长期困扰着奶牛的健康养殖。从妊娠期到泌乳期的过渡对高产奶牛来说是严峻的代谢挑战,母体需要更多的能量用于泌乳,大量的营养物质(如葡萄糖、氨基酸等)流向乳腺。受制于采食量抑制,泌乳早期奶牛主要通过降低肝外组织对胰岛素的敏感性,即产生胰岛素抵抗和体脂动员来调节营养与能量分配,为乳腺的生物合成提供更多的葡萄糖[1]。而脂肪组织脂肪合成减少,脂肪分解加速,使大量脂肪酸进入血液,被骨骼肌、肝脏等器官吸收或进入乳腺合成乳脂[1]。大量营养物质流入乳腺,而供应其他组织进行生命活动的营养物质减少,此时就会引起能量负平衡及代谢紊乱[2]。能量负平衡是围产期奶牛的正常生理过程,严重的能量负平衡[以高非酯化脂肪酸(nonestesterified fatty acid,NEFA)和高β-羟丁酸(β-hydroxybutyric acid,BHBA)为特征],则会增加酮病和脂肪肝的风险[3],常见于严重的产前肥胖和过度体脂动员的奶牛[4]。
脂肪组织不仅是脂肪酸的储存库、能量的蓄水池,还是重要的内分泌器官,可以分泌多种脂肪因子,如脂联素(adiponectin)、抵抗素(resistin)、瘦素(leptin)和肿瘤坏死因子-α(tumor necrosis factor α, TNF-α)等[5-6],作用于肝脏等器官,调节细胞的脂质积累及胰岛素敏感性等。同时,肝脏也可分泌细胞因子,如成纤维细胞生长因子21(fibroblast growth factor 21,FGF-21)等发挥调节糖脂代谢的作用[7]。在人类医学的研究中,脂肪组织在非酒精性脂肪肝(non-alcoholic fatty liver disease, NAFLD)等疾病的发生发展中起着重要作用[8]。围产期奶牛中肝脏-脂肪组织串扰对脂肪肝发展的影响及其机制尚不明确。因此,本文综述了围产期奶牛肝脏-脂肪串扰媒介在机体糖脂代谢紊乱及脂肪肝发展中的作用,旨在为预防和治疗奶牛脂肪肝提供参考。
1 围产期奶牛肝脏和脂肪组织串扰特征脂肪的从头合成始于脂肪酸的生物合成,一般从乙酰辅酶A开始生成丙二酸单酰辅酶A,之后在脂肪酸合酶的催化下生成软脂酰酰基载体蛋白(acyl carrier protein,ACP),再通过内质网途径或线粒体途径延长,生成所需脂肪酸[9]。反刍动物肝脏、脂肪组织和乳腺是脂肪合成的主要部位,乙酸是生成脂肪酸的主要底物[9]。除了从头合成外,脂肪和乳腺组织还分泌脂蛋白脂肪酶(lipoprotein lipase,LPL),使得这些组织能够从血液中乳糜微粒、极低密度脂蛋白(very low-density lipoprotein, VLDL)(分别由肠细胞和肝细胞分泌)和三酰甘油(triglyceride, TAG)中获得脂肪酸[9]。
肝脏既是脂肪生成的主要部位,又是脂肪分解的主要部位,这主要取决于肉碱棕榈酰转移酶-1(carnitine palmitoyltransferase-1, CPT-1)的活性,CPT-1是一种位于线粒体外膜的酶,催化长链脂肪酰基辅酶A酯转化为其酰基肉碱衍生物,从而使其被运输到线粒体进行脂肪酸β氧化。而反刍动物肝脏中脂肪酸从头合成速率非常低,其主要原因是乙酰辅酶A羧化酶的产物丙二酰辅酶A通过抑制CPT-1的活性来调节脂肪酸氧化[9-11]。肝脏在正常情况下是无法储存脂肪的,肝脏可将脂肪酸氧化,或将脂肪酸合成TAG并包装为VLDL从肝脏输出进入脂肪组织储存[12-13]。在奶牛能量负平衡和代谢应激期间,脂肪组织动员使大量NEFA释放进入血液,进一步加剧能量负平衡及脂肪组织炎症,造成脂肪分解失控。脂肪酸在肝脏中大量聚集超过肝脏三羧酸循环(tricarboxylic acid cycle,TCA cycle)的氧化能力,导致肝脏酮体大量生成和TAG沉积,诱导脂肪肝发展[9, 14]。
2 肝脏与脂肪组织的糖脂代谢串扰媒介“器官串扰”是指通过细胞和神经激素作用介导的不同器官中的复杂生物通信和反馈。尽管串扰对保持体内稳态至关重要,但一个或多个器官的病理状态可能会导致其他器官的功能和结构失调[15]。因此,肝脏与脂肪组织的糖脂代谢串扰过程复杂,涉及多种脂肪因子及激素,代谢串扰可能缓解胰岛素抵抗、利于机体健康[16-17](图 1),也可能由于脂肪代谢障碍导致肝脏糖脂代谢紊乱[18-19],见图 2。
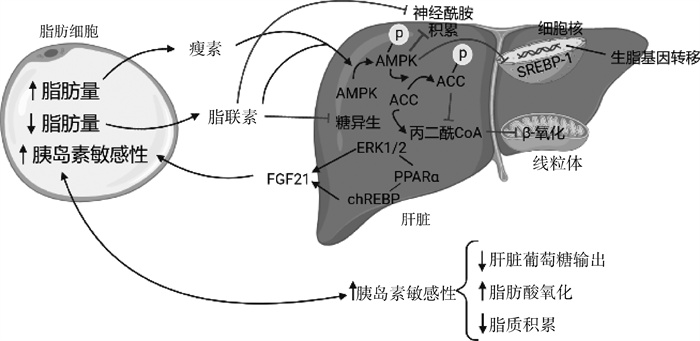
|
AMPK. 腺苷单磷酸激活蛋白激酶;ACC. 乙酰辅酶A羧化酶;ERK1/2. 细胞外调节蛋白激酶;PPARα. 过氧化物酶体增殖物激活受体α;chREBP. 糖类应答元件结合蛋白;FGF21. 成纤维细胞生长因子21;SREBP-1. 固醇反应元件结合蛋白-1 AMPK. Adenosine monophosphate activated protein kinase; ACC. Acetyl-coA carboxylase; ERK1/2. Extracellular regulatory protein kinase; PPARα. Peroxisome proliferator activated receptor α; ChREBP. Carbohydrate responsive element binding protein; FGF21. Fibroblast growth factor 21; SREBP-1. Sterol reaction element binding protein-1 图 1 肝脏-脂肪组织串扰维持糖脂代谢稳态[16-17] Fig. 1 Liver-adipose tissue crosstalk maintains glucose and lipid metabolism homeostasis[16-17] |
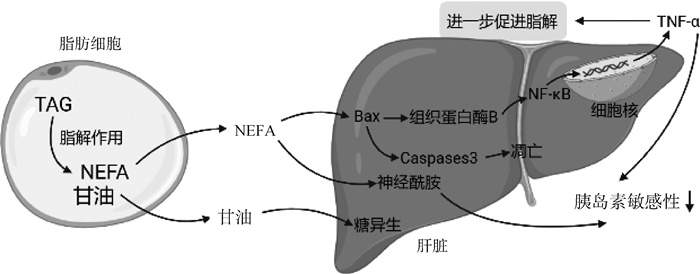
|
TAG. 三酰甘油;NEFA. 非酯化脂肪酸;Bax. 细胞凋亡促进基因;Caspases 3. 胱天蛋白酶3;NF-κB. 核因子κB;TNF-α. 肿瘤坏死因子α TAG. Triglyceride; NEFA. None esterified fatty acids; Bax. Apoptosis promoting genes; Caspase 3. Cysteine-aspartic acid protease 3; NF-κB. Nuclear factor κ B; TNF-α. Tumor necrosis factor α 图 2 肝脏-脂肪组织串扰加剧糖脂代谢紊乱[18-19] Fig. 2 Liver-adipose tissue crosstalk aggravates the disorder of glucose and lipid metabolism[18-19] |
神经酰胺(ceramide)是一种与胰岛素抵抗密切相关的鞘脂[20],已成为人类2型糖尿病(type 2 diabetes mellitus, T2DM)、非酒精性脂肪肝和心血管疾病等代谢性疾病的生物标志物[21]。同时,它也是连接某些营养素(如饱和脂肪酸)、炎性细胞因子(如TNF-α)和细胞功能调节的关键中间体[22]。神经酰胺可由多条途径合成,包括从头合成途径、鞘磷脂酶水解途径和补救途径[23-25]。肝脏是神经酰胺生成的主要器官,神经酰胺首先在细胞内质网中合成,之后到达高尔基体经细胞膜释放后进入血液循环[26-27]。神经酰胺的分解主要取决于神经酰胺酶的作用,神经酰胺酶可将神经酰胺分解为一种可变的游离脂肪酸和鞘氨醇[28]。
脂肪组织的过度脂解促使大量的游离脂肪酸进入血液循环,大量的饱和脂肪酸又可促进神经酰胺的形成[29]。过量的脂质及神经酰胺在肝脏中积累便会导致肝细胞功能障碍或死亡,这种异位脂质沉积导致细胞功能障碍和细胞死亡的现象被称为脂毒性[30]。研究表明,神经酰胺可通过与炎症因子相互作用来发挥脂毒性作用,可导致胰岛素抵抗,促进线粒体产生活性氧,促使炎症因子向肝脏的进一步募集,最终导致肝脏炎症恶化[31]。炎症因子(如TNF-α等)可选择性的增加鞘脂水平,而神经酰胺本身又可刺激炎症,这将形成一种典型的正反馈作用,从而加剧组织损伤[32-33]。肝脏中的神经酰胺还可进入循环到达脂肪组织,加剧脂肪组织胰岛素抵抗,促进脂解,加重能量负平衡[34]。
神经酰胺与肝脏疾病也有着密切的关联。肝脏中神经酰胺主要通过调节固醇调节元件结合蛋白-1(sterol regulatory element-binding protein-1,SREBP-1)表达诱导TAG合成[35],导致肝脏的脂肪沉积增加,胰岛素敏感性降低,加剧脂肪变性。神经酰胺还会破坏线粒体的呼吸能力,进一步促进组织细胞的凋亡。研究表明,鞘磷脂酶合酶2的耗竭导致线粒体呼吸受损,与细胞内神经酰胺的升高相关。同时,随着神经酰胺水平的持续升高,增加了线粒体外膜的通透性,这导致细胞色素c的释放和细胞凋亡的启动[36-37]。在肝脏中神经酰胺还可通过促进肝脏糖异生来产生胰岛素抵抗及肝脂肪变性,如用茴香醚处理小鼠可减少肝脏脂质积聚并抑制肝脏糖异生,主要是通过抑制二氢神经酰胺去饱和酶1即抑制神经酰胺的合成来发挥作用[38]。此外,神经酰胺还可通过阻断丝氨酸/苏氨酸激酶(AKT/PKB)磷酸化增强糖异生[35]。在反刍动物的研究中也在不断地证明神经酰胺对胰岛素抵抗及肝脏的影响,研究发现,营养限制造成奶牛机体胰岛素敏感性降低,增加了肝脏神经酰胺积累,循环神经酰胺含量与肝脏的胰岛素含量及胰岛素敏感性呈负相关[39]。Rico等[25]的研究也表明高体况奶牛更易产生系统胰岛素抵抗及神经酰胺积累,而神经酰胺也极有可能参与了奶牛胰岛素抵抗的病理发展。而产前过度饲喂也会进一步使脂肪组织和肝脏中神经酰胺积累,促使奶牛发生不同程度的胰岛素抵抗[40]。Rico等[41]发现静脉注射三酰甘油增加了肝脏中神经酰胺合酶2(ceramide synthase 2,CerS2)基因的mRNA表达,促进神经酰胺积累和脂肪肝的进一步发展。
总之,围产期奶牛脂肪过度脂解产生的大量NEFA,可造成神经酰胺在肝脏中积累并引发脂毒性作用,通过促进炎症因子的产生,破坏线粒体的功能和糖异生来促进细胞凋亡、胰岛素抵抗及肝脂肪变性,但神经酰胺在奶牛体内发挥作用的具体机制还有待进一步的研究。
2.2 TNF-αTNF-α在脂肪组织中产生,主要来源于驻留的巨噬细胞和单核细胞以及部分脂肪细胞,是一种多功能调节性脂肪细胞因子[42-43]。TNF-α通过与肿瘤坏死因子受体-1(TNF-R1)和肿瘤坏死因子受体-2(TNF-R2)相互作用促进肝脏和全身炎症、肝损伤和胰岛素抵抗:TNF-R1介导细胞凋亡和脂解,而TNF-R2诱导胰岛素抵抗[44]。
炎症因子在围产期奶牛中的研究多集中于能量负平衡所引发的炎症反应,而很少与糖脂代谢相关联。早期研究表明,血清TNF-α活性与脂肪肝奶牛的胰岛素抵抗相关,但具体机制并未阐明[45]。非反刍动物脂肪细胞中TNF-α主要通过抑制脂肪酸摄取的基因表达来发挥作用[46]。TNF-α还可抑制脂蛋白脂酶,并启动脂肪细胞中脂质分子的分解,由于脂肪分解作用,NEFA水平增加,导致胰岛素抵抗的发展[46]。
在肝脏及全身胰岛素抵抗中,c-Jun氨基末端蛋白激酶(c-Jun N-terminal kinase, JNK)通过诱导肝脏、脂肪细胞和外周组织中TNF-α的产生,TNF-α再通过诱导胰岛素受体底物1的丝氨酸磷酸化而抑制胰岛素刺激的酪氨酸激酶活性,从而产生胰岛素抵抗[47-48]。在脂肪性肝炎的蛋氨酸-胆碱缺乏动物模型中,抗TNF-α抗体给药改善了肝脏坏死、炎症和纤维化,表明TNF-α是肝损伤和脂肪性肝炎的关键媒介[49]。TNF-α可损害胰岛素敏感性并诱导牛脂肪细胞的脂肪分解和凋亡,这可能部分由NF-κB和JNK的激活介导[50],而在奶牛酮病等病理条件下通过激活肝细胞中的NF-κB信号通路来增加促炎因子的合成和表达,进一步导致酮症奶牛的炎症损伤[51]。石晓霞等[52]也发现较高浓度的BHBA可以通过NF-κB信号通路诱导TNF-α等炎症因子的表达造成牛肝细胞炎症损伤,进一步证实了奶牛体内介导炎症的机制,及其对肝脏的影响。
2.3 脂联素脂联素主要由白色脂肪组织(white adipose tissue,WAT)分泌[53],是血液中浓度最高的脂肪因子[54-57]。血液中全长脂联素有3种亚型,为三聚体(低分子量)、六聚体(中等分子量)和多达18个分子组成的高分子量(HMW)形式[58-62]。HMW被认为是脂联素活性最高的形式,被视为胰岛素抵抗和代谢综合征的生物标志物[63-64]。脂联素主要与三种受体结合发挥作用:脂联素受体1(adiponectin receptor 1, AdipoR1)、AdipoR2和T-钙黏蛋白。其中AdipoR1、AdipoR2发挥主要作用,而T-钙黏蛋白被认为是一种脂联素结合蛋白,但其功能意义尚未完全确定[65-66]。AdipoR1在骨骼肌中高度表达,AdipoR2主要在肝中表达,腺苷单磷酸激活蛋白激酶(adenosine monophosphate activated protein kinase, AMPK)和过氧化物酶体增殖物激活受体α(peroxisome proliferator-activated receptor alpha, PPARα)分别是AdipoR1和AdipoR2激活的主要靶点[67-69]。
脂联素通过自分泌/旁分泌形式在脂肪细胞中发挥重要作用,可促进前脂肪细胞分化为脂肪细胞,增加负责脂肪生成的程序化基因表达,最后通过促进葡萄糖转运蛋白4(glucose transporter type 4,GLUT4)基因表达增加脂肪细胞中葡萄糖转运系统的脂质含量和胰岛素敏感性[70]。并且,脂联素优先促进皮下脂肪中的脂质积累,而不是内脏脂肪,这种优先脂质积累减少了内脏脂肪组织质量和全身炎症,改善了葡萄糖和脂肪代谢,进一步增加了胰岛素敏感性[71]。当脂肪组织炎症发生时也会显著抑制胰岛素信号的传导,并且脂肪细胞炎症和巨噬细胞浸润与巨噬细胞极性从M2(抗炎)表型转变为M1(促炎)表型有关[72-73]。而脂联素可以刺激抗炎型脂肪因子白细胞介素-10(IL-10)的释放[73],并将巨噬细胞极化转变为M2表型[74],促进脂肪组织扩张、减少脂肪组织和全身炎症有助于提高全身胰岛素敏感性。脂联素进入血液循环后可到达其靶器官,肝脏是脂联素作用的主要靶器官[71, 75]。
研究表明,脂肪肝等肝脏疾病会使动物机体内脂联素水平降低[76],脂联素可通过抗炎、抗纤维化和缓解脂质积累等作用显著缓解脂肪肝的发展[77]。脂联素主要通过激活AMPK抑制活性氧产生和随后的AKT途径来对主要作用为调节和促进纤维化的肝星状细胞增殖的作用产生抑制,从而产生抗纤维化作用[78]。在糖脂代谢方面,脂联素可以通过抑制磷酸烯醇式丙酮酸羧激酶和葡萄糖-6-磷酸酶的mRNA表达来抑制肝脏糖异生[79],通过与AdipoR1结合激活下游靶点AMPK,AMPK通过磷酸化从头脂肪生成的限速酶乙酰辅酶A羧化酶-1(acetyl-CoA carboxylase 1,ACC-1)来抑制脂肪生成。这降低了ACC-1活性并减少了丙二酰辅酶A的产生,从而缓解了CPT-1活性的抑制,并增强了脂肪酸向线粒体的转运,以进行β氧化(图 1)。这种通过AdipoR1的脂联素信号传导诱导肝激酶B(liver kinase, LKB)-AMPK途径,来抑制固醇反应元件结合蛋白1c(SREBP1c)表达的方式降低了参与肝脏脂肪生成和胆固醇合成的基因表达,从而减少了肝脏脂质积累[16, 77, 80]。陈辉等[81]研究发现脂联素通过激活AMPK信号通路介导奶牛肝脏的脂质代谢,其可促进脂质氧化、抑制脂质合成和减少肝脏脂质积累。沈丽等[82]发现在酮病发生期间,酮病奶牛脂联素水平高于健康奶牛,脂联素很可能在调节能量平衡、体脂肪动员和酮病发生中发挥作用。
此外,脂联素与脂联素受体结合后会促进神经酰胺酶活性升高,神经酰胺酶将神经酰胺脱乙酰化生成鞘氨醇,鞘氨醇激酶又可将鞘氨醇磷酸化为1-磷酸鞘氨醇(sphingosine 1-phosphate,S1P),S1P/神经酰胺比率的增加可有效地抑制细胞凋亡,甚至诱导增殖[83-85]。S1P主要通过激活PPARa和AMPK即脂联素的下游介质来发挥作用[85]。过表达脂联素受体1和2可显著增强神经酰胺酶的活性,改善全身葡萄糖代谢及胰岛素敏感性,抑制肝脏脂肪变性[86]。并且,当过表达酸性神经酰胺酶后也产生了同样的效果[87]。然而,现在依然没有足够的证据证明在奶牛脂肪肝的发展过程中二者是否同样存在代谢耦合,对脂联素和神经酰胺关系的进一步研究也有助于深层次揭示二者在围产期奶牛糖脂代谢中的作用机制。
2.4 FGF-21FGF-21是FGF超家族内分泌分支的成员,对动物能量稳态、糖脂代谢和胰岛素敏感性发挥重要的调控作用[7]。肝脏是合成FGF-21的主要器官,禁食或生酮饮食以及通过激活PPARα、cAMP响应元件结合蛋白H和去乙酰化酶1可诱导FGF-21的产生[88-90]。FGF-21可以缓解胰岛素抵抗,减少肝脏等器官的脂质积累,药理剂量的FGF-21对一些代谢性疾病也有一定的治疗作用[91]。对高脂饮食诱导肥胖小鼠模型延长FGF-21给药可显著降低体重、缓解肥胖、降低肝脏TAG和胆固醇含量,同时逆转高血糖和高TAG血症[92]。临床酮症以及脂肪肝的发生与奶牛肝脏FGF-21表达增加和血浆FGF-21浓度升高有关[93-94]。Caixeta等[95]研究表明,静脉输注脂肪乳注射液(一种脂肪酸补充剂)导致血浆NEFA浓度增加,提高了奶牛肝脏FGF-21基因表达水平,使得血浆及肝脏FGF-21浓度急剧增加。并且Caixeta等[95]和Khan等[96]也发现过度饲喂的奶牛产后血浆中NEFA升高,可诱导肝脏中FGF-21表达的增加,由此可知FGF-21对奶牛的代谢应激具有示警作用。
FGF-21是迄今发现的最有效的急性胰岛素敏化剂之一。其可以使血浆葡萄糖水平明显降低,并且通过对脂肪组织的直接作用显著增强胰岛素敏感性[97]。FGF-21可以通过诱导葡萄糖转运蛋白1的表达促进脂肪细胞的葡萄糖摄取[98];通过刺激白细胞分化抗原36介导的脂肪酸摄取,加速脂肪组织中的脂蛋白代谢,从而降低血浆TAG水平[99]。研究表明在奶牛体内FGF-21无法防止脂肪组织脂解,对胰岛素抵抗的效果也不明显[96]。但Caixeta等[100]却发现外源性FGF-21可通过减少脂肪组织来源的脂肪酸进入肝脏来缓解牛肝脏脂质的积累,说明FGF-21对奶牛的脂质代谢依然具有调节作用。
2.5 瘦素瘦素(leptin)是一种由Lepob基因编码的肽激素,主要在WAT中合成[101-102]。细胞内葡萄糖代谢产物和循环因子(如胰岛素)可刺激瘦素分泌,而下丘脑前皮内啡肽神经元中的瘦素信号则会在禁食期间抑制瘦素分泌[103-105]。瘦素需要与质膜上的受体结合发挥作用,小鼠和人类的瘦素受体有多种亚型[106-107]。研究表明,奶牛的瘦素受体也具有多态性,瘦素主要通过中枢神经系统发挥作用,下丘脑是瘦素控制食物摄入和能量消耗的主要部位[108]。奶牛产后一周体内NEFA含量升高时血浆瘦素的含量明显减少,并且体脂动员程度更高的奶牛产前产后血浆瘦素的变化幅度也更大,这表明血浆瘦素与泌乳早期奶牛的体脂动员密切相关[109-111]。
瘦素对肝脏和脂肪组织的糖脂代谢也具有明显的调节作用。奶牛瘦素受体基因主要在肝脏和脂肪组织中表达,激活后可启动防御机制防止奶牛肝脏的脂肪沉积[112-113]。肝脏可直接从循环瘦素接收调节脂质和葡萄糖代谢的指令,如用瘦素灌注分离的大鼠肝脏发现瘦素会通过胰岛素受体底物-2阻碍糖异生[114]。并且,瘦素可促进脂肪酸氧化,与脂联素类似,瘦素通过ACC-1的磷酸化增加肝脏脂肪酸氧化并减少脂肪的从头合成[115](图 1)。瘦素还可通过脑-迷走神经-肝轴抑制异位脂质积累,改善肥胖相关的肝脏脂肪变性[116],并对NAFLD诱导的肝损伤具有预防作用[117]。瘦素在体内对脂肪组织的影响主要通过交感神经活动[118]。研究表明,瘦素减弱了脂肪细胞中的胰岛素反应,导致WAT细胞中胰岛素诱导的葡萄糖摄取和脂肪生成减少[119-121]。在棕色脂肪细胞中,瘦素信号通过降低胰岛素受体激酶活性抑制胰岛素刺激的葡萄糖摄取[122]。
瘦素浓度的变化与奶牛过渡期的代谢适应有着密切关系。研究发现,围产期奶牛瘦素水平降低,有利于奶牛机体对葡萄糖的储存及减少能量的额外消耗,将大量的营养和能量用于泌乳,有助于奶牛适应代谢变化[123-124]。泌乳早期能量负平衡的奶牛瘦素水平要普遍低于未产生能量负平衡的奶牛,随着泌乳期的延长瘦素水平需要慢慢恢复,而负平衡奶牛的恢复主要取决于脂解程度,过度脂解的奶牛瘦素水平恢复很慢,长时间未恢复就会加剧负平衡并引发代谢性疾病[123, 125]。酮病奶牛的瘦素水平要显著低于健康奶牛恰恰能解释这一点,泌乳早期酮病的发生往往伴随着过度的体脂动员,及胰岛素抵抗,瘦素水平的显著下降说明其在体脂动员和酮症发生的调节中发挥了重要作用[82]。同时,瘦素基因的表达与牛的脂肪沉积显著相关,在奶牛肝细胞中甲状腺激素可以调节瘦素基因的表达,防止肝脏的脂肪沉积,预防脂肪肝的发生[112-113]。
3 小结奶牛脂肪组织和肝脏可形成负责代谢稳态的关键代谢回路,这两个器官之间存在着复杂的双向串扰关系。泌乳早期奶牛往往出现能量负平衡,这是奶牛适应代谢变化的正常机制,但过度的体脂动员则会使奶牛脂肪细胞发生代谢应激,导致血浆NEFA浓度显著增加。大量脂肪酸流入肝脏促使神经酰胺生成,神经酰胺在肝脏中积累促进脂毒性的发生,神经酰胺通过募集炎症因子、促进糖异生等方式使肝脏发生脂肪变性并向脂肪肝等疾病发展。除了神经酰胺对TNF-α的募集,发生功能障碍的脂肪细胞也可分泌TNF-α,TNF-α促进脂解并介导肝损伤和脂肪性肝炎。
同为脂肪因子的脂联素却可以促进抗炎脂肪因子的产生缓解脂肪及肝脏炎症、抑制糖异生、减少肝脏脂质积累以及通过促进神经酰胺酶活性升高降解神经酰胺,对奶牛的肝脏脂质积累具有一定的缓解作用,然而脂肪肝的进一步发展会使其含量降低。FGF-21主要由肝脏生成,同样对肝脏脂质积累有显著的缓解作用,其含量会随着脂肪肝的发展而升高,具有一定的示警作用。另外,瘦素与脂联素相似,均可促进肝脏脂肪酸氧化,减少脂肪的从头合成,减少牛肝脏的脂肪沉积,防止其进一步向脂肪肝发展。因此,这些脂肪因子作为肝脏-脂肪组织串扰的媒介,在脂肪肝的发展中扮演着关键角色。
然而,在奶牛上这些脂肪因子发挥作用的主要靶点和机制依然不够清晰,如FGF-21和瘦素的研究依然停留在体内试验所观察到的其对糖脂代谢的调节作用,在奶牛体内它们发挥作用的具体通路和机制尚不明确,脂联素和神经酰胺的代谢耦合已在小鼠和人类的研究中得到证实,在奶牛体内是否会发挥同样的作用?如今,脂质组学、代谢组学和蛋白组学等研究方法为研究奶牛体内脂联素、FGF21及瘦素与神经酰胺等相互作用的靶点和机制提供了方法路径,未来深入探究肝脏与脂肪组织串扰机制,或可为改善围产期奶牛糖脂代谢稳态、预防和治疗脂肪肝等代谢性疾病提供新思路和新靶点。
| [1] |
BAUMGARD L H, COLLIER R J, BAUMAN D E. A 100-year review: Regulation of nutrient partitioning to support lactation[J]. J Dairy Sci, 2017, 100(12): 10353-10366. DOI:10.3168/jds.2017-13242 |
| [2] |
MCFADDEN J W. Review: Lipid biology in the periparturient dairy cow: contemporary perspectives[J]. Animal, 2020, 14(S1): s165-s175. |
| [3] |
BOBE G, YOUNG J W, BEITZ D C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows[J]. J Dairy Sci, 2004, 87(10): 3105-3124. DOI:10.3168/jds.S0022-0302(04)73446-3 |
| [4] |
ZACHUT M, HONIG H, STRIEM S, et al. Periparturient dairy cows do not exhibit hepatic insulin resistance, yet adipose-specific insulin resistance occurs in cows prone to high weight loss[J]. J Dairy Sci, 2013, 96(9): 5656-5669. DOI:10.3168/jds.2012-6142 |
| [5] |
ARNER P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones[J]. Trends Endocrinol Metab, 2003, 14(3): 137-145. DOI:10.1016/S1043-2760(03)00024-9 |
| [6] |
HAVEL P J. Section Ⅳ: Lipid modulators of islet function update on adipocyte hormones regulation of energy balance and carbohydrate/lipid metabolism[J]. Diabetes, 2004, 53(S1): s143-s151. |
| [7] |
KHARITONENKOV A, SHIYANOVA T L, KOESTER A, et al. FGF-21 as a novel metabolic regulator[J]. J Clin Invest, 2005, 115(6): 1627-1635. DOI:10.1172/JCI23606 |
| [8] |
SHUBHAM K, VINAY L, VINOD P K. Systems-level organization of non-alcoholic fatty liver disease progression network[J]. Mol BioSyst, 2017, 13(9): 1898-1911. DOI:10.1039/C7MB00013H |
| [9] |
VERNON R G. Lipid metabolism during lactation: a review of adipose tissue-liver interactions and the development of fatty liver[J]. J Dairy Res, 2005, 72(4): 460-469. DOI:10.1017/S0022029905001299 |
| [10] |
ZAMMIT V A. Ketogenesis in the liver of ruminants-adaptations to a challenge[J]. J Agric Sci, 1990, 115(2): 155-162. DOI:10.1017/S0021859600075080 |
| [11] |
ZAMMIT V A. The malonyl-CoA-long-chain acyl-CoA axis in the maintenance of mammalian cell function[J]. Biochem J, 1999, 343(Pt 3): 505-515. |
| [12] |
GRUMMER R R. Etiology of lipid-related metabolic disorders in periparturient dairy cows[J]. J Dairy Sci, 1993, 76(12): 3882-3896. DOI:10.3168/jds.S0022-0302(93)77729-2 |
| [13] |
LUCY M C, JIANG H, KOBAYASHI Y. Changes in the somatotrophic axis associated with the initiation of lactation[J]. J Dairy Sci, 2001, 84: E113-E119. DOI:10.3168/jds.S0022-0302(01)70205-6 |
| [14] |
WHITE H. The role of TCA cycle anaplerosis in ketosis and fatty liver in periparturient dairy cows[J]. Animals, 2015, 5(3): 793-802. DOI:10.3390/ani5030384 |
| [15] |
HUSAIN-SYED F, MCCULLOUGH P A, BIRK H W, et al. Cardio-pulmonary-renal interactions[J]. J Am Coll Cardiol, 2015, 65(22): 2433-2448. DOI:10.1016/j.jacc.2015.04.024 |
| [16] |
STERN J H, RUTKOWSKI J M, SCHERER P E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk[J]. Cell Metab, 2016, 23(5): 770-784. DOI:10.1016/j.cmet.2016.04.011 |
| [17] |
SPANN R A, MORRISON C D, DEN HARTIGH L J. The nuanced metabolic functions of endogenous FGF21 depend on the nature of the stimulus, tissue source, and experimental model[J]. Front Endocrinol, 2022, 12: 802541. DOI:10.3389/fendo.2021.802541 |
| [18] |
PETERSEN M C, SHULMAN G I. Mechanisms of insulin action and insulin resistance[J]. Physiol Rev, 2018, 98(4): 2133-2223. DOI:10.1152/physrev.00063.2017 |
| [19] |
CARTER-KENT C, ZEIN N N, FELDSTEIN A E. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment[J]. Am J Gastroenterol, 2008, 103(4): 1036-1042. DOI:10.1111/j.1572-0241.2007.01709.x |
| [20] |
RICO J E, SAED SAMⅡ S, MATHEWS A T, et al. Temporal changes in sphingolipids and systemic insulin sensitivity during the transition from gestation to lactation[J]. PLoS ONE, 2017, 12(5): e0176787. DOI:10.1371/journal.pone.0176787 |
| [21] |
BORODZICZ S, CZARZASTA K, KUCH M, et al. Sphingolipids in cardiovascular diseases and metabolic disorders[J]. Lipids Health Dis, 2015, 14(1): 55. DOI:10.1186/s12944-015-0053-y |
| [22] |
SUMMERS S. Ceramides in insulin resistance and lipotoxicity[J]. Prog Lipid Res, 2006, 45(1): 42-72. DOI:10.1016/j.plipres.2005.11.002 |
| [23] |
BARTKE N, HANNUN Y A. Bioactive sphingolipids: metabolism and function[J]. J Lipid Res, 2009, 50: S91-S96. DOI:10.1194/jlr.R800080-JLR200 |
| [24] |
MARCHESINI N, HANNUN Y A. Acid and neutral sphingomyelinases: roles and mechanisms of regulation[J]. Biochem Cell Biol, 2004, 82(1): 27-44. DOI:10.1139/o03-091 |
| [25] |
RICO J E, BANDARU V V R, DORSKIND J M, et al. Plasma ceramides are elevated in overweight Holstein dairy cows experiencing greater lipolysis and insulin resistance during the transition from late pregnancy to early lactation[J]. J Dairy Sci, 2015, 98(11): 7757-7770. DOI:10.3168/jds.2015-9519 |
| [26] |
GREEN C D, MACEYKA M, COWART L A, et al. Sphingolipids in metabolic disease: The good, the bad, and the unknown[J]. Cell Metab, 2021, 33(7): 1293-1306. DOI:10.1016/j.cmet.2021.06.006 |
| [27] |
BOON J, HOY A J, STARK R, et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance[J]. Diabetes, 2013, 62(2): 401-410. DOI:10.2337/db12-0686 |
| [28] |
COANT N, SAKAMOTO W, MAO C G, et al. Ceramidases, roles in sphingolipid metabolism and in health and disease[J]. Adv Biol Regul, 2017, 63: 122-131. DOI:10.1016/j.jbior.2016.10.002 |
| [29] |
CHAVEZ J A, SUMMERS S A. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes[J]. Arch Biochem Biophys, 2003, 419(2): 101-109. DOI:10.1016/j.abb.2003.08.020 |
| [30] |
TRAUNER M, ARRESE M, WAGNER M. Fatty liver and lipotoxicity[J]. Biochim Biophys Acta Mol Cell Biol Lipids, 2010, 1801(3): 299-310. |
| [31] |
SCHWABE R F, BRENNER D A. Mechanisms of liver injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways[J]. Am J Physiol Gastrointest Liver Physiol, 2006, 290(4): G583-G589. DOI:10.1152/ajpgi.00422.2005 |
| [32] |
DE MELLO V D F, LANKINEN M, SCHWAB U, et al. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease[J]. Diabetologia, 2009, 52(12): 2612-2615. DOI:10.1007/s00125-009-1482-9 |
| [33] |
VANDANMAGSAR B, YOUM Y H, RAVUSSIN A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance[J]. Nat Med, 2011, 17(2): 179-188. DOI:10.1038/nm.2279 |
| [34] |
LI Y, TALBOT C L, CHAURASIA B. Ceramides in adipose tissue[J]. Front Endocrinol, 2020, 11: 407. DOI:10.3389/fendo.2020.00407 |
| [35] |
CHAURASIA B, TIPPETTS T S, MAYORAL MONIBAS R, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis[J]. Science, 2019, 365(6451): 386-392. DOI:10.1126/science.aav3722 |
| [36] |
PARK M, KADDAI V, CHING J, et al. A role for ceramides, but not sphingomyelins, as antagonists of insulin signaling and mitochondrial metabolism in C2C12 myotubes[J]. J Biol Chem, 2016, 291(46): 23978-23988. DOI:10.1074/jbc.M116.737684 |
| [37] |
OBEID L M, LINARDIC C M, KAROLAK L A, et al. Programmed cell death induced by ceramide[J]. Science, 1993, 259(5102): 1769-1771. DOI:10.1126/science.8456305 |
| [38] |
BIKMAN B T, GUAN Y G, SHUI G H, et al. Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis[J]. J Biol Chem, 2012, 287(21): 17426-17437. DOI:10.1074/jbc.M112.359950 |
| [39] |
DAVIS A N, CLEGG J L, PERRY C A, et al. Nutrient restriction increases circulating and hepatic ceramide in dairy cows displaying impaired insulin tolerance[J]. Lipids, 2017, 52(9): 771-780. DOI:10.1007/s11745-017-4287-5 |
| [40] |
QIN N B, KOKKONEN T, SALIN S, et al. Prepartal overfeeding alters the lipidomic profiles in the liver and the adipose tissue of transition dairy cows[J]. Metabolomics, 2017, 13(2): 21. DOI:10.1007/s11306-016-1160-0 |
| [41] |
RICO J E, GIESY S L, HAUGHEY N J, et al. Intravenous triacylglycerol infusion promotes ceramide accumulation and hepatic steatosis in dairy cows[J]. J Nutr, 2018, 148(10): 1529-1535. DOI:10.1093/jn/nxy155 |
| [42] |
HOTAMISLIGIL G S, SHARGILL N S, SPIEGELMAN B M. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance[J]. Science, 1993, 259(5091): 87-91. DOI:10.1126/science.7678183 |
| [43] |
OLEFSKY J M, GLASS C K. Macrophages, inflammation, and insulin resistance[J]. Annu Rev Physiol, 2010, 72(1): 219-246. DOI:10.1146/annurev-physiol-021909-135846 |
| [44] |
GAMBINO R, MUSSO G, CASSADER M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: mechanisms and therapeutic opportunities[J]. Antioxid Redox Signal, 2011, 15(5): 1325-1365. DOI:10.1089/ars.2009.3058 |
| [45] |
OHTSUKA H, KOIWA M, HATSUGAYA A, et al. Relationship between serum TNF activity and insulin resistance in dairy cows affected with naturally occurring fatty liver[J]. J Vet Med Sci, 2001, 63(9): 1021-1025. DOI:10.1292/jvms.63.1021 |
| [46] |
KERN P A, RANGANATHAN S, LI C L, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance[J]. Am J Physiol Endocrinol Metab, 2001, 280(5): E745-E751. DOI:10.1152/ajpendo.2001.280.5.E745 |
| [47] |
HOTAMISLIGIL G S, PERALDI P, BUDAVARI A, et al. IRS-I-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α-and obesity-induced insulin resistance[J]. Science, 1996, 271(5249): 665-670. DOI:10.1126/science.271.5249.665 |
| [48] |
AGUIRRE V, UCHIDA T, YENUSH L, et al. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307[J]. J Biol Chem, 2000, 275(12): 9047-9054. DOI:10.1074/jbc.275.12.9047 |
| [49] |
KOCA S S, BAHCECIOGLU I H, POYRAZOGLU O K, et al. The treatment with antibody of TNF-α reduces the inflammation, necrosis and fibrosis in the non-alcoholic steatohepatitis induced by methionine-and choline-deficient diet[J]. Inflammation, 2008, 31(2): 91-98. DOI:10.1007/s10753-007-9053-z |
| [50] |
LI Y, DING H Y, WANG X C, et al. High levels of acetoacetate and glucose increase expression of cytokines in bovine hepatocytes, through activation of the NF-κB signalling pathway[J]. J Dairy Res, 2016, 83(1): 51-57. DOI:10.1017/S0022029915000680 |
| [51] |
DU X L, LIU M C, TAI W J, et al. Tumor necrosis factor-α promotes lipolysis and reduces insulin sensitivity by activating nuclear factor kappa B and c-Jun N-terminal kinase in primary bovine adipocytes[J]. J Dairy Sci, 2022, 105(10): 8426-8438. DOI:10.3168/jds.2022-22009 |
| [52] |
SHI X X, LI X W, LI D D, et al. β-hydroxybutyrate activates the NF-κB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes[J]. Cell Physiol Biochem, 2014, 33(4): 920-932. DOI:10.1159/000358664 |
| [53] |
WANG Y, XU A M, KNIGHT C, et al. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin[J]. J Biol Chem, 2002, 277(22): 19521-19529. DOI:10.1074/jbc.M200601200 |
| [54] |
MAEDA K, OKUBO K, SHIMOMURA I, et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1)[J]. Biochem Biophys Res Commun, 1996, 221(2): 286-289. DOI:10.1006/bbrc.1996.0587 |
| [55] |
NAKANO Y, TOBE T, CHOI-MIURA N H, et al. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma[J]. J Biochem, 1996, 120(4): 803-812. DOI:10.1093/oxfordjournals.jbchem.a021483 |
| [56] |
SCHERER P E, WILLIAMS S, FOGLIANO M, et al. A novel serum protein similar to C1q, produced exclusively in adipocytes[J]. J Biol Chem, 1995, 270(45): 26746-26749. DOI:10.1074/jbc.270.45.26746 |
| [57] |
HU E D, LIANG P, SPIEGELMAN B M. AdipoQ is a novel adipose-specific gene dysregulated in obesity[J]. J Biol Chem, 1996, 271(18): 10697-10703. DOI:10.1074/jbc.271.18.10697 |
| [58] |
PAJVANI U B, DU X L, COMBS T P, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin[J]. J Biol Chem, 2003, 278(11): 9073-9085. DOI:10.1074/jbc.M207198200 |
| [59] |
WAKI H, YAMAUCHI T, KAMON J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes[J]. J Biol Chem, 2003, 278(41): 40352-40363. DOI:10.1074/jbc.M300365200 |
| [60] |
YAMAUCHI T, KAMON J, WAKI H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity[J]. Nat Med, 2001, 7(8): 941-946. DOI:10.1038/90984 |
| [61] |
PAJVANI U B, HAWKINS M, COMBS T P, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity[J]. J Biol Chem, 2004, 279(13): 12152-12162. DOI:10.1074/jbc.M311113200 |
| [62] |
QIANG L, WANG H, FARMER S R. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-Lα[J]. Mol Cell Biol, 2007, 27(13): 4698-4707. DOI:10.1128/MCB.02279-06 |
| [63] |
SEINO Y, HIROSE H, SAITO I, et al. High-molecular-weight adiponectin is a predictor of progression to metabolic syndrome: a population-based 6-year follow-up study in Japanese men[J]. Metabolism, 2009, 58(3): 355-360. DOI:10.1016/j.metabol.2008.10.008 |
| [64] |
HARA K, HORIKOSHI M, YAMAUCHI T, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome[J]. Diabetes Care, 2006, 29(6): 1357-1362. DOI:10.2337/dc05-1801 |
| [65] |
YAMAUCHI T, KAMON J, ITO Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects[J]. Nature, 2003, 423(6941): 762-769. DOI:10.1038/nature01705 |
| [66] |
HUG C, WANG J, AHMAD N S, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin[J]. Proc Natl Acad Sci U S A, 2004, 101(28): 10308-10313. DOI:10.1073/pnas.0403382101 |
| [67] |
张辉. 脂联素对围产期奶牛脂肪动员的相关性研究[D]. 长春: 吉林大学, 2007. ZHANG H. The interrelation of ADPN and fat mobilization of dairy cows in peripartum[D]. Changchun: Jilin University, 2007. (in Chinese) |
| [68] |
YAMAUCHI T, KAMON J, MINOKOSHI Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase[J]. Nat Med, 2002, 8(11): 1288-1295. DOI:10.1038/nm788 |
| [69] |
MAO X M, KIKANI C K, RIOJAS R A, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function[J]. Nat Cell Biol, 2006, 8(5): 516-523. DOI:10.1038/ncb1404 |
| [70] |
FU Y C, LUO N L, KLEIN R L, et al. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation[J]. J Lipid Res, 2005, 46(7): 1369-1379. DOI:10.1194/jlr.M400373-JLR200 |
| [71] |
KIM J Y, VAN DE WALL E, LAPLANTE M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue[J]. J Clin Invest, 2007, 117(9): 2621-2637. DOI:10.1172/JCI31021 |
| [72] |
LUMENG C N, BODZIN J L, SALTIEL A R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization[J]. J Clin Invest, 2007, 117(1): 175-184. DOI:10.1172/JCI29881 |
| [73] |
KUMADA M, KIHARA S, OUCHI N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages[J]. Circulation, 2004, 109(17): 2046-2049. DOI:10.1161/01.CIR.0000127953.98131.ED |
| [74] |
OHASHI K, PARKER J L, OUCHI N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype[J]. J Biol Chem, 2010, 285(9): 6153-6160. DOI:10.1074/jbc.M109.088708 |
| [75] |
CHENG K K Y, LAM K S L, WANG B L, et al. Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin[J]. Best Pract Res Clin Endocrinol Metab, 2014, 28(1): 3-13. DOI:10.1016/j.beem.2013.06.006 |
| [76] |
BIANCHI G, BUGIANESI E, FRYSTYK J, et al. Adiponectin isoforms, insulin resistance and liver histology in nonalcoholic fatty liver disease[J]. Dig Liver Dis, 2011, 43(1): 73-77. DOI:10.1016/j.dld.2010.05.011 |
| [77] |
XU A M, WANG Y, KESHAW H, et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice[J]. J Clin Invest, 2003, 112(1): 91-100. DOI:10.1172/JCI200317797 |
| [78] |
ADACHI M, BRENNER D A. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase[J]. Hepatology, 2008, 47(2): 677-685. DOI:10.1002/hep.21991 |
| [79] |
RYU J, HADLEY J T, LI Z, et al. Adiponectin alleviates diet-induced inflammation in the liver by suppressing MCP-1 expression and macrophage infiltration[J]. Diabetes, 2021, 70(6): 1303-1316. DOI:10.2337/db20-1073 |
| [80] |
AWAZAWA M, UEKI K, INABE K, et al. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway[J]. Biochem Biophys Res Commun, 2009, 382(1): 51-56. DOI:10.1016/j.bbrc.2009.02.131 |
| [81] |
CHEN H, ZHANG L, LI X W, et al. Adiponectin activates the AMPK signaling pathway to regulate lipid metabolism in bovine hepatocytes[J]. J Steroid Biochem Mol Biol, 2013, 138: 445-454. DOI:10.1016/j.jsbmb.2013.08.013 |
| [82] |
SHEN L, QIAN B, XIAO J, et al. Characterization of serum adiponectin and leptin in healthy perinatal dairy cows or cows with ketosis, and their effects on ketosis involved indices[J]. Pol J Vet Sci, 2020, 23(3): 373-381. |
| [83] |
KUPCHAK B R, GARITAONANDIA I, VILLA N Y, et al. Antagonism of human adiponectin receptors and their membrane progesterone receptor paralogs by TNFα and a ceramidase inhibitor[J]. Biochemistry, 2009, 48(24): 5504-5506. DOI:10.1021/bi9006258 |
| [84] |
SHARMA A X, HOLLAND W L. Adiponectin and its hydrolase-activated receptors[J]. J Nat Sci, 2017, 3(6): e396. |
| [85] |
HOLLAND W L, MILLER R A, WANG Z V, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin[J]. Nat Med, 2011, 17(1): 55-63. DOI:10.1038/nm.2277 |
| [86] |
HOLLAND W L, XIA J Y, JOHNSON J A, et al. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis[J]. Mol Metab, 2017, 6(3): 267-275. DOI:10.1016/j.molmet.2017.01.002 |
| [87] |
XIA J Y, HOLLAND W L, KUSMINSKI C M, et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis[J]. Cell Metab, 2015, 22(2): 266-278. DOI:10.1016/j.cmet.2015.06.007 |
| [88] |
BADMAN M K, PISSIOS P, KENNEDY A R, et al. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states[J]. Cell Metab, 2007, 5(6): 426-437. DOI:10.1016/j.cmet.2007.05.002 |
| [89] |
INAGAKI T, DUTCHAK P, ZHAO G X, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21[J]. Cell Metab, 2007, 5(6): 415-425. DOI:10.1016/j.cmet.2007.05.003 |
| [90] |
FISHER F M, MARATOS-FLIER E. Understanding the physiology of FGF21[J]. Annu Rev Physiol, 2016, 78(1): 223-241. DOI:10.1146/annurev-physiol-021115-105339 |
| [91] |
GENG L L, LAM K S L, XU A M. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic[J]. Nat Rev Endocrinol, 2020, 16(11): 654-667. DOI:10.1038/s41574-020-0386-0 |
| [92] |
XU J, LLOYD D J, HALE C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice[J]. Diabetes, 2009, 58(1): 250-259. DOI:10.2337/db08-0392 |
| [93] |
AKBAR H, BATISTEL F, DRACKLEY J K, et al. Alterations in hepatic FGF21, co-regulated genes, and upstream metabolic genes in response to nutrition, ketosis and inflammation in peripartal holstein cows[J]. PLoS ONE, 2015, 10(10): e0139963. DOI:10.1371/journal.pone.0139963 |
| [94] |
WANG J G, ZHU X Y, SHE G H, et al. Serum hepatokines in dairy cows: periparturient variation and changes in energy-related metabolic disorders[J]. BMC Vet Res, 2018, 14(1): 236. DOI:10.1186/s12917-018-1560-7 |
| [95] |
CAIXETA L S, GIESY S L, KRUMM C S, et al. Effect of circulating glucagon and free fatty acids on hepatic FGF21 production in dairy cows[J]. Am J Physiol Regul Integr Comp Physiol, 2017, 313(5): R526-R534. DOI:10.1152/ajpregu.00197.2017 |
| [96] |
KHAN M J, JACOMETO C B, GRAUGNARD D E, et al. Overfeeding dairy cattle during late-pregnancy alters hepatic PPARα-regulated pathways including hepatokines: impact on metabolism and peripheral insulin sensitivity[J]. Gene Regul Syst Biol, 2014, 8: 97-111. |
| [97] |
BONDURANT L D, AMEKA M, NABER M C, et al. FGF21 regulates metabolism through adipose-dependent and-independent mechanisms[J]. Cell Metab, 2017, 25(4): 935-944. DOI:10.1016/j.cmet.2017.03.005 |
| [98] |
GE X, CHEN C, HUI X Y, et al. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes[J]. J Biol Chem, 2011, 286(40): 34533-34541. DOI:10.1074/jbc.M111.248591 |
| [99] |
SCHLEIN C, TALUKDAR S, HEINE M, et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues[J]. Cell Metab, 2016, 23(3): 441-453. DOI:10.1016/j.cmet.2016.01.006 |
| [100] |
CAIXETA L S, GIESY S L, KRUMM C S, et al. Fibroblast growth factor-21 (FGF21) administration to early-lactating dairy cows. Ⅱ. Pharmacokinetics, whole-animal performance, and lipid metabolism[J]. J Dairy Sci, 2019, 102(12): 11597-11608. DOI:10.3168/jds.2019-16696 |
| [101] |
ZHANG Y Y, PROENCA R, MAFFEI M, et al. Positional cloning of the mouse obese gene and its human homologue[J]. Nature, 1994, 372(6505): 425-432. DOI:10.1038/372425a0 |
| [102] |
HARRIS R B S. Direct and indirect effects of leptin on adipocyte metabolism[J]. Biochim Biophys Acta, 2014, 1842(3): 414-423. DOI:10.1016/j.bbadis.2013.05.009 |
| [103] |
WANG J L, LIU R, HAWKINS M, et al. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat[J]. Nature, 1998, 393(6686): 684-688. DOI:10.1038/31474 |
| [104] |
BARR V A, MALIDE D, ZARNOWSKI M J, et al. Insulin stimulates both leptin secretion and production by rat white adipose tissue[J]. Endocrinology, 1997, 138(10): 4463-4472. DOI:10.1210/endo.138.10.5451 |
| [105] |
CARON A, DUNGAN LEMKO H M, CASTORENA C M, et al. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels[J]. eLife, 2018, 7: e33710. DOI:10.7554/eLife.33710 |
| [106] |
FEI H, OKANO H J, LI C, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues[J]. Proc Natl Acad Sci U S A, 1997, 94(13): 7001-7005. DOI:10.1073/pnas.94.13.7001 |
| [107] |
BURGUERA B, COUCE M E, LONG J, et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain[J]. Neuroendocrinology, 2000, 71(3): 187-195. DOI:10.1159/000054536 |
| [108] |
LIEFERS S C, VEERKAMP R F, PAS M F W, et al. A missense mutation in the bovine leptin receptor gene is associated with leptin concentrations during late pregnancy[J]. Anim Genet, 2004, 35(2): 138-141. DOI:10.1111/j.1365-2052.2004.01115.x |
| [109] |
SADRI H, MIELENZ M, MOREL I, et al. Plasma leptin and mRNA expression of lipogenesis and lipolysis-related factors in bovine adipose tissue around parturition[J]. J Anim Physiol Anim Nutr (Berl), 2011, 95(6): 790-797. DOI:10.1111/j.1439-0396.2010.01111.x |
| [110] |
KOKKONEN T, TAPONEN J, ANTTILA T, et al. Effect of body fatness and glucogenic supplement on lipid and protein mobilization and plasma leptin in dairy cows[J]. J Dairy Sci, 2005, 88(3): 1127-1141. DOI:10.3168/jds.S0022-0302(05)72779-X |
| [111] |
SCHUH K, SADRI H, HÄUSSLER S, et al. Comparison of performance and metabolism from late pregnancy to early lactation in dairy cows with elevated v. normal body condition at dry-off[J]. Animal, 2019, 13(7): 1478-1488. DOI:10.1017/S1751731118003385 |
| [112] |
RAZA S H A, LIU G Y, ZHOU L, et al. Detection of polymorphisms in the bovine leptin receptor gene affects fat deposition in two Chinese beef cattle breeds[J]. Gene, 2020, 758: 144957. DOI:10.1016/j.gene.2020.144957 |
| [113] |
GYÖRFFY A, KERESZTES M, FAIGL V, et al. Glycogenic induction of thyroid hormone conversion and leptin system activation in the liver of postpartum dairy cows[J]. Acta Vet Hung, 2009, 57(1): 139-146. DOI:10.1556/avet.57.2009.1.14 |
| [114] |
ANDERWALD C, MULLER G, KOCA G, et al. Short-term leptin-dependent inhibition of hepatic gluconeogenesis is mediated by insulin receptor substrate-2[J]. Mol Endocrinol, 2002, 16(7): 1612-1628. DOI:10.1210/mend.16.7.0867 |
| [115] |
HUANG W, DEDOUSIS N, BANDI A, et al. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo[J]. Endocrinology, 2006, 147(3): 1480-1487. DOI:10.1210/en.2005-0731 |
| [116] |
HACKL M T, FVRNSINN C, SCHUH C M, et al. Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis[J]. Nat Commun, 2019, 10(1): 2717. DOI:10.1038/s41467-019-10684-1 |
| [117] |
POLYZOS S A, KOUNTOURAS J, MANTZOROS C S. Leptin in nonalcoholic fatty liver disease: A narrative review[J]. Metabolism, 2015, 64(1): 60-78. DOI:10.1016/j.metabol.2014.10.012 |
| [118] |
ZENG W W, PIRZGALSKA R M, PEREIRA M M A, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis[J]. Cell, 2015, 163(1): 84-94. DOI:10.1016/j.cell.2015.08.055 |
| [119] |
PÉREZ C, FERNÁNDEZ-GALAZ C, FERNÁNDEZ-AGULLÓ T, et al. Leptin impairs insulin signaling in rat adipocytes[J]. Diabetes, 2004, 53(2): 347-353. DOI:10.2337/diabetes.53.2.347 |
| [120] |
ELIMAM A, KAMEL A, MARCUS C. In vitro effects of leptin on human adipocyte metabolism[J]. Horm Res, 2002, 58(2): 88-93. |
| [121] |
MVLLER G, ERTL J, GERL M, et al. Leptin impairs metabolic actions of insulin in isolated rat adipocytes[J]. J Biol Chem, 1997, 272(16): 10585-10593. DOI:10.1074/jbc.272.16.10585 |
| [122] |
KRAUS D, FASSHAUER M, OTT V, et al. Leptin secretion and negative autocrine crosstalk with insulin in brown adipocytes[J]. J Endocrinol, 2002, 175(1): 185-191. DOI:10.1677/joe.0.1750185 |
| [123] |
BLOCK S S, BUTLER W R, EHRHARDT R A, et al. Decreased concentration of plasma leptin in periparturient dairy cows is caused by negative energy balance[J]. J Endocrinol, 2001, 171(2): 339-348. DOI:10.1677/joe.0.1710339 |
| [124] |
EHRHARDT R A, FOSKOLOS A, GIESY S L, et al. Increased plasma leptin attenuates adaptive metabolism in early lactating dairy cows[J]. J Endocrinol, 2016, 229(2): 145-157. DOI:10.1530/JOE-16-0031 |
| [125] |
LIEFERS S C, VEERKAMP R F, TE PAS M F W, et al. Leptin concentrations in relation to energy balance, milk yield, intake, live weight, and estrus in dairy cows[J]. J Dairy Sci, 2003, 86(3): 799-807. DOI:10.3168/jds.S0022-0302(03)73662-5 |
(编辑 白永平)



