2. 福建省畜禽遗传育种重点实验室, 福州 350013
2. Fujian Key Laboratory of Animal Genetics and Breeding, Fuzhou 350013, China
闽西南黑兔(Oryctolagus cuniculus)是福建省少数几个进入国家畜禽遗传资源目录的地方肉兔品种资源,其毛色全黑,体躯短小,两耳直立,眼大有神,主要分布于福建省龙岩市和泉州市等地区的山区。闽西南黑兔肉质鲜美,在闽西和闽南一带常以白宰和炖酒食用,是民间酒席上的佳肴,深受当地市场的欢迎[1]。
促性腺激素抑制素(gonadotropin-inhibitory hormone,GnIH)是最早由Tsutsui等[2]发现的一种抑制鹌鹑垂体促性腺激素(gonadotropin, GTH)释放的下丘脑RF-酰胺神经肽(SIKPSAYLPLRFa),由GnIH前体蛋白裂解产生。不同动物的GnIH成熟肽均是由GnIH基因编码的前体蛋白经过酶切加工产生,具有C末端LPXRFa酰胺特征基序[3]。GnIH主要分布于包括鸟类、哺乳动物以及鱼类在内的脊椎动物下丘脑-垂体-性腺轴(hypothalamic-pituitary-gonadal axis, HPG),其通过减少脊椎动物的GTH的合成和释放,进而影响生殖激素的分泌、性腺的发育和形成以及性行为的表现[4-9]。鸟类GnIH神经元细胞体主要集中在下丘脑室旁核,其神经纤维几乎延伸至整个前脑、中脑和间脑等区域,GnIH神经元投射最密集区域为正中隆起外层,在此区域与促性腺激素释放激素(gonadotropin releasing hormone, GnRH)、阿片黑皮质激素原(proopiomelanocortin,POMC)、神经肽Y(neuropeptide Y,NPY)、亲吻肽(kisspeptin,Kiss1)和食欲素(orexin,OX)等诸多神经元紧密联接。哺乳动物的GnIH神经纤维的细胞体集中在下丘脑背内侧核,神经纤维投射到视前核和杏仁核中部、终纹床核、室旁核、弓状核、腹内侧核以及正中隆起[9-10]。在小鼠、仓鼠、绵羊、灵长类动物的大脑内,40%~80%的GnRH细胞与GnIH纤维紧密联系,GnIH可以通过作用于下丘脑GnRH神经元进而调控GTH的合成与分泌[11-16]。除了作用于哺乳动物下丘脑外,GnIH被报道可以通过结合垂体上受体G蛋白耦联受体147(G-protein coupled receptor 147,GPR147)对GTH及其下游胆固醇激素进行调控[4, 17-20]。在羊、大鼠、牛的原代垂体细胞均检测到GPR147的存在,添加GnIH都能抑制GnRH刺激的促黄体生成素(luteinizing hormone,LH)的释放[5, 13, 21-23]。在羊中,GnIH还能直接降低原代垂体细胞卵泡刺激素β亚基(follicle stimulating hormone subunit beta,FSHβ)和促黄体素β亚基(luteinizing hormone subunit beta,LHβ)的合成,消除GTH细胞内GnRH激活的Ca2+活动,中和GnRH刺激的LH mRNA升高[13]。此外,将GnIH过表达质粒转染至小鼠睾丸间质细胞中会降低睾酮合成相关酶-类固醇合成快速调节蛋白(steroidogenic acute regulatory,StAR)、P450侧链裂解酶(cytochrome P450 side chain cleavage enzyme,P450 scc)和3β-羟基类固醇脱氢酶(3β-hydroxysteroid dehydrogenase,3β-HSD)基因的表达量进而抑制睾酮的分泌[24]。综上所述,GnIH可以调控哺乳动物下丘脑神经元释放GnRH以及抑制垂体GTH细胞合成和释放LH和FSH。
目前,鹌鹑(967 bp)[25]、小鼠(818 bp)[26]、人(1 190 bp)[26]、猪(1 124 bp)[27]等物种的GnIH cDNA全长陆续被克隆和测序,家兔方面,只有刘晶[28]获得了家兔GnIH cDNA部分序列(389 bp),然而未获得完整的cDNA序列。因此,本试验以闽西南黑兔为研究对象,通过RACE(rapid-amplification of cDNA ends)技术克隆GnIH基因完整cDNA序列,分析其基因功能,再根据获得序列信息设计引物,检测GnIH基因在各个组织以及不同发育阶段下丘脑中的表达水平,确定GnIH基因表达规律,并在此基础上通过腹腔注射鹌鹑GnIH相关肽,研究其对幼龄公兔血清中生殖激素的影响,以期为下一步探究GnIH对兔生殖发育的调控机理奠定基础。
1 材料与方法 1.1 试验设计本试验分为兔GnIH基因cDNA全长克隆与序列分析、GnIH基因mRNA表达分析以及GnIH相关肽对幼龄公兔生殖激素的影响3部分。其中:1)GnIH基因cDNA克隆与序列分析试验主要以90日龄公兔为研究对象,通过RACE技术克隆兔GnIH基因cDNA序列,并对序列进行生物信息学分析;2)GnIH基因mRNA表达分析试验,主要分析该基因在90日龄公兔的不同组织表达水平以及11~150日龄公兔下丘脑中的表达水平;3)GnIH相关肽对公兔生殖激素的影响试验,主要研究腹腔注射公兔0、0.5、5以及50 μg鹌鹑GnIH相关肽后,公兔血清中包括睾酮在内的生殖激素以及睾丸睾酮合成相关酶基因mRNA的变化。
1.2 试验动物与样品采集本次试验的样品均采自福建省农业科学院畜牧兽医研究所智能化兔舍试验平台的闽西南黑兔群体。样品包括:1)5只90日龄健康公兔的心、肝、脾、肺、肾、胃、小肠、睾丸、下丘脑、垂体、背肌、脂肪;2)同一批次公兔分别在11、30、60、90、120、150日龄时随机选择6只屠宰,采集下丘脑组织备用;3)同一批次40只公兔随机分为4组,在80日龄时分别腹腔注射0、0.5、5和50 μg的GnIH相关肽(Quail GnIH related peptide 1,Phoenix Pharmaceuticals, 美国),连续注射10 d,第11天早上采集耳静脉血液,随后屠宰,剥离睾丸组织备用。
1.3 RNA的提取和cDNA合成利用总RNA提取试剂盒(Invitrogen, 美国)提取兔各组织的总RNA,加入DNase I(Takara,日本)进行消化,RNA的含量和纯度使用凝胶电泳和Nanodrop 2000紫外分光光度计(Thermo Scientific,美国)进行检测,并调整总RNA质量浓度至1 μg·μL-1。取出2 μL总RNA,利用SuperScriptTM Ⅱ反转录酶(Invitrogen, 美国)和Oligo (dT)18反转录成cDNA,用于克隆GnIH全长cDNA序列的中间片段或荧光定量PCR(real-time quantitative PCR, qPCR)检测。使用闽西黑兔下丘脑组织RNA,根据SMARTer RACE 5′/3′ Kit(TaKaRa,日本)说明书分别合成5′-RACE和3′-RACE的cDNA模板,用于5′和3′末端序列的克隆。
1.4 兔GnIH基因全长cDNA序列克隆参考兔GnIH基因预测序列(GenBank:XM_ 002713827.2)设计保守区段的扩增引物(表 1),以兔下丘脑cDNA为模板,通过PCR扩增获得其中间序列。PCR反应体系(50 μL):模板(cDNA原液)1 μL、2×PCR Buffer 25 μL、20 μmol·L-1上、下游引物各1 μL、1.25 U·μL-1 DNA聚合酶1 μL,灭菌超纯水21 μL。反应条件:94 ℃预变性1 min;98 ℃ 10 s,55 ℃ 15 s,68 ℃ 1 min,35个循环。PCR产物在1%的琼脂糖凝胶中检测,然后送上海博尚生物技术有限公司进行测序。根据所获得兔GnIH基因的中间序列,设计5′-RACE和3′-RACE特异引物(表 1)。5′-RACE和3′-RACE PCR反应按照SMARTer RACE 5′/3′ Kit(TaKaRa,日本)说明书进行。将扩增的片段分别切胶纯化回收后,连接到T-Vector pMDTM20载体(TaKaRa,日本),经菌落PCR筛选后挑取克隆送上海博尚生物技术有限公司进行测序。
|
|
表 1 引物信息 Table 1 Primer information |
用DNAStar 8.0中的SeqMan拼接5′-RACE、3′-RACE和中间序列,得到GnIH基因的cDNA全长,并根据获得cDNA全长序列重新设计引物,通过PCR扩增后测序验证。验证PCR反应体系(50 μL):模板2 μL、2×PCR Buffer 25 μL、20 μmol·L-1上、下游引物各1 μL、1.25 U·μL-1 DNA聚合酶1 μL, 灭菌超纯水20 μL。验证PCR反应条件:94 ℃预变性1 min;98 ℃ 10 s,55 ℃ 15 s,68 ℃ 1 min,35个循环。
1.5 兔GnIH生物信息学分析使用Expasy网站(https://web.expasy.org/)在线软件ProtParam和Protscale分别分析兔GnIH前体蛋白的理化性质和亲-疏水性。利用PSIPRED(http://bioinf.cs.ucl.ac.uk/psipred)预测GnIH前体蛋白的二级结构以及用Phyre2(http://www.sbg.bio.ic.ac.uk/~phyre2/html/page.cgi?id=index)预测其三级结构。使用SignalP-5.0(https://services.healthtech.dtu.dk/service.php?SignalP-5.0)进行信号肽预测。使用MegAlign软件对闽西南黑兔、人(NP_071433.3)、猕猴(ACF58053.1)、黑猩猩(PNI40345.1)、马(XP_ 001498898.1)、虎鲸(XP_004265697.1)、熊猫(XP_002912906.2)、树鼩(XP_006162460.1)、灰海豹(XP_035953085.1)、九带犰狳(XP_004447617.1)、北极熊(XP_008684635.1)、犬(NP_001279989.1)、绵羊(NP_001120740.1)、大鼠(NP_076442.1)、鸡(BAC87781.1)、猪(ADM12803)、日本鹌鹑(BAB15932.1)以及斑马鱼(NP_001076418.1)的GnIH前体蛋白的氨基酸序列进行同源性比对,应用MEGA-X软件中的邻接法(Neighbor-joining,NJ)构建GnIH前体蛋白的系统进化树,并基于Ubuka和Parhar(2017)[29]报道现有脊椎动物的GnIH前体蛋白分解产生的RF-酰胺相关肽(RFamide-related peptide,RFRPs)和GnIH序列,预测闽西南黑兔相关RFRPs序列。
1.6 GnIH在公兔不同组织的表达谱根据克隆获得的GnIH基因序列设计引物(表 1),使用qPCR方法检测GnIH基因在90日龄健康公兔的不同组织(心、肝、脾、肺、肾、胃、小肠、睾丸、下丘脑、垂体、背肌、脂肪)以及不同日龄(11、30、60、90、120、150日龄)健康公兔下丘脑组织中的表达水平。以兔GAPDH基因(NM_001082253.1)为内参基因,引物表见表 1。qPCR扩增体系:模板cDNA 1 μL,Power SYBRⓇ Green PCR Master Mix(ABI,美国)10 μL,上、下游引物各0.5 μL,灭菌超纯水8 μL。反应条件:95 ℃预变性1 min;95 ℃变性15 s,60 ℃退火25 s,40个循环;熔解曲线为55 ~95 ℃,每10 s增加0.5 ℃。每个样本重复3次,各个基因的相对表达水平以2-ΔΔCt法进行后续分析。
1.7 GnIH对公兔生殖激素分泌的调控将采集的耳静脉血4 ℃ 3 000 r·min-1离心20 min后取其上清,-20 ℃贮存。采用上海纪宁兔促性腺激素释放激素(GnRH)试剂盒(ELISA)、兔促卵泡素(FSH)试剂盒(ELISA)、兔黄体生成素(LH)试剂盒(ELISA)、兔睾酮(T)试剂盒(ELISA)和兔抑制素B(INHB)试剂盒(ELISA)检测血清中GnRH、FSH、LH、睾酮(testosterone,T)和抑制素B亚基(inhibin β subunit,INHB)的含量。
1.8 GnIH多肽对睾酮合成相关酶基因mRNA表达的影响检测兔StAR、p450scc和3β-HSD基因在睾丸中的mRNA表达量。根据NCBI提供的兔StAR(XM_017350352.1)、p450scc(XM_008253734.2)和3β-HSD(XM_002715682.3)基因序列设计引物(表 1),并以兔GAPDH基因(NM_001082253.1)为内参基因,引物表见表 1。qPCR扩增体系:模板cDNA 1 μL,Power SYBRⓇ Green PCR Master Mix(ABI,美国)10 μL,上、下游引物各0.5 μL,灭菌超纯水8 μL。反应条件:95 ℃预变性1 min;95 ℃变性15 s,63 ℃退火25 s,40个循环;熔解曲线为55 ℃~95 ℃,每10 s增加0.5 ℃。每个样本重复3次,各个基因的相对表达水平以2-ΔΔCt法进行后续分析。
1.9 数据分析用SAS 9.4软件GLM模型分析qPCR和激素检测结果,结果以“平均数±标准误”表示,以P < 0.05表示差异显著,以P < 0.01和P < 0.001表示差异极显著。
2 结果 2.1 兔GnIH cDNA全长序列克隆克隆获得的cDNA序列片段经DNAMAN拼接后,得到兔GnIH cDNA全长序列为904 bp,其中5′UTR、cDNA和3′UTR分别为41、606和227 bp,还有一个poly(A)尾巴;编码201个氨基酸,起始密码子为ATG,终止密码子为TAA。序列已提交到GenBank,登录号为MK403675.1。
2.2 兔GnIH前体蛋白氨基酸序列理化性质分析兔GnIH前体蛋白的理化性质和亲-疏水性分析结果显示,蛋白分子式为C995H1 592N294O305S11,分子量为22.9 ku,共编码201个氨基酸,理论等电点为9.1。带正电荷残基总数(精氨酸+赖氨酸,Arg+Lys)为29个,带负电荷的残基总数(天冬氨酸+谷氨酸,Asp+Glu)为24个。在哺乳动物网织红细胞体外半衰期为30 h。不稳定系数为74.85,亲水性平均系数为-0.838,脂肪系数为61.89。
2.3 GnIH前体蛋白质结构域和信号肽预测二级结构预测结果显示,GnIH前体蛋白只含有α螺旋和无规则卷曲2种二级结构(图 1)。预测GnIH前体蛋白潜在的Sec信号肽(Sec/SPI)概率分值为0.686 2,裂解位点在第26位半胱氨酸和27位天冬氨酸之间,概率分值在0.400 5,此位置恰好在α螺旋与无规则卷曲连接处(图 2)。

|
A.二级结构预测;B.三级结构预测 A. Secondary structure prediction; B. Tertiary structure prediction 图 1 兔GnIH前体蛋白结构分析 Fig. 1 Structure analysis of rabbit GnIH precursor |
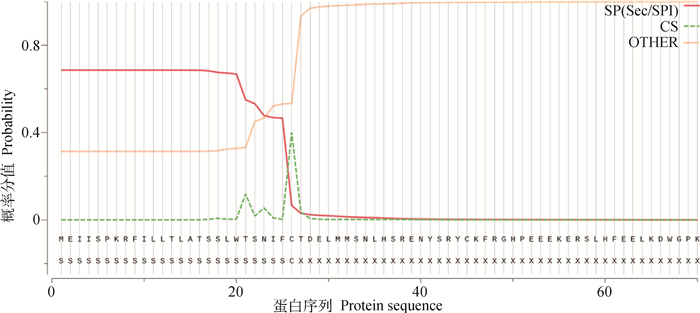
|
图 2 兔GnIH前体蛋白信号肽预测 Fig. 2 Predicted signal peptide of rabbit GnIH precursor |
在进化上,兔GnIH前体蛋白与啮齿目大鼠的关系最近,与鸡、日本鹌鹑以及九带犰狳和斑马鱼关系较远(图 3)。与大鼠、人、猕猴、马以及鹌鹑和鸡等动物GnIH前体蛋白中RFRPs和GnIH短肽氨基酸序列比较,发现兔GnIH前体蛋白中可能存在RFRP1(RFamide-related peptide 1,RF氨基相关肽1)、RFRP2(RFamide-related peptide 2,RF氨基相关肽2)和RFRP3(RFamide-related peptide 3,RF氨基相关肽3)3种GnIH同源类似物,其氨基酸序列分别为MPHAAATLPLRF、SPQPVANLPLRF和IPNLPQRF(图 4)。

|
图 3 GnIH前体蛋白系统进化树 Fig. 3 Phylogenetic tree of GnIH precursor |

|
方框表示已知的RFRPs和GnIHs氨基酸序列;虚线表示预测闽西南黑兔RFRPs氨基酸序列 The identified amino-acid sequences of RFRPs and GnIHs are boxed in solid lines; The predicted amino-acid sequences of RFRPs from Minxinan black rabbits are boxed in dashed lines 图 4 兔GnIH前体蛋白的RFRPs氨基酸序列预测 Fig. 4 Prediction of amino-acid sequences of rabbit RFRPs in GnIH precursor |
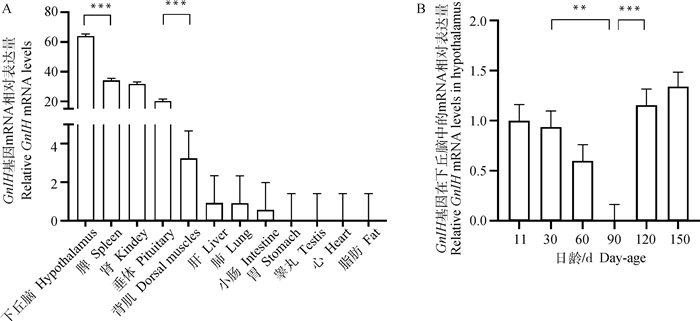
使用qPCR法检测GnIH基因在90日龄公兔不同组织中的表达情况,结果如图 5A所示,GnIH在下丘脑、脾、肾、垂体、背肌、肝、肺和小肠中均有表达,其中,下丘脑中的相对表达量最高,并且极其显著高于其他组织(P < 0.001),表示GnIH基因可能在下丘脑中发挥重要的生理作用。
|
*.表示差异显著(P < 0.05),**、***.表示差异极显著(P < 0.01, P < 0.001),下同。A. GnIH基因在不同组织中的表达;B. GnIH基因在出生后不同发育阶段下丘脑中的表达 *. indicate significant differences (P < 0.05), **, ***. indicate extremely significant differences (P < 0.01, P < 0.001), the same as below. A. Expression pattern of GnIH gene in different tissues; B. Expression pattern of GnIH gene at different developmental phases of hypothalamus 图 5 GnIH基因在公兔不同组织和不同发育阶段下丘脑中表达 Fig. 5 Expression patterns of GnIH gene in different tissues and hypothalamus at different developmental phases from male rabbits |
使用qPCR法检测GnIH基因在不同日龄公兔下丘脑中的表达,结果见图 5B,出生后GnIH基因的表达量随着日龄增长而降低,在90日龄GnIH的表达量降至最低(P < 0.01),随后出现拐点,表达量随着日龄增长极显著增加(P < 0.001)。
2.7 GnIH多肽对公兔体内生殖激素分泌的影响通过检测血清中生殖激素的浓度发现,GnRH浓度各剂量组间差异不显著(P>0.05,图 6A);FSH浓度,5和50 μg剂量组均高于0.5 μg剂量组(P < 0.05),其中50 μg剂量组和0.5 μg剂量组差异极显著(P < 0.01,图 6B);LH浓度,50 μg剂量组显著高于对照组(P < 0.05,图 6C);INHB浓度,各剂量组间差异不显著(P>0.05,图 6D);T浓度,50 μg剂量组极显著低于对照组和0.5 μg剂量组(P < 0.001),5 μg剂量组显著低于对照组和0.5 μg剂量组(P < 0.05,图 6E)。

|
A.GnIH对GnRH浓度的影响;B.GnIH对FSH浓度的影响;C.GnIH对LH浓度的影响;D.GnIH对INHB浓度的影响;E.GnIH对T的影响 A. Effect of GnIH on the concentration of GnRH; B. Effect of GnIH on the concentration of FSH; C. Effect of GnIH on the concentration of LH; D. Effect of GnIH on the concentration of INHB; E. Effect of GnIH on the concentration of T 图 6 不同注射剂量GnIH相关肽对生殖激素浓度的影响 Fig. 6 Effects of different injected doses of GnIH related peptide on the concentrations of reproductive hormones |
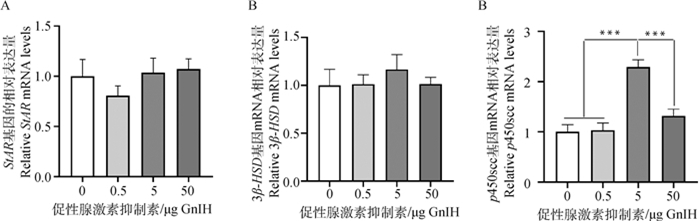
经qPCR检测发现,5 μg剂量组的p450scc mRNA表达量极其显著高于其他剂量组(P < 0.001),而注射GnIH相关肽并未引起StarR和3β-HSD这两个基因mRNA表达量发生显著变化(P>0.05,图 7)。
|
A.GnIH对StAR基因mRNA表达量的影响;B.GnIH对3β-HSD基因mRNA表达量的影响;C.GnIH对p450scc基因mRNA表达量的影响 A. Effect of GnIH on the relative StAR mRNA levels; B. Effect of GnIH on the relative 3β-HSD mRNA levels; C. Effect of GnIH on the relative p450scc mRNA levels 图 7 不同注射剂量GnIH对睾酮合成相关酶基因mRNA表达量的影响 Fig. 7 Effects of different injected doses of GnIH on the relative mRNA levels of testosterone synthetic enzyme genes |
刘晶[28]通过RT-PCR方法克隆得到了新西兰兔GnIH基因389 bp的cDNA序列,且核苷酸序列与绵羊、牛、猕猴、人、猪、小鼠有较高的同源性(>75%),可见GnIH基因在不同物种间具有一定的保守性,然而,该报道仅仅上传了GnIH基因部分cDNA序列,影响了后续验证。本研究以闽西南黑兔为试验材料,利用RACE技术克隆得到了兔GnIH基因的cDNA全长(904 bp),通过序列比对发现,一部分序列与前人上传的序列完全一致,这填补了兔GnIH基因cDNA不完整的缺陷,从而为后续相关研究奠定了基础。GnIH前体蛋白有较高的同源性,本研究根据已知的人、猕猴、大鼠、马以及鸟类GnIH相关肽及RFRPs序列[29],预测兔GnIH前体上可能存在3种同源蛋白,分别为RFRP1、RFRP2和RFRP3。
本研究通过qPCR检测了GnIH基因在闽西南黑兔公兔不同组织中的表达,组织表达谱分析显示,该基因在下丘脑、脾、肾、垂体、背肌、肝、肺和小肠中均有表达,在下丘脑中表达最高,与鸟类、猪及小鼠[7, 9, 12, 30-31]中的研究结果相类似。鹌鹑、家雀等鸟类免疫组化和原位杂交结果显示,GnIH神经元主要分布于下丘脑室旁核[28, 31-32],小啮齿类动物下丘脑也是GnIH阳性细胞高度分布区域[4-5, 11, 16, 31, 33-37],此外,绵羊的室旁核和恒河猴的视上核、室旁核和弓状核以及下丘脑背侧区均发现GnIH阳性纤维[13, 15]。本试验检测到GnIH基因在闽西南黑兔公兔下丘脑中表达水平呈现先下降后上升的趋势,表达量随着出生后日龄的增加逐渐降低,直至90日龄后才逐渐升高。根据《福建省地方畜禽品种资源志》上的记载,闽西南黑兔公兔在3月龄有性行为表现,通常3.5 ~4.5月龄达到性成熟[1]。猪和仓鼠GnIH表达量与发育状态显著相关[38-39],小丑鱼成熟期下丘脑GnIH mRNA表达量远高于发育期[40]。因此,GnIH的表达量可能与闽西南黑兔公兔生殖发育有一定的相关性。
GnIH对HPG轴抑制或刺激作用取决于动物生殖发育阶段和内源性胆固醇激素水平[3]。在小鼠电生理研究中,GnIH对41%的成年小鼠GnRH神经元放电有抑制作用以及对12%的GnRH神经元放电有刺激效果,其中,对发情前期18%小鼠GnRH神经元有刺激作用,但是对发情期的GnRH小鼠神经元没有刺激作用[41]。GnRH神经元和GTH细胞上的GnRH和GnIH受体及雌激素膜受体(membrane estrogen receptor, mER)均属于A类G蛋白偶联受体(class A GCPRs,G-protein coupled receptor),它们除了在细胞膜上以单体或者同源二聚体的形式存在,还能与其他GPCR组成异聚体,但是会导致与配体结合的亲和力、信号传导及受体内化发生改变[3, 42-43]。研究表明,发育前的阉割母鼠经过E2处理后,注射GnIH引起血液中LH浓度的降低;而给未经E2处理的阉割母鼠注射GnIH,血液中LH浓度则未发生变化[44]。此外,据Iqbal等[45]报道,低浓度E2能阻止GnRH诱导的绵羊垂体细胞内自由Ca2+浓度上升及LH的释放,Rudolf和Kadokawa[46]在牛腺垂体促性腺细胞中也发现类似现象。长期注射GnRH或其拮抗剂会使金钱鱼和鲳参鱼垂体GTH细胞产生耐受性,下调GnRH受体,抑制血清中LH、FSH和类固醇激素的水平[47-48]。在鱼类中,GnIH对垂体的刺激作用可能与给药时间、浓度或不同种类LPXRFa多肽的拮抗作用有关[49]。长期注射GnIH的短日照西伯利亚仓鼠公鼠的睾丸重量和血清中的睾酮浓度会逐渐恢复到正常值,可能也是因为下丘脑和垂体中GnIH受体下调引起的[50]。本试验通过给发情前期公兔注射GnIH相关肽后发现,5和50 μg剂量组血清中T浓度显著低于对照组和0.5 μg剂量组(P < 0.05),可能是GnIH在E2的配合下短时间内对GnRH神经元和GTH细胞负调控导致的。连续10 d给药后,外周血中的50 μg剂量组LH浓度和5 μg剂量组睾酮合成限速酶p450scc基因的表达量显著上升(P < 0.05),可能由于长期注射GnIH会下调GnRH神经元和GTH细胞上的GnIH受体数量,导致GnRH神经元和GTH细胞对GnIH不敏感。此外,外周血中T浓度显著降低后,脑部芳香化酶神经元合成的E2减少,导致GnIH抑制效果显著减弱。
4 结论本研究克隆得到兔GnIH基因cDNA序列,全长904 bp,编码201个氨基酸,序列比对发现该基因在进化过程中具有较高的保守性。GnIH基因在大部分组织均有表达,其中在下丘脑中表达最高,出生后随着日龄增加逐渐减少,90日龄后则逐渐增加。在鹌鹑GnIH相关肽注射幼龄公兔后,短时间促使外周血中T浓度显著下降,同时由于长期注射药物,公兔对GnIH不敏感,血液中的LH浓度和睾丸中睾酮合成相关酶基因p450scc的mRNA表达量显著上升,基于以上结果推测,GnIH基因可能与兔LH和睾酮的分泌与合成有关,并参与公兔的生殖发育调控,长期注射GnIH相关肽会导致公兔对药物不敏感,其具体调控机制还需后续研究。
| [1] |
《福建省地方畜禽品种资源志》编委会. 福建省地方畜禽品种资源志[M]. 福州: 福建科学技术出版社, 2019. Editorial Board of Fujian Local Livestock and Poultry Variety Resources. Indigenous animal genetic resources in Fujian[M]. Fuzhou: Fujian Science & Technology Publishing House, 2019. (in Chinese) |
| [2] |
TSUTSUI K, SAIGOH E, UKENA K, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release[J]. Biochem Biophys Res Commun, 2000, 275(2): 661-667. DOI:10.1006/bbrc.2000.3350 |
| [3] |
TSUTSUI K, UBUKA T, UKENA K. Advancing reproductive neuroendocrinology through research on the regulation of GnIH and on its diverse actions on reproductive physiology and behavior[J]. Front Neuroendocrinol, 2022, 64: 100955. DOI:10.1016/j.yfrne.2021.100955 |
| [4] |
PINELLI C, SCANDURRA A, TSUTSUI K, et al. Comparative insights of the neuroanatomical distribution of the gonadotropin-inhibitory hormone (GnIH) in fish and amphibians[J]. Front Neuroendocrinol, 2022, 65: 100991. DOI:10.1016/j.yfrne.2022.100991 |
| [5] |
KHAMIS T, ABDELALIM A F, ABDALLAH S H, et al. Early intervention with breast milk mesenchymal stem cells attenuates the development of diabetic-induced testicular dysfunction via hypothalamic Kisspeptin/Kiss1r-GnRH/GnIH system in male rats[J]. Biochim Biophys Acta Mol Basis Dis, 2020, 1866(1): 165577. DOI:10.1016/j.bbadis.2019.165577 |
| [6] |
ZHANG X, LI M, HUANG M G, et al. Effect of RFRP-3, the mammalian ortholog of GnIH, on apoptosis and autophagy in porcine ovarian granulosa cells via the p38MAPK pathway[J]. Theriogenology, 2022, 180: 137-145. DOI:10.1016/j.theriogenology.2021.12.024 |
| [7] |
WILSTERMAN K, ALONGE M M, BAO X M, et al. Food access modifies GnIH, but not CRH, cell number in the hypothalamus in a female songbird[J]. Gen Comp Endocrinol, 2020, 292: 113438. DOI:10.1016/j.ygcen.2020.113438 |
| [8] |
MA Y, LADISA C, CHANG J P, et al. Multifactorial control of reproductive and growth axis in male goldfish: influences of GnRH, GnIH and thyroid hormone[J]. Mol Cell Endocrinol, 2020, 500: 110629. DOI:10.1016/j.mce.2019.110629 |
| [9] |
VAN WYK B, FRALEY G. Ontogeny of OPN4, OPN5, GnRH and GnIH mRNA expression in the posthatch male and female Pekin duck (Anas platyrhynchos domesticus) suggests OPN4 may have additional functions beyond reproduction[J]. Animals (Basel), 2021, 11(4): 1121. |
| [10] |
QASIMI M I, NAGAOKA K, WATANABE G. Feeding of phytosterols reduced testosterone production by modulating GnRH and GnIH expression in the brain and testes of male Japanese quail (Coturnix coturnix japonica)[J]. Poult Sci, 2018, 97(3): 1066-1072. DOI:10.3382/ps/pex370 |
| [11] |
ANJUM S, KRISHNA A, TSUTSUI K. Inhibitory roles of the mammalian GnIH ortholog RFRP3 in testicular activities in adult mice[J]. J Endocrinol, 2014, 223(1): 79-91. DOI:10.1530/JOE-14-0333 |
| [12] |
SOGA T, DALPATADU S L, WONG D W, et al. Neonatal dexamethasone exposure down-regulates GnRH expression through the GnIH pathway in female mice[J]. Neuroscience, 2012, 218: 56-64. DOI:10.1016/j.neuroscience.2012.05.023 |
| [13] |
CLARKE I J, SMITH J T. The role of kisspeptin and gonadotropin inhibitory hormone (GnIH) in the seasonality of reproduction in sheep[J]. Soc Reprod Fertil Suppl, 2010, 67: 159-169. |
| [14] |
UBUKA T, MORGAN K, PAWSON A J, et al. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis[J]. PLoS One, 2009, 4(12): e8400. DOI:10.1371/journal.pone.0008400 |
| [15] |
UBUKA T, LAI H, KITANI M, et al. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain[J]. J Comp Neurol, 2009, 517(6): 841-855. DOI:10.1002/cne.22191 |
| [16] |
CÁZAREZ-MÁRQUEZ F, LARAN-CHICH M P, KLOSEN P, et al. RFRP3 increases food intake in a sex-dependent manner in the seasonal hamster Phodopus sungorus[J]. J Neuroendocrinol, 2020, 32(5): e12845. |
| [17] |
LAMM M S, LAMB A D, KLAPHEKE B P, et al. Characterization and distribution of kisspeptins, kisspeptin receptors, GnIH, and GnRH1 in the brain of the protogynous bluehead wrasse (Thalassoma bifasciatum)[J]. J Chem Neuroanat, 2022, 121: 102087. DOI:10.1016/j.jchemneu.2022.102087 |
| [18] |
TSUTSUI K, UBUKA T. Gonadotropin-inhibitory hormone (GnIH): a new key neurohormone controlling reproductive physiology and behavior[J]. Front Neuroendocrinol, 2021, 61: 100900. DOI:10.1016/j.yfrne.2021.100900 |
| [19] |
张克山, 高广亮, 李琴, 等. NPFFR1基因对鹅卵泡颗粒细胞激素分泌和细胞凋亡的影响研究[J]. 畜牧兽医学报, 2021, 52(10): 2822-2831. ZHANG K S, GAO G L, LI Q, et al. The function of NPFFR1 on hormone secretion and apoptosis of follicle granulosa cell in geese[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(10): 2822-2831. DOI:10.11843/j.issn.0366-6964.2021.010.013 (in Chinese) |
| [20] |
陈世健, 刘文俊, 杨晨, 等. 不同浓度GnIH对鸭颗粒细胞周期和增殖的影响[J]. 畜牧兽医学报, 2021, 52(1): 116-125. CHEN S J, LIU W J, YANG C, et al. Effects of different concentrations of GnIH on cell cycle and proliferation of duck granulosa cells[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(1): 116-125. (in Chinese) |
| [21] |
FERNANDES J R D, MOITRA A, TSUTSUI K, et al. Regulation of the hypothalamic GnRH-GnIH system by putrescine in adult female rats and GT1-7 neuronal cell line[J]. J Exp Zool A Ecol Integr Physiol, 2020, 333(4): 214-229. DOI:10.1002/jez.2351 |
| [22] |
SUN W, LI S H, TIAN Z Z, et al. Dynamic changes of RFRP3/GPR147 in the precocious puberty model female rats[J]. Curr Mol Med, 2019, 19(10): 766-775. DOI:10.2174/1566524019666190906142445 |
| [23] |
BENTLEY G E, TSUTSUI K, KRIEGSFELD L J. Recent studies of gonadotropin-inhibitory hormone (GnIH) in the mammalian hypothalamus, pituitary and gonads[J]. Brain Res, 2010, 1364: 62-71. DOI:10.1016/j.brainres.2010.10.001 |
| [24] |
汤亚茹, 阳美霞, 贾金美, 等. GnIH过表达载体构建及其对小鼠睾丸间质细胞的作用研究[J]. 畜牧兽医学报, 2021, 52(10): 2969-2977. TANG Y R, YANG M X, JIA J M, et al. Construction of GnIH overexpression vector and its effect on mouse Leydig cells[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(10): 2969-2977. DOI:10.11843/j.issn.0366-6964.2021.010.028 (in Chinese) |
| [25] |
UBUKA T, UENO M, UKENA K, et al. Developmental changes in gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica) hypothalamo-hypophysial system[J]. J Endocrinol, 2003, 178(2): 311-318. DOI:10.1677/joe.0.1780311 |
| [26] |
HINUMA S, SHINTANI Y, FUKUSUMI S, et al. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals[J]. Nat Cell Biol, 2000, 2(10): 703-708. DOI:10.1038/35036326 |
| [27] |
LI X, SU J, LEI Z H, et al. Gonadotropin-inhibitory hormone (GnIH) and its receptor in the female pig: cDNA cloning, expression in tissues and expression pattern in the reproductive axis during the estrous cycle[J]. Peptides, 2012, 36(2): 176-185. DOI:10.1016/j.peptides.2012.05.008 |
| [28] |
刘晶. 兔GnIH及其受体基因的克隆和mRNA在组织器官的表达[D]. 南京: 南京农业大学, 2011: 29-37. LIU J. The cloning and mRNA expression of GnIH and its receptors in rabbit[D]. Nanjing: Nanjing Agricultural University, 2011: 29-37. (in Chinese) |
| [29] |
UBUKA T, PARHAR I. Dual actions of mammalian and piscine gonadotropin-inhibitory hormones, RFamide-related peptides and LPXRFamide peptides, in the hypothalamic-pituitary-gonadal axis[J]. Front Endocrinol (Lausanne), 2017, 8: 377. |
| [30] |
DIXIT A S, BYRSAT S, SINGH N S. Circadian rhythm in photoperiodic expressions of GnRH-I and GnIH regulating seasonal reproduction in the Eurasian tree sparrow, Passer montanus[J]. J Photochem Photobiol B Biol, 2020, 211: 111993. DOI:10.1016/j.jphotobiol.2020.111993 |
| [31] |
WANG X Y, LI X, HU C H. Distribution of gonadotropin-inhibitory hormone (GnIH) in male Luchuan piglets[J]. Gene Expr Patterns, 2018, 28: 42-53. DOI:10.1016/j.gep.2018.02.004 |
| [32] |
BANERJEE S, SHAHIN S, CHATURVEDI C M. Age dependent variations in the deep brain photoreceptors (DBPs), GnRH-GnIH system and testicular steroidogenesis in Japanese quail, Coturnix coturnix japonica[J]. Exp Gerontol, 2018, 108: 7-17. DOI:10.1016/j.exger.2018.03.018 |
| [33] |
DIXIT A S, BYRSAT S, KATAKI B. Hypothalamic expression of GnRH-I and GnIH in the Eurasian tree sparrow over a single long day[J]. Photochem Photobiol Sci, 2022, 21(2): 147-158. DOI:10.1007/s43630-021-00143-6 |
| [34] |
TEO C H, SOGA T, PARHAR I. Lithium chloride enhances serotonin induced calcium activity in EGFP-GnIH neurons[J]. Sci Rep, 2020, 10(1): 13876. DOI:10.1038/s41598-020-70710-x |
| [35] |
WANG H M, KHORADMEHR A, JALALI M, et al. The roles of RFamide-related peptides (RFRPs), mammalian gonadotropin-inhibitory hormone (GnIH) orthologues in female reproduction[J]. Iran J Basic Med Sci, 2018, 21(12): 1210-1220. |
| [36] |
HAN X F, LI J L, CAO X H, et al. Surgical castration but not immuncastration is associated with reduced hypothalamic GnIH and GHRH/GH/IGF-I axis function in male rats[J]. Theriogenology, 2016, 86(2): 657-665. |
| [37] |
CALISI R M, GERAGHTY A C, AVILA A, et al. Patterns of hypothalamic GnIH change over the reproductive period in starlings and rats[J]. Gen Comp Endocrinol, 2016, 237: 140-146. |
| [38] |
ZHAO L, ZHONG M, XUE H L, et al. Effect of RFRP-3 on reproduction is sex- and developmental status-dependent in the striped hamster (Cricetulus barabensis)[J]. Gene, 2014, 547(2): 273-279. |
| [39] |
ZHENG L C, SU J, FANG R, et al. Developmental changes in the role of gonadotropin-inhibitory hormone (GnIH) and its receptors in the reproductive axis of male Xiaomeishan pigs[J]. Anim Reprod Sci, 2015, 154: 113-120. |
| [40] |
CHOI Y J, HABIBI H R, CHOI C Y. Profiles of gonadotropin-inhibitory hormone and melatonin during the sex change and maturation of cinnamon clownfish, Amphiprion melanopus[J]. Biochem Biophys Res Commun, 2016, 475(2): 189-193. |
| [41] |
DUCRET E, ANDERSON G M, HERBISON A E. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse[J]. Endocrinology, 2009, 150(6): 2799-2804. |
| [42] |
WANG H M, BU S Y, TANG J J, et al. PTPN5 promotes follicle-stimulating hormone secretion through regulating intracellular calcium homeostasis[J]. FASEB J, 2021, 35(8): e21756. |
| [43] |
KOKLIČ T, HROVAT A, GUIXÀ-GONZÁLEZ R, et al. Electron paramagnetic resonance gives evidence for the presence of type 1 gonadotropin-releasing hormone receptor (GnRH-R) in subdomains of lipid rafts[J]. Molecules, 2021, 26(4): 973. |
| [44] |
XIANG W, ZHANG B Y, LV F L, et al. The inhibitory effects of RFamide-related peptide 3 on luteinizing hormone release involves an estradiol-dependent manner in prepubertal but not in adult female mice[J]. Biol Reprod, 2015, 93(2): 30. |
| [45] |
IQBAL J, LATCHOUMANIN O, SARI I P, et al. Estradiol-17β inhibits gonadotropin-releasing hormone-induced Ca2+ in gonadotropes to regulate negative feedback on luteinizing hormone release[J]. Endocrinology, 2009, 150(9): 4213-4220. |
| [46] |
RUDOLF F O, KADOKAWA H. Cytoplasmic kinases downstream of GPR30 suppress gonadotropin-releasing hormone (GnRH)-induced luteinizing hormone secretion from bovine anterior pituitary cells[J]. J Reprod Dev, 2016, 62(1): 65-69. |
| [47] |
CHEN H P, CUI X F, WANG Y R, et al. Identification, functional characterization, and estrogen regulation on gonadotropin-releasing hormone in the spotted scat, Scatophagus argus[J]. Fish Physiol Biochem, 2020, 46(5): 1743-1757. |
| [48] |
REN X L, HUANG Y L, LI X M, et al. Identification and functional characterization of gonadotropin-releasing hormone in pompano (Trachinotus ovatus)[J]. Gen Comp Endocrinol, 2022, 316: 113958. |
| [49] |
SHAHJAHAN M, IKEGAMI T, OSUGI T, et al. Synchronised expressions of LPXRFamide peptide and its receptor genes: seasonal, diurnal and circadian changes during spawning period in grass puffer[J]. J Neuroendocrinol, 2011, 23(1): 39-51. |
| [50] |
HENNINGSEN J B, GAUER F, SIMONNEAUX V. RFRP neurons-the doorway to understanding seasonal reproduction in mammals[J]. Front Endocrinol (Lausanne), 2016, 7: 36. |
(编辑 郭云雁)



